Impact of Enhanced Recovery After Surgery with Neuromuscular Monitoring and Sugammadex on Healthcare Costs and Effectiveness of Recovery in Patients Following Anterior Cervical Spine Discectomy
Abstract
:1. Introduction
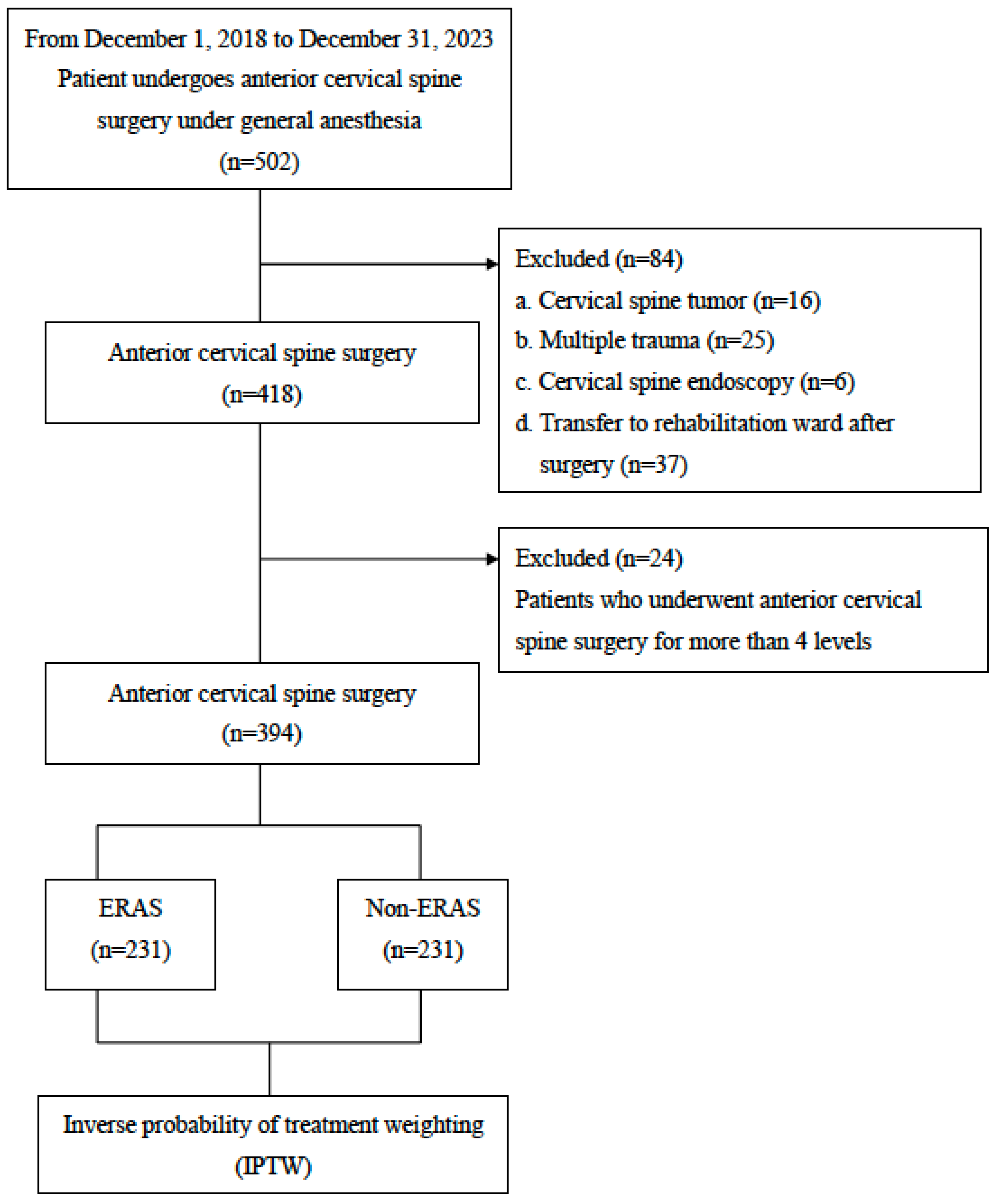
2. Materials and Methods
2.1. Data Resources
2.2. Anesthesia
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Descriptive Analysis Between ERAS Group and Non-ERAS Group After IPTW
3.3. Outcomes of Linear Regression Analysis Comparing ERAS and Non-ERAS Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Debkowska, M.P.; Butterworth, J.F.; Moore, J.E.; Kang, S.; Appelbaum, E.N.; Zuelzer, W.A. Acute post-operative airway complications following anterior cervical spine surgery and the role for cricothyrotomy. J. Spine Surg. 2019, 5, 142–154. [Google Scholar] [CrossRef]
- Kim, M.; Rhim, S.C.; Roh, S.W.; Jeon, S.R. Analysis of the Risk Factors Associated with Prolonged Intubation or Reintubation after Anterior Cervical Spine Surgery. J. Korean Med. Sci. 2018, 33, e77. [Google Scholar] [CrossRef] [PubMed]
- Sagi, H.C.; Beutler, W.; Carroll, E.; Connolly, P.J. Airway complications associated with surgery on the anterior cervical spine. Spine (Phila. Pa. 1976) 2002, 27, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Manninen, P.H.; Jose, G.B.; Lukitto, K.; Venkatraghavan, L.; El Beheiry, H. Management of the airway in patients undergoing cervical spine surgery. J. Neurosurg. Anesthesiol. 2007, 19, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, Y.; Shen, B.; Ba, Z.; Wu, D. Multivariate analysis of airway obstruction and reintubation after anterior cervical surgery: A retrospective cohort study of 774 patients. Int. J. Surg. 2017, 41, 28–33. [Google Scholar] [CrossRef]
- Boddapati, V.; Lee, N.J.; Mathew, J.; Held, M.B.; Peterson, J.R.; Vulapalli, M.M.; Lombardi, J.M.; Dyrszka, M.D.; Sardar, Z.M.; Lehman, R.A.; et al. Respiratory Compromise After Anterior Cervical Spine Surgery: Incidence, Subsequent Complications, and Independent Predictors. Global Spine J. 2022, 12, 1647–1654. [Google Scholar] [CrossRef]
- Jing, X.; Zhu, Z.; Fan, H.; Wang, J.; Fu, Q.; Kong, R.; Long, Y.; Wang, S.; Wang, Q. Impact of delay extubation on the reintubation rate in patients after cervical spine surgery: A retrospective cohort study. J. Orthop. Surg. Res. 2023, 18, 557. [Google Scholar] [CrossRef]
- Ljungqvist, O.; Scott, M.; Fearon, K.C. Enhanced Recovery After Surgery: A Review. JAMA Surg. 2017, 152, 292–298. [Google Scholar] [CrossRef]
- Zaed, I.; Bossi, B.; Ganau, M.; Tinterri, B.; Giordano, M.; Chibbaro, S. Current state of benefits of Enhanced Recovery After Surgery (ERAS) in spinal surgeries: A systematic review of the literature. Neurochirurgie 2022, 68, 61–68. [Google Scholar] [CrossRef]
