Comparative Changes in Fecal Microbiome After Endoscopic Resection and Surgical Resection in Gastric Cancer Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants and Sample Collection
2.2. DNA Extraction and NGS Library Preparation for Sequencing
2.3. Taxonomic Assignment and Profiling
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Enrolled Participants
3.2. Comparative Analysis of the Microbiomes of the Pre-Treatment Samples in the ESD and Surgery Groups
3.3. Integrated Analysis of Microbiome Alterations Between the Pre- and Post-Treatment Samples
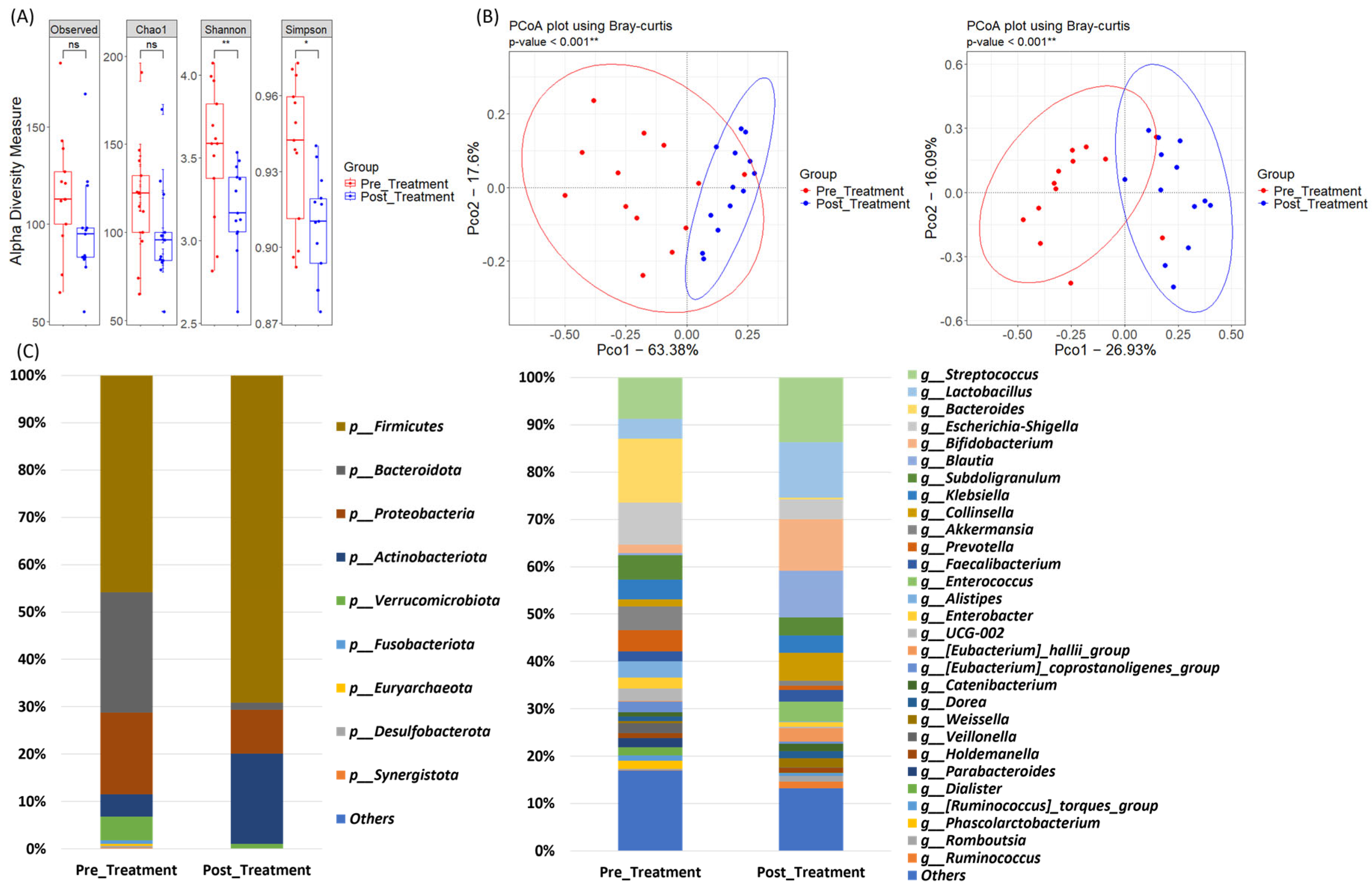
3.4. Microbiome Dynamics in the ESD Group: Pre- and Post-Treatment Samples
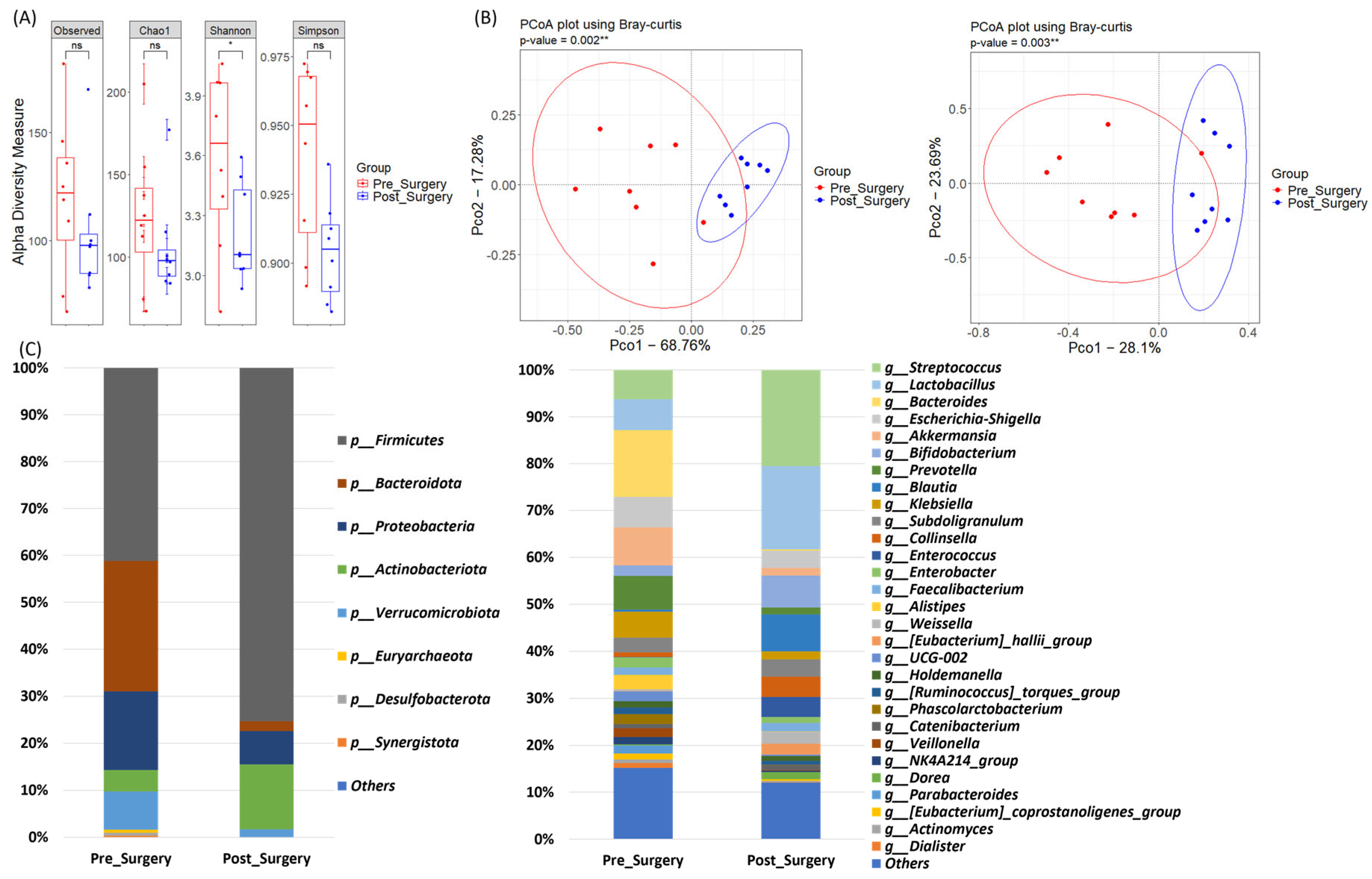
3.5. Microbiome Dynamics in the Surgery Group: Pre- and Post-Treatment Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, N.; Wu, H.; Cao, M.; Yang, F.; Yan, X.; He, S.; Cao, M.; Zhang, S.; Teng, Y.; Li, Q.; et al. Global, regional, and national burden of early-onset gastric cancer. Cancer Biol. Med. 2024, 21, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Shirani, M.; Pakzad, R.; Haddadi, M.H.; Akrami, S.; Asadi, A.; Kazemian, H.; Moradi, M.; Kaviar, V.H.; Zomorodi, A.R.; Khoshnood, S.; et al. The global prevalence of gastric cancer in Helicobacter pylori-infected individuals: A systematic review and meta-analysis. BMC Infect. Dis. 2023, 23, 543. [Google Scholar] [CrossRef]
- Zeng, R.; Gou, H.; Lau, H.C.H.; Yu, J. Stomach microbiota in gastric cancer development and clinical implications. Gut 2024, 73, 2062–2073. [Google Scholar] [CrossRef]
- Ağagündüz, D.; Cocozza, E.; Cemali, Ö.; Bayazıt, A.D.; Nanì, M.F.; Cerqua, I.; Morgillo, F.; Saygılı, S.K.; Canani, R.B.; Amero, P.; et al. Understanding the role of the gut microbiome in gastrointestinal cancer: A review. Front. Pharmacol. 2023, 14, 1130562. [Google Scholar] [CrossRef]
- Kim, I.-H.; Kang, S.J.; Choi, M.; Kim, B.-H.; Eom, B.W.; Kim, B.J.; Min, B.-H.; Choi, C.I.; Shin, C.M.; Tae, C.H.; et al. Korean Practice Guidelines for Gastric Cancer 2022: An Evidence-based, Multidisciplinary Approach. J. Gastric Cancer 2023, 23, 3–106. [Google Scholar] [CrossRef]
