Vimentin and p53 Immunoreactivity in Cases of Traumatic Brain Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Group Selection
2.2. Sample Preparation and Microscopic Analysis
2.3. Semi-Quantitative Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hardman, J.M.; Manoukian, A. Pathology of head trauma. Neuroimaging Clin. N. Am. 2002, 12, 175–187. [Google Scholar] [PubMed]
- Menon, D.K.; Schwab, K.; Wright, D.W.; Maas, A.I. Demographics and Clinical Assessment Working Group of the International and Interagency Initiative toward Common Data Elements for Research on Traumatic Brain Injury and Psychological Health. Position statement: Definition of traumatic brain injury. Arch. Phys. Med. Rehabil. 2010, 91, 1637–1640. [Google Scholar] [PubMed]
- Turillazzi, E.; Manetti, A.C.; Turco, S.; Parte, V. Capitolo 2, La medicina legale. In Diritto Penale della Circolazione Stradale, a Cura di Balzani Simone, Trinci Alessandro; CEDAM, Wolters Kluwer: Bitritto, Italy, 2021; pp. 593–1002. [Google Scholar]
- Skandsen, T.; Kvistad, K.A.; Solheim, O.; Strand, I.H.; Folvik, M.; Vik, A. Prevalence and impact of diffuse axonal injury in patients with moderate and severe head injury: A cohort study of early magnetic resonance imaging findings and 1-year outcome. J. Neurosurg. 2010, 113, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.H.; Meaney, D.F.; Shull, W.H. Diffuse axonal injury in head trauma. J. Head Trauma Rehabil. 2003, 18, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.H.; Graham, D.I.; Murray, L.S.; Scott, G. Diffuse axonal injury due to nonmissile head injury in humans: An analysis of 45 cases. Ann. Neurol. 1982, 12, 557–563. [Google Scholar]