- Debono, B.; Sabatier, P.; Boniface, G.; Bousquet, P.; Lescure, J.P.; Garnaud, V.; Hamel, O.; Lonjon, G. Implementation of enhanced recovery after surgery (ERAS) protocol for anterior cervical discectomy and fusion: A propensity score-matched analysis. Eur. Spine J. 2021, 30, 560–567. [Google Scholar] [CrossRef]
- Wang, P.; Kong, C.; Teng, Z.; Zhang, S.; Cui, P.; Wang, S.; Zhao, G.; Lu, S. Enhanced Recovery After Surgery (ERAS) Program for Anterior Cervical Discectomy and Fusion (ACDF) in Patients Over 60 Years Old. Clin. Interv. Aging 2023, 18, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Soffin, E.M.; Wetmore, D.S.; Barber, L.A.; Vaishnav, A.S.; Beckman, J.D.; Albert, T.J.; Gang, C.H.; Qureshi, S.A. An enhanced recovery after surgery pathway: Association with rapid discharge and minimal complications after anterior cervical spine surgery. Neurosurg. Focus. 2019, 46, E9. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, H.; Xv, Z.K.; Wang, J.; Yu, Q.F.; Chen, G.; Li, F.C.; Ren, Y.; Chen, Q.X. Enhanced recovery care versus traditional care following laminoplasty: A retrospective case-cohort study. Medicine 2018, 97, e13195. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.C. Enhanced Recovery After Surgery (ERAS) Spine Pathways and the Role of Perioperative Checklists. Adv. Tech. Stand. Neurosurg. 2024, 49, 73–94. [Google Scholar] [CrossRef]
- Moskven, E.; McIntosh, G.; Nataraj, A.; Christie, S.D.; Kumar, R.; Phan, P.; Wang, Z.; Tarabay, B.; Weber, M.H.; Singh, S.; et al. Factors associated with increased length of stay in degenerative cervical spine surgery: A cohort analysis from the Canadian Spine Outcomes and Research Network. J. Neurosurg. Spine 2024, 41, 46–55. [Google Scholar] [CrossRef]
- Debono, B.; Corniola, M.V.; Pietton, R.; Sabatier, P.; Hamel, O.; Tessitore, E. Benefits of Enhanced Recovery After Surgery for fusion in degenerative spine surgery: Impact on outcome, length of stay, and patient satisfaction. Neurosurg. Focus. 2019, 46, E6. [Google Scholar] [CrossRef]
- Qin, X.; Li, H.; Long, J.; Feng, C. A meta-analysis of the implementation of enhanced recovery after surgery pathways in anterior cervical spine surgery for degenerative cervical spine diseases. Eur. Spine J. 2024, 33, 1283–1291. [Google Scholar] [CrossRef]
- Mesfin, F.B.; Hoang, S.; Ortiz Torres, M.; Ngnitewe Massa’a, R.; Castillo, R. Retrospective Data Analysis and Literature Review for a Development of Enhanced Recovery after Surgery Pathway for Anterior Cervical Discectomy and Fusion. Cureus 2020, 12, e6930. [Google Scholar] [CrossRef]
- Lied, B.; Sundseth, J.; Helseth, E. Immediate (0-6 h), early (6-72 h) and late (>72 h) complications after anterior cervical discectomy with fusion for cervical disc degeneration; discharge six hours after operation is feasible. Acta Neurochir. 2008, 150, 111–118. [Google Scholar] [CrossRef]
- Tasiou, A.; Giannis, T.; Brotis, A.G.; Siasios, I.; Georgiadis, I.; Gatos, H.; Tsianaka, E.; Vagkopoulos, K.; Paterakis, K.; Fountas, K.N. Anterior cervical spine surgery-associated complications in a retrospective case-control study. J. Spine Surg. 2017, 3, 444–459. [Google Scholar] [CrossRef] [PubMed]