- Kim, S.G.; Park, C.M.; Lee, N.R.; Kim, J.; Lyu, D.H.; Park, S.-H.; Choi, I.J.; Lee, W.S.; Park, S.J.; Kim, J.J.; et al. Long-Term Clinical Outcomes of Endoscopic Submucosal Dissection in Patients with Early Gastric Cancer: A Prospective Multicenter Cohort Study. Gut Liver 2018, 12, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Kim, E.S.; Lee, Y.J.; Cho, K.B.; Park, K.S.; Jang, B.K.; Chung, W.J.; Hwang, J.S.; Ryu, S.W. Comparison of quality of life and worry of cancer recurrence between endoscopic and surgical treatment for early gastric cancer. Gastrointest. Endosc. 2015, 82, 299–307. [Google Scholar] [CrossRef]
- Erawijantari, P.P.; Mizutani, S.; Shiroma, H.; Shiba, S.; Nakajima, T.; Sakamoto, T.; Saito, Y.; Fukuda, S.; Yachida, S.; Yamada, T. Influence of gastrectomy for gastric cancer treatment on faecal microbiome and metabolome profiles. Gut 2020, 69, 1404–1415. [Google Scholar] [CrossRef]
- Maksimaityte, V.; Bausys, A.; Kryzauskas, M.; Luksta, M.; Stundiene, I.; Bickaite, K.; Bausys, B.; Poskus, T.; Bausys, R.; Strupas, K. Gas-trectomy impact on the gut microbiome in patients with gastric cancer: A comprehensive review. World J. Gastrointest. Surg. 2021, 13, 678–688. [Google Scholar]
- Paganelli, F.L.; Luyer, M.; Hazelbag, C.M.; Uh, H.W.; Rogers, M.R.C.; Adriaans, D.; Berbers, R.-M.; Hendrickx, A.P.A.; Viveen, M.C.; Groot, J.A.; et al. Roux-Y Gastric Bypass and Sleeve Gastrectomy directly change gut microbiota composition independent of surgery type. Sci. Rep. 2019, 9, 10979. [Google Scholar]
- Münzker, J.; Haase, N.; Till, A.; Sucher, R.; Haange, S.-B.; Nemetschke, L.; Gnad, T.; Jäger, E.; Chen, J.; Riede, S.J.; et al. Functional changes of the gastric bypass microbiota reactivate thermogenic adipose tissue and systemic glucose control via intestinal FXR-TGR5 crosstalk in diet-induced obesity. Microbiome 2022, 10, 96. [Google Scholar] [CrossRef]