- Hortobágyi, T.; Wise, S.; Hunt, N.; Cary, N.; Djurovic, V.; Fegan-Earl, A.; Shorrock, K.; Rouse, D.; Al-Sarraj, S. Traumatic axonal damage in the brain can be detected using beta-APP immunohistochemistry within 35 min after head injury to human adults. Neuropathol. Appl. Neurobiol. 2007, 33, 226–237. [Google Scholar] [CrossRef]
- Beer, R.; Franz, G.; Srinivasan, A.; Hayes, R.L.; Pike, B.R.; Newcomb, J.K.; Zhao, X.; Schmutzhard, E.; Poewe, W.; Kampfl, A. Temporal profile and cell subtype distribution of activated caspase-3 following experimental traumatic brain injury. J. Neurochem. 2000, 75, 1264–1273. [Google Scholar]
- Franz, G.; Beer, R.; Intemann, D.; Krajewski, S.; Reed, J.C.; Engelhardt, K.; Pike, B.R.; Hayes, R.L.; Wang, K.K.; Schmutzhard, E.; et al. Temporal and spatial profile of Bid cleavage after experimental traumatic brain injury. J. Cereb. Blood Flow Metab. 2002, 22, 951–958. [Google Scholar]
- Esposti, M.D. The roles of Bid. Apoptosis 2002, 7, 433–440. [Google Scholar]
- Larner, S.F.; McKinsey, D.M.; Hayes, R.L.; Wang, K.K.W. Caspase 7: Increased expression and activation after traumatic brain injury in rats. J. Neurochem. 2005, 94, 97–108. [Google Scholar] [CrossRef]
- Dressler, J.; Hanisch, U.; Kuhlisch, E.; Geiger, K.D. Neuronal and glial apoptosis in human traumatic brain injury. Int. J. Legal Med. 2007, 121, 365–375. [Google Scholar] [PubMed]
- Napoletano, G.; Marinelli, E.; Palla, L.; Zaami, S.; Maiese, A. Expression of RIPK-1 and S-100B in Traumatic Brain Injury—Exploring a Forensic Cases Series. Int. J. Legal Med. 2024. [Google Scholar] [CrossRef]
- Maiese, A.; Spina, F.; Visi, G.; Del Duca, F.; De Matteis, A.; La Russa, R.; Di Paolo, M.; Frati, P.; Fineschi, V. The Expression of FOXO3a as a Forensic Diagnostic Tool in Cases of Traumatic Brain Injury: An Immunohistochemical Study. Int. J. Mol. Sci. 2023, 24, 2584. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.J.; Kaiser, A.; Yu, J.W.; Natale, J.E.; Al-Abdulla, N.A. Injury-induced apoptosis of neurons in adult brain is mediated by p53-dependent and p53-independent pathways and requires bax. J. Comp. Neurol. 2001, 433, 299–311. [Google Scholar]