- Nagoshi, N.; Fehlings, M.G.; Nakashima, H.; Tetreault, L.; Gum, J.L.; Smith, Z.A.; Hsu, W.K.; Tannoury, C.A.; Tannoury, T.; Traynelis, V.C.; et al. Prevalence and Outcomes in Patients Undergoing Reintubation After Anterior Cervical Spine Surgery: Results from the AOSpine North America Multicenter Study on 8887 Patients. Global Spine J. 2017, 7, 96s–102s. [Google Scholar] [CrossRef] [PubMed]
- Epstein, N. Frequency, recognition, and management of postoperative hematomas following anterior cervical spine surgery: A review. Surg. Neurol. Int. 2020, 11, 356. [Google Scholar] [CrossRef]
- Raksakietisak, M.; Keawsai, T.; Sirivanasandha, B. Factors Related to Delayed Extubation in Cervical Spine Surgery in an Academic Hospital: A Retrospective Study of 506 Patients. Asian J. Anesthesiol. 2019, 57, 111–116. [Google Scholar] [CrossRef]
- Fuchs-Buder, T.; Romero, C.S.; Lewald, H.; Lamperti, M.; Afshari, A.; Hristovska, A.M.; Schmartz, D.; Hinkelbein, J.; Longrois, D.; Popp, M.; et al. Peri-operative management of neuromuscular blockade: A guideline from the European Society of Anaesthesiology and Intensive Care. Eur. J. Anaesthesiol. 2023, 40, 82–94. [Google Scholar] [CrossRef]
- Thilen, S.R.; Weigel, W.A.; Todd, M.M.; Dutton, R.P.; Lien, C.A.; Grant, S.A.; Szokol, J.W.; Eriksson, L.I.; Yaster, M.; Grant, M.D.; et al. 2023 American Society of Anesthesiologists Practice Guidelines for Monitoring and Antagonism of Neuromuscular Blockade: A Report by the American Society of Anesthesiologists Task Force on Neuromuscular Blockade. Anesthesiology 2023, 138, 13–41. [Google Scholar] [CrossRef]
- Carr, S.G.; Clifton, J.C.; Freundlich, R.E.; Fowler, L.C.; Sherwood, E.R.; McEvoy, M.D.; Robertson, A.; Dunworth, B.A.; McCarthy, K.Y.; Shotwell, M.S.; et al. Improving Neuromuscular Monitoring Through Education-Based Interventions and Studying Its Association with Adverse Postoperative Outcomes: A Retrospective Observational Study. Anesth. Analg. 2024, 138, 517–529. [Google Scholar] [CrossRef]
- Cheng, K.I.; Tse, J.; Li, T.Y. The Strategy to Use Sugammadex to Reduce Postoperative Pulmonary Complications after da Vinci Surgery: A Retrospective Study. J. Pers. Med. 2022, 12, 52. [Google Scholar] [CrossRef]
- Min, B.H.; Oh, T.K.; Song, I.A.; Jeon, Y.T. Comparison of the effects of sugammadex and neostigmine on hospital stay in robot-assisted laparoscopic prostatectomy: A retrospective study. BMC Anesthesiol. 2020, 20, 178. [Google Scholar] [CrossRef]
- Bardia, A.; Treggiari, M.M.; Dai, F.; Johnson, C.; Singh, M.; Kunze, K.; Tickoo, M.; Tantawy, H.; Giersson, A.; Darr, U.; et al. Efficacy and Safety of Sugammadex to Shorten Time-to-Extubation Following Cardiac Surgery: A Single-Center Randomized Placebo-Controlled Trial. Crit. Care Explor. 2022, 4, e0821. [Google Scholar] [CrossRef]