- Park, J.-Y.; Kang, C.-S.; Seo, H.-C.; Shin, J.-C.; Kym, S.-M.; Park, Y.-S.; Shin, T.-S.; Kim, J.-G.; Kim, Y.-K. Bacteria-Derived Extracellular Vesicles in Urine as a Novel Biomarker for Gastric Cancer: Integration of Liquid Biopsy and Metagenome Analysis. Cancers 2021, 13, 4687. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [PubMed]
- Christodoulidis, G.; Koumarelas, K.-E.; Tsagkidou, K.; Agko, E.-S.; Bartzi, D.; Koumarelas, K.; Zacharoulis, D. The Impact of Gastrectomy on Inflammatory Bowel Disease Risk in Gastric Cancer Patients: A Critical Analysis. Curr. Oncol. 2024, 31, 5789–5801. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, X.; Liu, X.; Ling, Z.; Ji, F. Role of the Gastric Microbiome in Gastric Cancer: From Carcinogenesis to Treatment. Front. Microbiol. 2021, 12, 641322. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Q.; Xia, S.; He, Y.; Liu, Y.; Yang, J.; Xiao, X. Proton Pump Inhibitors and Oral–Gut Microbiota: From Mechanism to Clinical Significance. Biomedicines 2024, 12, 2271. [Google Scholar] [CrossRef]
- Ventura, I.; Chomon-García, M.; Tomás-Aguirre, F.; Palau-Ferré, A.; Legidos-García, M.E.; Murillo-Llorente, M.T.; Pérez-Bermejo, M. Therapeutic and Immunologic Effects of Short-Chain Fatty Acids in Inflammatory Bowel Disease: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 10879. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De La Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Carretta, M.D.; Quiroga, J.; López, R.; Hidalgo, M.A.; Burgos, R.A. Participation of Short-Chain Fatty Acids and Their Receptors in Gut Inflammation and Colon Cancer. Front. Physiol. 2021, 12, 662739. [Google Scholar] [CrossRef]
- van der Beek, C.M.; Dejong, C.H.C.; Troost, F.J.; Masclee, A.A.M.; Lenaerts, K. Role of short-chain fatty acids in colonic inflammation, carcinogenesis, and mucosal protection and healing. Nutr. Rev. 2017, 75, 286–305. [Google Scholar] [CrossRef] [PubMed]