- Grady, M.S.; Charleston, J.S.; Maris, D.; Witgen, B.M.; Lifshitz, J. Neuronal and glial cell number in the hippocampus after experimental traumatic brain injury: Analysis by stereological estimation. J. Neurotrauma 2003, 20, 929–941. [Google Scholar]
- Schuler, M.; Green, D.R. Mechanisms of p53-dependent apoptosis. Biochem. Soc. Trans. 2001, 29, 684–688. [Google Scholar]
- Nicolai, S.; Rossi, A.; Di Daniele, N.; Melino, G.; Annicchiarico-Petruzzelli, M.; Raschellà, G. DNA repair and aging: The impact of the p53 family. Aging 2015, 7, 1050–1065. [Google Scholar]
- Ng, S.Y.; Lee, A.Y.W. Traumatic Brain Injuries: Pathophysiology and Potential Therapeutic Targets. Front. Cell Neurosci. 2019, 13, 528. [Google Scholar]
- Ivaska, J.; Pallari, H.-M.; Nevo, J.; Eriksson, J.E. Novel functions of vimentin in cell adhesion, migration, and signaling. Exp. Cell Res. 2007, 313, 2050–2062. [Google Scholar]
- Schaffeld, M.; Herrmann, H.; Schultess, J.; Markl, J. Vimentin and desmin of a cartilaginous fish, the shark Scyliorhinus stellaris: Sequence, expression patterns and in vitro assembly. Eur. J. Cell Biol. 2001, 80, 692–702. [Google Scholar]
- Colucci-Guyon, E.; Giménez Y Ribotta, M.; Maurice, T.; Babinet, C.; Privat, A. Cerebellar defect and impaired motor coordination in mice lacking vimentin. Glia 1999, 25, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Eckes, B.; Colucci-Guyon, E.; Smola, H.; Nodder, S.; Babinet, C.; Krieg, T.; Martin, P. Impaired wound healing in embryonic and adult mice lacking vimentin. J. Cell Sci. 2000, 113 Pt 13, 2455–2462. [Google Scholar] [CrossRef] [PubMed]