- Song, S.W.; Yoo, K.Y.; Ro, Y.S.; Pyeon, T.; Bae, H.B.; Kim, J. Sugammadex is associated with shorter hospital length of stay after open lobectomy for lung cancer: A retrospective observational study. J. Cardiothorac. Surg. 2021, 16, 45. [Google Scholar] [CrossRef] [PubMed]
- Olesnicky, B.L.; Farrell, C.; Clare, P.; Wen, S.; Leslie, K.; Delaney, A. The effect of sugammadex on patient morbidity and quality of recovery after general anaesthesia: A systematic review and meta-analysis. Br. J. Anaesth. 2024, 132, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Wu, E.B.; Li, Y.Y.; Hung, K.C.; Illias, A.M.; Tsai, Y.F.; Yang, Y.L.; Chin, J.C.; Wu, S.C. The Impact of Rocuronium and Sugammadex on Length of Stay in Patients Undergoing Open Spine Surgery: A Propensity Score-Matched Analysis. Bioengineering 2023, 10, 959. [Google Scholar] [CrossRef] [PubMed]
- Mraovic, B.; Timko, N.J.; Choma, T.J. Comparison of recovery after sugammadex or neostigmine reversal of rocuronium in geriatric patients undergoing spine surgery: A randomized controlled trial. Croat. Med. J. 2021, 62, 606–613. [Google Scholar] [CrossRef]
| Variable | Before IPTW | After IPTW | |||||
|---|---|---|---|---|---|---|---|
| ERAS (N = 231) Mean ± SD N(%) | Non-ERAS (N = 163) Mean ± SD N(%) | p-Value | ERAS Mean ± SD (%) | Non-ERAS Mean ± SD (%) | p-Value | ||
| Demographic characteristics | |||||||
| Age | 54.72 ± 11.63 | 57.51 ± 11.02 | 0.014 | 56.58 ± 11.13 | 56.39 ± 11.48 | 0.8 | |
| Sex | male | 113 (48.9) | 85 (52.1) | 0.6 | (53.2) | (50.4) | 0.5 |
| female | 118 (51.1) | 78 (47.9) | (46.8) | (49.6) | |||
| BMI | 25.24 ± 3.91 | 25.63 ± 4.35 | 0.4 | 26.00 ± 4.23 | 25.67 ± 4.11 | 0.3 | |
| Smoking | 30(13.0) | 33 (20.2) | 0.072 | (20.8) | (17.8) | 0.3 | |
| ASA | I and II | 120 (51.9) | 69 (42.3) | 0.075 | (51.2) | (47.0) | 0.3 |
| III | 111 (48.1) | 94 (57.7) | (48.8) | (53.0) | |||
| Comorbid factors | |||||||
| Hypertension | 56(24.2) | 55 (33.7) | 0.5 | (36.6) | (30.5) | 0.084 | |
| Diabetes | 30(13.0) | 36 (22.1) | 0.025 | (20.5) | (18.6) | 0.6 | |
| CAD | 6(2.6) | 4 (2.5) | 1.0 | (2.0) | (2.0) | 1.0 | |
| Clinical characteristics | |||||||
| Level | I | 103 (44.6) | 27 (16.6) | <0.001 | (28.5) | (31.1) | 0.4 |
| II | 94 (40.7) | 60 (36.8) | (34.4) | (36.0) | |||
| III | 34 (14.7) | 76 (46.6) | (37.1) | (32.9) | |||
| Anesthesia method | intravenous | 77 (33.3) | 25 (15.3) | <0.001 | (22.3) | (18.9) | 0.3 |
| inhalational | 154 (66.7) | 138 (84.7) | (77.7) | (81.1) | |||
| Postoperative analgesics | 0–1 | 122 (52.8) | 140 (85.9) | <0.001 | (70.1) | (72.5) | 0.5 |
| ≥2 | 109 (47.2) | 23 (14.1) | (29.9) | (27.5) | |||
| Anesthesia time (min) | 218.53 ± 59.36 | 265.62 ± 71.26 | <0.001 | 251.10 ± 75.86 | 244.01 ± 68.24 | 0.2 | |
| Surgical time (min) | 166.18 ± 57.82 | 214.34 ± 67.27 | <0.001 | 198.56 ± 73.74 | 193.03 ± 63.49 | 0.3 | |
| Variable | After IPTW | ||