- Facchin, S.; Bertin, L.; Bonazzi, E.; Lorenzon, G.; De Barba, C.; Barberio, B.; Zingone, F.; Maniero, D.; Scarpa, M.; Ruffolo, C.; et al. Short-Chain Fatty Acids and Human Health: From Metabolic Pathways to Current Therapeutic Implications. Life 2024, 14, 559. [Google Scholar] [CrossRef] [PubMed]
- Giambra, V.; Pagliari, D.; Rio, P.; Totti, B.; Di Nunzio, C.; Bosi, A.; Giaroni, C.; Gasbarrini, A.; Gambassi, G.; Cianci, R. Gut Microbiota, Inflammatory Bowel Disease, and Cancer: The Role of Guardians of Innate Immunity. Cells 2023, 12, 2654. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Anderson, S.M.; Sears, C.L. The Role of the Gut Microbiome in Cancer: A Review, With Special Focus on Colorectal Neoplasia and Clostridioides difficile. Clin. Infect. Dis. 2023, 77 (Suppl. S6), S471–S478. [Google Scholar] [CrossRef]
- Zhang, R.; Wu, Y.; Ju, W.; Wang, S.; Liu, Y.; Zhu, H. Gut microbiome alterations during gastric cancer: Evidence assessment of case–control studies. Front. Microbiol. 2024, 15, 1406526. [Google Scholar] [CrossRef]
- Wu, M.; Tian, C.; Zou, Z.; Jin, M.; Liu, H. Gastrointestinal Microbiota in Gastric Cancer: Potential Mechanisms and Clinical Applications—A Literature Review. Cancers 2024, 16, 3547. [Google Scholar] [CrossRef]
- Olteanu, G.; Ciucă-Pană, M.A.; Busnatu, Ș.S.; Lupuliasa, D.; Neacșu, S.M.; Mititelu, M.; Musuc, A.M.; Ioniță-Mîndrican, C.B.; Boroghină, S.C. Unraveling the Microbiome-Human Body Axis: A Comprehensive Examination of Therapeutic Strategies, Interactions and Implications. Int. J. Mol. Sci. 2024, 25, 5561. [Google Scholar] [CrossRef]
- Chen, X.-H.; Wang, A.; Chu, A.-N.; Gong, Y.-H.; Yuan, Y. Mucosa-Associated Microbiota in Gastric Cancer Tissues Compared With Non-cancer Tissues. Front. Microbiol. 2019, 10, 1261. [Google Scholar] [CrossRef]
- Yang, M. Interaction between intestinal flora and gastric cancer in tumor microenvironment. Front. Oncol. 2024, 14, 1402483. [Google Scholar] [CrossRef]
- Farrugia, A.; Arasaradnam, R. Bile acid diarrhoea: Pathophysiology, diagnosis and management. Front. Gastroenterol. 2020, 12, 500–507. [Google Scholar] [CrossRef]