- Eckes, B.; Dogic, D.; Colucci-Guyon, E.; Wang, N.; Maniotis, A.; Ingber, D.; Merckling, A.; Langa, F.; Aumailley, M.; Delouvée, A.; et al. Impaired mechanical stability, migration and contractile capacity in vimentin-deficient fibroblasts. J. Cell Sci. 1998, 111 Pt 13, 1897–1907. [Google Scholar] [CrossRef] [PubMed]
- Larsson, A.; Wilhelmsson, U.; Pekna, M.; Pekny, M. Increased cell proliferation and neurogenesis in the hippocampal dentate gyrus of old GFAP(−/−)Vim(−/−) mice. Neurochem. Res. 2004, 29, 2069–2073. [Google Scholar] [CrossRef] [PubMed]
- Satelli, A.; Li, S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol. Life Sci. 2011, 68, 3033–3046. [Google Scholar] [CrossRef]
- Paulin, D.; Lilienbaum, A.; Kardjian, S.; Agbulut, O.; Li, Z. Vimentin: Regulation and pathogenesis. Biochimie 2022, 197, 96–112. [Google Scholar] [CrossRef]
- Rahaman, P.; Del Bigio, M.R. Histology of Brain Trauma and Hypoxia-Ischemia. Acad. Forensic Pathol. 2018, 8, 539–554. [Google Scholar] [CrossRef]
- O’Leary, L.A.; Davoli, M.A.; Belliveau, C.; Tanti, A.; Ma, J.C.; Farmer, W.T.; Turecki, G.; Murai, K.K.; Mechawar, N. Characterization of Vimentin-Immunoreactive Astrocytes in the Human Brain. Front. Neuroanat. 2020, 14, 31. [Google Scholar] [CrossRef]
- Hausmann, R.; Betz, P. Course of glial immunoreactivity for vimentin, tenascin and alpha1-antichymotrypsin after traumatic injury to human brain. Int. J. Legal Med. 2001, 114, 338–342. [Google Scholar] [CrossRef]
- Danielsson, F.; Peterson, M.K.; Caldeira Araújo, H.; Lautenschläger, F.; Gad, A.K.B. Vimentin Diversity in Health and Disease. Cells 2018, 7, 147. [Google Scholar] [CrossRef]
- Lopez-Rodriguez, A.B.; Acaz-Fonseca, E.; Viveros, M.-P.; Garcia-Segura, L.M. Changes in cannabinoid receptors, aquaporin 4 and vimentin expression after traumatic brain injury in adolescent male mice. Association with edema and neurological deficit. PLoS ONE 2015, 10, e0128782. [Google Scholar] [CrossRef] [PubMed]