|---|---|---|---|
| ERAS Mean ± SD (%) | Non-ERAS Mean ± SD (%) | p-Value | |
| Medical expenses | |||
| LOS | 5.69 ± 1.86 | 6.32 ± 3.06 | <0.001 |
| Cost (NTD) | 134,436.66 ± 45,005.77 | 147,070.22 ± 60,946.94 | 0.001 |
| Ventilator time (minutes) | 0 | 161.06 ± 200.91 | <0.001 |
| ICU admission | (28.9) | (100) | <0.001 |
| Complications | |||
| Neck hematoma | (0.7) | (0.6) | 1.000 |
| Reoperation | (0.2) | (0.6) | 0.8 |
| Pulmonary complications | (0) | (1.1) | 0.080 |
| Postoperative recovery indicators | |||
| First oral intake time (hours) | 8.02 ± 3.10 | 12.91 ± 4.72 | <0.001 |
| First ambulation time (hours) | 18.19 ± 8.70 | 25.95 ± 12.11 | <0.001 |
| Variable | Unadjusted Model | p-Value | Adjusted Model | p-Value | ||
|---|---|---|---|---|---|---|
| Beta | 95%CI | Beta | 95%CI | |||
| LOS | ||||||
| ERAS vs. non-ERAS | −0.63 | −0.98~−0.29 | <0.001 | −0.62 | −0.94~−0.29 | <0.001 |
| Cost (NTD) | ||||||
| ERAS vs. non-ERAS | −12,633.60 | −20,032.01~−5235.10 | 0.001 | −13,174.40 | −20,306.10~−6042.60 | <0.001 |
| Ventilator time (min) | ||||||
| ERAS vs. non-ERAS | −161.56 | −179.80~−142.30 | <0.001 | −149.40 | −166.30~−132.60 | <0.001 |
| First oral intake time (hrs) | ||||||
| ERAS vs. non-ERAS | −4.89 | −5.44~−4.34 | <0.001 | −4.71 | −5.22~−4.20 | <0.001 |
| First ambulation time (hrs) | ||||||
| ERAS vs. non-ERAS | −7.76 | −9.21~−6.30 | <0.001 | −8.00 | −9.38~−6.62 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, H.-T.; Chen, S.-Y.; Huang, Y.-K.; Cheng, K.-I.; Weng, S.-F.; Wu, Z.-F. Impact of Enhanced Recovery After Surgery with Neuromuscular Monitoring and Sugammadex on Healthcare Costs and Effectiveness of Recovery in Patients Following Anterior Cervical Spine Discectomy. J. Pers. Med. 2025, 15, 87. https://doi.org/10.3390/jpm15030087
Hsu H-T, Chen S-Y, Huang Y-K, Cheng K-I, Weng S-F, Wu Z-F. Impact of Enhanced Recovery After Surgery with Neuromuscular Monitoring and Sugammadex on Healthcare Costs and Effectiveness of Recovery in Patients Following Anterior Cervical Spine Discectomy. Journal of Personalized Medicine. 2025; 15(3):87. https://doi.org/10.3390/jpm15030087
Chicago/Turabian StyleHsu, Hung-Te, Szu-Yu Chen, Yu-Kai Huang, Kuang-I Cheng, Shih-Feng Weng, and Zhi-Fu Wu. 2025. "Impact of Enhanced Recovery After Surgery with Neuromuscular Monitoring and Sugammadex on Healthcare Costs and Effectiveness of Recovery in Patients Following Anterior Cervical Spine Discectomy" Journal of Personalized Medicine 15, no. 3: 87. https://doi.org/10.3390/jpm15030087
APA StyleHsu, H.-T., Chen, S.-Y., Huang, Y.-K., Cheng, K.-I., Weng, S.-F., & Wu, Z.-F. (2025). Impact of Enhanced Recovery After Surgery with Neuromuscular Monitoring and Sugammadex on Healthcare Costs and Effectiveness of Recovery in Patients Following Anterior Cervical Spine Discectomy. Journal of Personalized Medicine, 15(3), 87. https://doi.org/10.3390/jpm15030087