- Acevedo-Román, A.; Pagán-Zayas, N.; Velázquez-Rivera, L.I.; Torres-Ventura, A.C.; Godoy-Vitorino, F. Insights into Gut Dysbiosis: Inflammatory Diseases, Obesity, and Restoration Approaches. Int. J. Mol. Sci. 2024, 25, 9715. [Google Scholar] [CrossRef]
- Seto, C.T.; Jeraldo, P.; Orenstein, R.; Chia, N.; DiBaise, J.K. Prolonged use of a proton pump inhibitor reduces microbial diversity: Implications for Clostridium difficile susceptibility. Microbiome 2014, 2, 42. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Acharya, C.; Fagan, A.; White, M.B.; Gavis, E.; Heuman, D.M.; Hylemon, P.B.; Fuchs, M.; Puri, P.; Schubert, M.L.; et al. Proton Pump Inhibitor Initiation and Withdrawal affects Gut Microbiota and Readmission Risk in Cirrhosis. Am. J. Gastroenterol. 2018, 113, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.; Deng, J.; Ang, D.; Hsiang, J.; Lee, L.; Aazmi, S.; Mohamed, E.; Yang, H.; Yap, S.; Teh, L.; et al. Effects of proton pump inhibitor on the human gut microbiome profile in multi-ethnic groups in Singapore. Singap. Med. J. 2019, 60, 512–521. [Google Scholar] [CrossRef]
- Palleja, A.; Mikkelsen, K.H.; Forslund, S.K.; Kashani, A.; Allin, K.H.; Nielsen, T.; Hansen, T.H.; Liang, S.; Feng, Q.; Zhang, C.; et al. Recovery of gut microbiota of healthy adults following antibiotic exposure. Nat. Microbiol. 2018, 3, 1255–1265. [Google Scholar] [CrossRef]
- Du, L.; Chen, B.; Cheng, F.; Kim, J.; Kim, J.J. Effects of Helicobacter pylori Therapy on Gut Microbiota: A Systematic Review and Meta-Analysis. Dig. Dis. 2022, 42, 102–112. [Google Scholar] [CrossRef]


| Variables | Total (n = 13) | ESD Group (n = 5) | Surgery Group (n = 8) | p-Value |
|---|---|---|---|---|
| Age, year | 66.5 ± 10.3 | 60.6 ± 10 | 70.1 ± 9.3 | 0.1064 |
| Sex, male/female | 8/5 (61.5%/38.5%) | 4/1 (80%/20%) | 4/4 (50%/50%) | 0.5649 |
| Histology | 0.2929 | |||
| W/D and M/D | 8 (61.5%) | 5 (100%) | 3 (37.5%) | |
| P/D | 2 (15.4%) | 0 (0%) | 2 (25%) | |
| PCC | 2 (15.4%) | 0 (0%) | 2 (25%) | |
| MiNEN | 1 (7.7%) | 0 (0%) | 1 (12.5%) | |
| Stage | 0.2308 | |||