- Ekmark-Lewén, S.; Lewén, A.; Israelsson, C.; Li, G.L.; Farooque, M.; Olsson, Y.; Ebendal, T.; Hillered, L. Vimentin and GFAP responses in astrocytes after contusion trauma to the murine brain. Restor. Neurol. Neurosci. 2010, 28, 311–321. [Google Scholar] [PubMed]
- Sarkis, G.A.; Lees-Gayed, N.; Banoub, J.; Abbatielo, S.E.; Robertson, C.; Haskins, W.E.; Yost, R.A.; Wang, K.K.W. Generation and Release of Neurogranin, Vimentin, and MBP Proteolytic Peptides, Following Traumatic Brain Injury. Mol. Neurobiol. 2022, 59, 731–747. [Google Scholar] [PubMed]

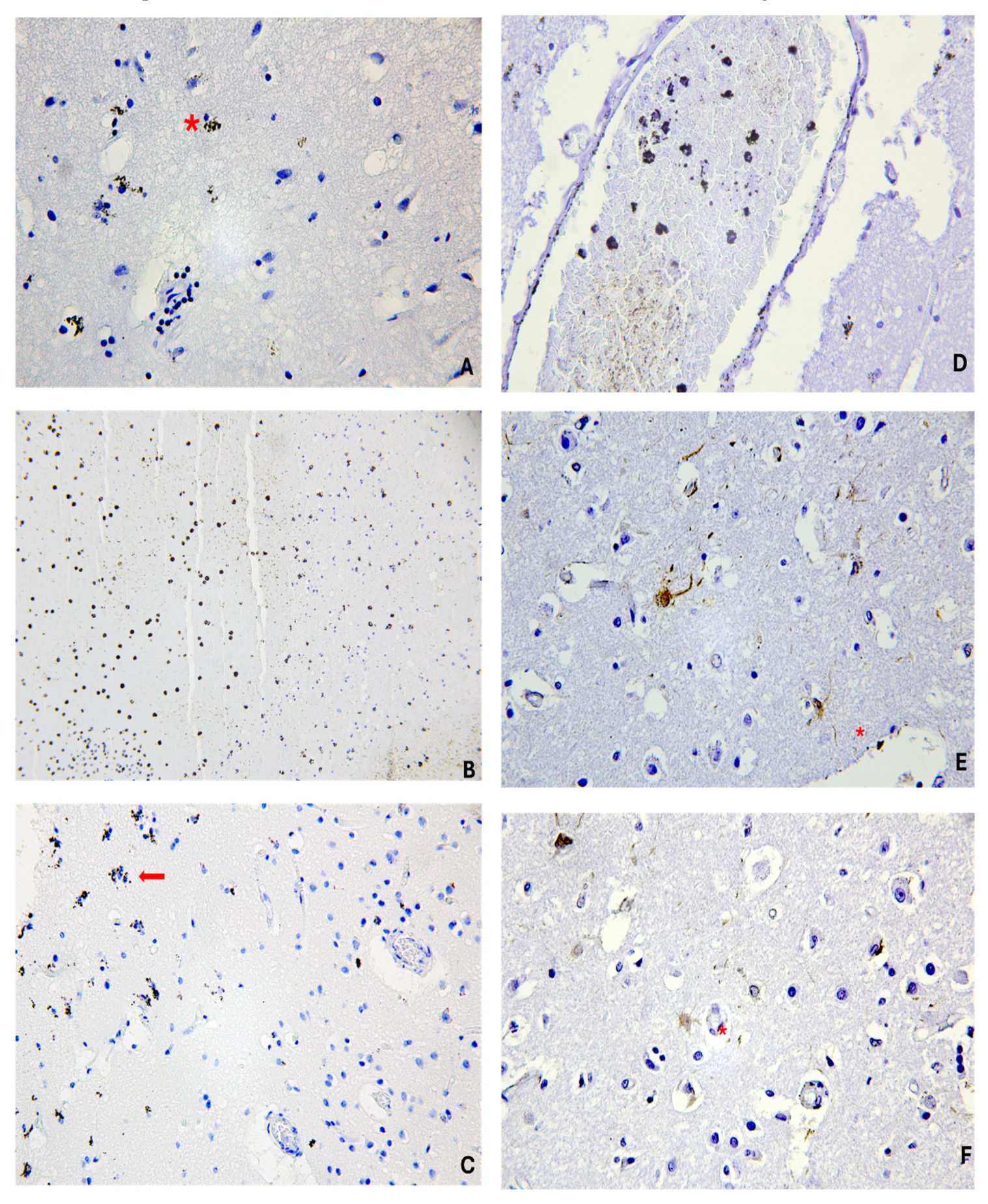
| Case | Age/Sex | Traumatic Event | PTI/PMI | Autopsy Findings |
|---|---|---|---|---|
| 1 | 56/m | Neck and head trauma after assault | 4 days/24 h | Brain contusion, subarachnoid hemorrhage, and carotid dissection |
| 2 | 16/m | Traffic accident with traumatic brain injury | 0 h/26 h | Brain contusion and laceration, subarachnoid hemorrhage, edema, head fracture, leg fracture and various skin wounds |
| 3 | 71/f | Traffic accident | 12 days/30 h | Brain contusion and hypoxic-ischemic brain injury following cardiac arrest |
| 4 | 48/m | Traffic accident with cerebral death | 2 days/25 h | Brain contusion |
| 5 | 49/m | Traffic accident | 4 days/25 h | Brain contusion |
| 6 | 49/m | Fall from height and heroin abuse | 0 h/24 h | Brain swelling and pulmonary edema |
| 7 | 5/m | Fall from height | 0 h/24 h | Brain contusion and head fracture |
| 8 | 47/m | Head assault | 0 h/28 h | Brain contusion and laceration, subarachnoid hemorrhage, edema |
| 9 | 41/m | Head assault | 0 h/24 h | Bran contusion and laceration, subarachnoid hemorrhage, edema |