| IA | 10 (76.9%) | 5 (100%) | 5 (62.5%) | |
| IB | 3 (23.1%) | 0 (0%) | 3 (37.5%) | |
| T stage | 0.3147 | |||
| 1a | 9 (69.2%) | 4 (80%) | 5 (62.5%) | |
| 1b | 1 (7.7%) | 1 (20%) | 0 (0%) | |
| 2 | 3 (23.1%) | 0 (0%) | 3 (37.5%) | |
| N stage | - | |||
| 0 | 13 (100%) | 5 (100%) | 8 (100%) | |
| 1 | 0 (0%) | 0 (0%) | 0 (0%) | |
| Lymphovascular invasion | 0.4872 | |||
| Absent | 11 (84.6%) | 5 (100%) | 6 (75%) | |
| Present | 2 (15.4%) | 0 (0%) | 2 (25%) | |
| Gross type | 1 | |||
| Elevated | 2 (15.4%) | 1 (20%) | 1 (12.5%) | |
| Flat | 3 (23.1%) | 1 (20%) | 2 (25%) | |
| Depressed | 8 (61.5%) | 3 (60%) | 5 (62.5%) | |
| Location | 0.4872 | |||
| Upper third | 0 (0%) | 0 (0%) | 0 (0%) | |
| Middle third | 2 (15.4%) | 0 (0%) | 2 (25%) | |
| Lower third | 11 (84.6%) | 5 (100%) | 6 (75%) | |
| Anastomosis method | - | |||
| Billroth I | - | - | 2 (25%) | |
| Billroth II | - | - | 1 (12.5%) | |
| Roux-en-Y | - | - | 5 (62.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, H.; Park, J.Y.; You, H.S.; Kim, B.J.; Kim, J.G. Comparative Changes in Fecal Microbiome After Endoscopic Resection and Surgical Resection in Gastric Cancer Patients. J. Pers. Med. 2025, 15, 144. https://doi.org/10.3390/jpm15040144
Seo H, Park JY, You HS, Kim BJ, Kim JG. Comparative Changes in Fecal Microbiome After Endoscopic Resection and Surgical Resection in Gastric Cancer Patients. Journal of Personalized Medicine. 2025; 15(4):144. https://doi.org/10.3390/jpm15040144
Chicago/Turabian StyleSeo, Hochan, Jae Yong Park, Hee Sang You, Beom Jin Kim, and Jae Gyu Kim. 2025. "Comparative Changes in Fecal Microbiome After Endoscopic Resection and Surgical Resection in Gastric Cancer Patients" Journal of Personalized Medicine 15, no. 4: 144. https://doi.org/10.3390/jpm15040144
APA StyleSeo, H., Park, J. Y., You, H. S., Kim, B. J., & Kim, J. G. (2025). Comparative Changes in Fecal Microbiome After Endoscopic Resection and Surgical Resection in Gastric Cancer Patients. Journal of Personalized Medicine, 15(4), 144. https://doi.org/10.3390/jpm15040144