| 10 | 71/m | Traffic accident car-car | 5 days/27 h | Subdural hematoma, bran contusions, subarachnoid hemorrhage and secondary brain injury |
| 11 | 29/m | Fall from height with alcohol intoxication | 0 h/36 h | Brain swelling and contusion, thorax fracture, heart rupture |
| 12 | 80/f | Traffic accident car-car | 0 h/25 h | Brain contusion, subdural hemorrhage, hands and foot fracture, sternal bruises |
| Grade | Meaning |
|---|---|
| 0 | Absence of staining |
| +1 | Minimal staining in minor areas/spots |
| +2 | Clear staining in more diffuse areas |
| +3 | Diffuse intense staining |
| Cases | Age | PTI | Vimentin | p53 |
|---|---|---|---|---|
| 1 | 56 y.o. | 4 days | Positive astrocytes near the vessels | Positive neurons near the hemorrhagic areas |
| 2 | 16 y.o. | 0 h | Negative | Negative |
| 3 | 71 y.o. | 12 days | Positive astrocytes within the cortex | Positive spots within the neurons |
| 4 | 48 y.o. | 2 days | Positive astrocytes near the vessels | Positive neurons near the hemorrhagic areas |
| 5 | 49 y.o. | 4 days | Positive astrocytes near the vessels | Positive spots within the neurons |
| 6 | 49 y.o. | 0 h | Negative | Negative |
| 7 | 5 y.o. | 0 h | Negative | Positive spots within the neurons |
| 8 | 47 y.o. | 0 h | Negative | Positive neurons near the hemorrhagic areas |
| 9 | 41 y.o. | 0 h | Negative | Positive neurons near the hemorrhagic areas |
| 10 | 71 y.o. | 5 days | Positive astrocytes near the vessels | Positive spots within the neurons |
| 11 | 29 y.o. | 0 h | Negative | Negative |
| 12 | 80 y.o. | 0 h | Positive astrocytes near the vessels | Positive neurons near the hemorrhagic areas |
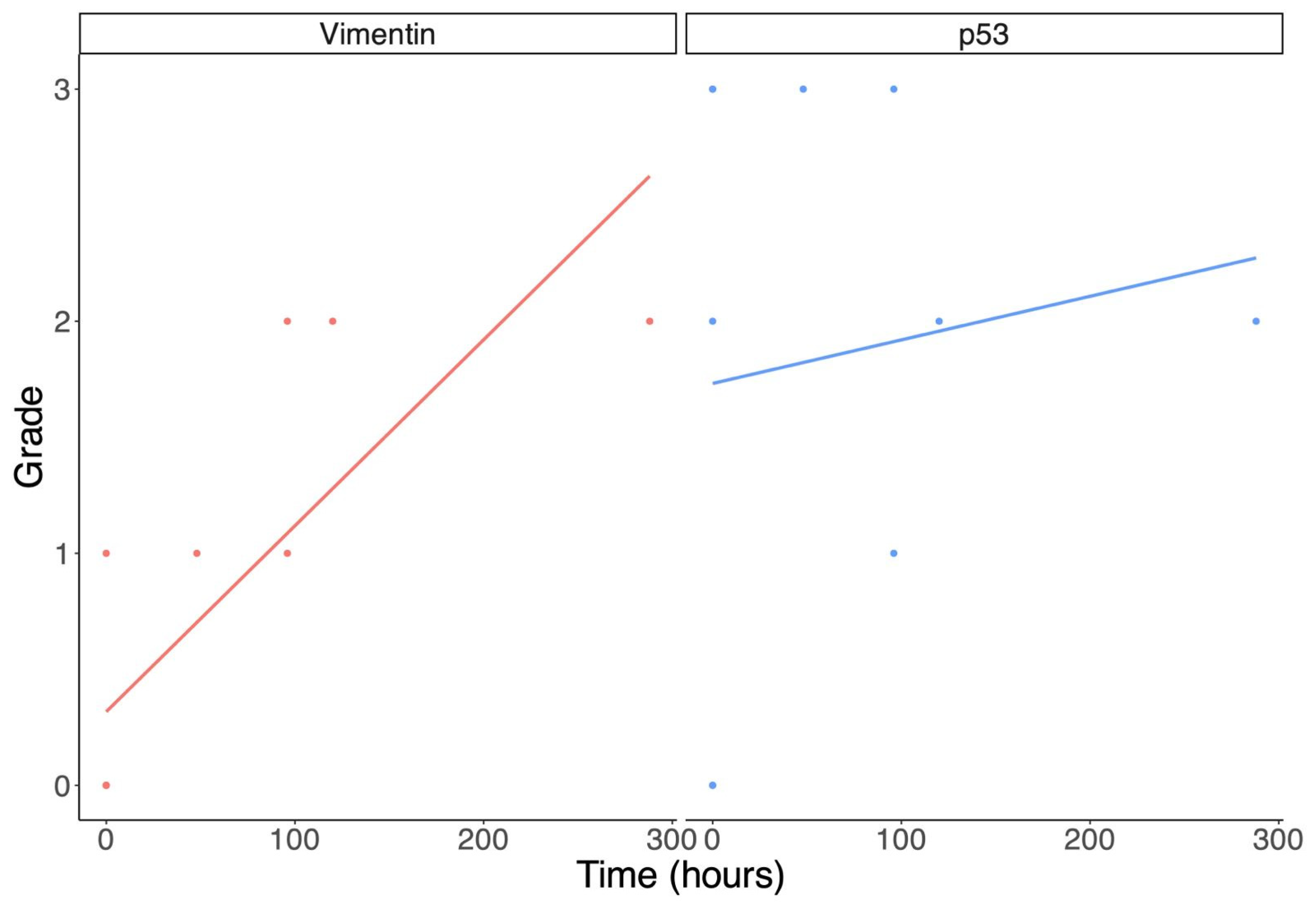
| Cases | Age | PTI | Vimentin Grade | p53 Grade |
|---|---|---|---|---|
| 1 | 56 y.o. | 4 days | +1 | +3 |
| 2 | 16 y.o. | 0 h | 0 | 0 |
| 3 | 71 y.o. | 12 days | +2 | +2 |
| 4 | 48 y.o. | 2 days | +1 | + 3 |
| 5 | 49 y.o. | 4 days | +2 | +1 |
| 6 | 49 y.o. | 0 h | 0 | 0 |
| 7 | 5 y.o. | 0 h | 0 | +2 |
| 8 | 47 y.o. | 0 h | 0 | +3 |
| 9 | 41 y.o. | 0 h | 0 | +3 |
| 10 | 71 y.o. | 5 days | +2 | +2 |
| 11 | 29 y.o. | 0 h | 0 | 0 |
| 12 | 80 y.o. | 0 h | +1 | +3 |
| Controls | Vimentin Grade | p53 Grade |
|---|---|---|
| 13 | 0 | 0 |
| 14 | 0 | 0 |
| 15 | 0 | 0 |
| 16 | 0 | +1 |
| 17 | 0 | 0 |
| 18 | 0 | 0 |
| 19 | 0 | 0 |
| 20 | 0 | 0 |
| 21 | 0 | 0 |
| 22 | 0 | 0 |
| 23 | 0 | 0 |
| 24 | +1 | 0 |
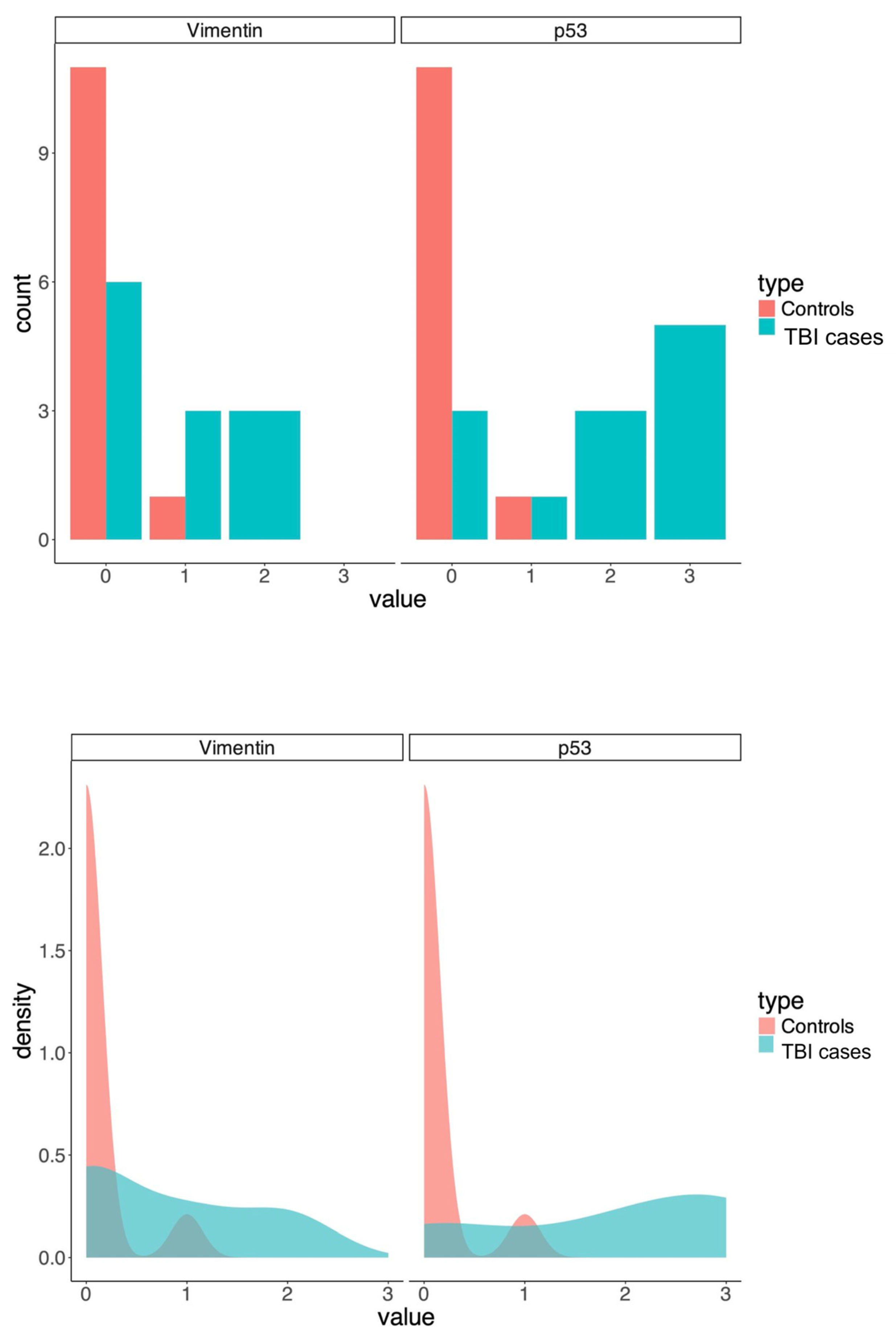
| Vimentin Positive | Vimentin Negative | p53 Positive | p53 Negative | Total | |
|---|---|---|---|---|---|
| TBI group | 6 | 6 | 9 | 3 | 12 |
| Control group | 1 | 11 | 1 | 11 | 12 |
| Total | 7 | 17 | 10 | 14 | 24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manetti, A.C.; De Matteis, A.; Napoletano, G.; La Russa, R.; Maiese, A.; Frati, P. Vimentin and p53 Immunoreactivity in Cases of Traumatic Brain Injury. J. Pers. Med. 2025, 15, 135. https://doi.org/10.3390/jpm15040135
Manetti AC, De Matteis A, Napoletano G, La Russa R, Maiese A, Frati P. Vimentin and p53 Immunoreactivity in Cases of Traumatic Brain Injury. Journal of Personalized Medicine. 2025; 15(4):135. https://doi.org/10.3390/jpm15040135
Chicago/Turabian StyleManetti, Alice Chiara, Alessandra De Matteis, Gabriele Napoletano, Raffaele La Russa, Aniello Maiese, and Paola Frati. 2025. "Vimentin and p53 Immunoreactivity in Cases of Traumatic Brain Injury" Journal of Personalized Medicine 15, no. 4: 135. https://doi.org/10.3390/jpm15040135
APA StyleManetti, A. C., De Matteis, A., Napoletano, G., La Russa, R., Maiese, A., & Frati, P. (2025). Vimentin and p53 Immunoreactivity in Cases of Traumatic Brain Injury. Journal of Personalized Medicine, 15(4), 135. https://doi.org/10.3390/jpm15040135







