Impact of Carotid Artery Geometry and Clinical Risk Factors on Carotid Atherosclerotic Plaque Prevalence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Demographic
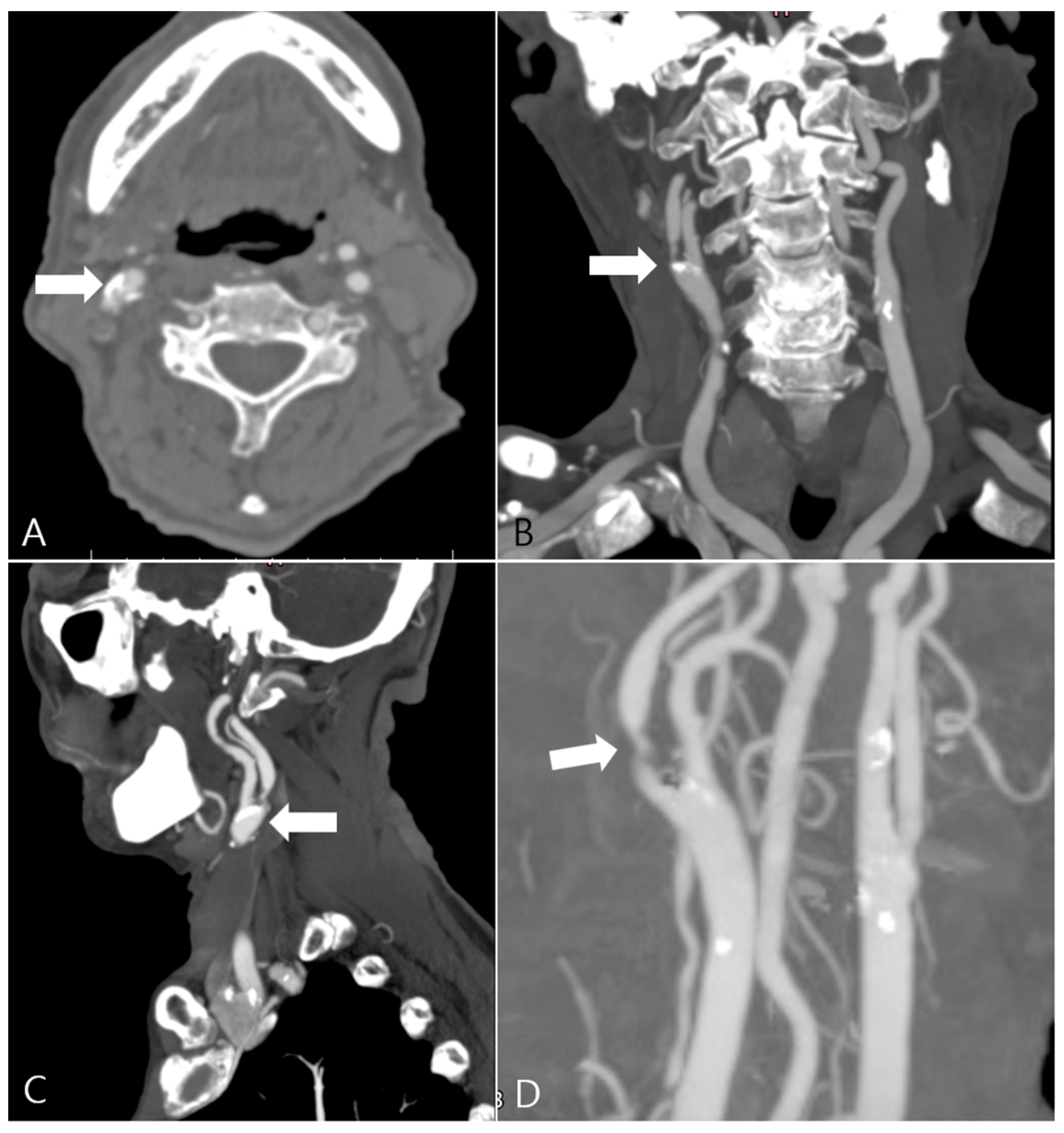
2.2. CTA Imaging Protocol
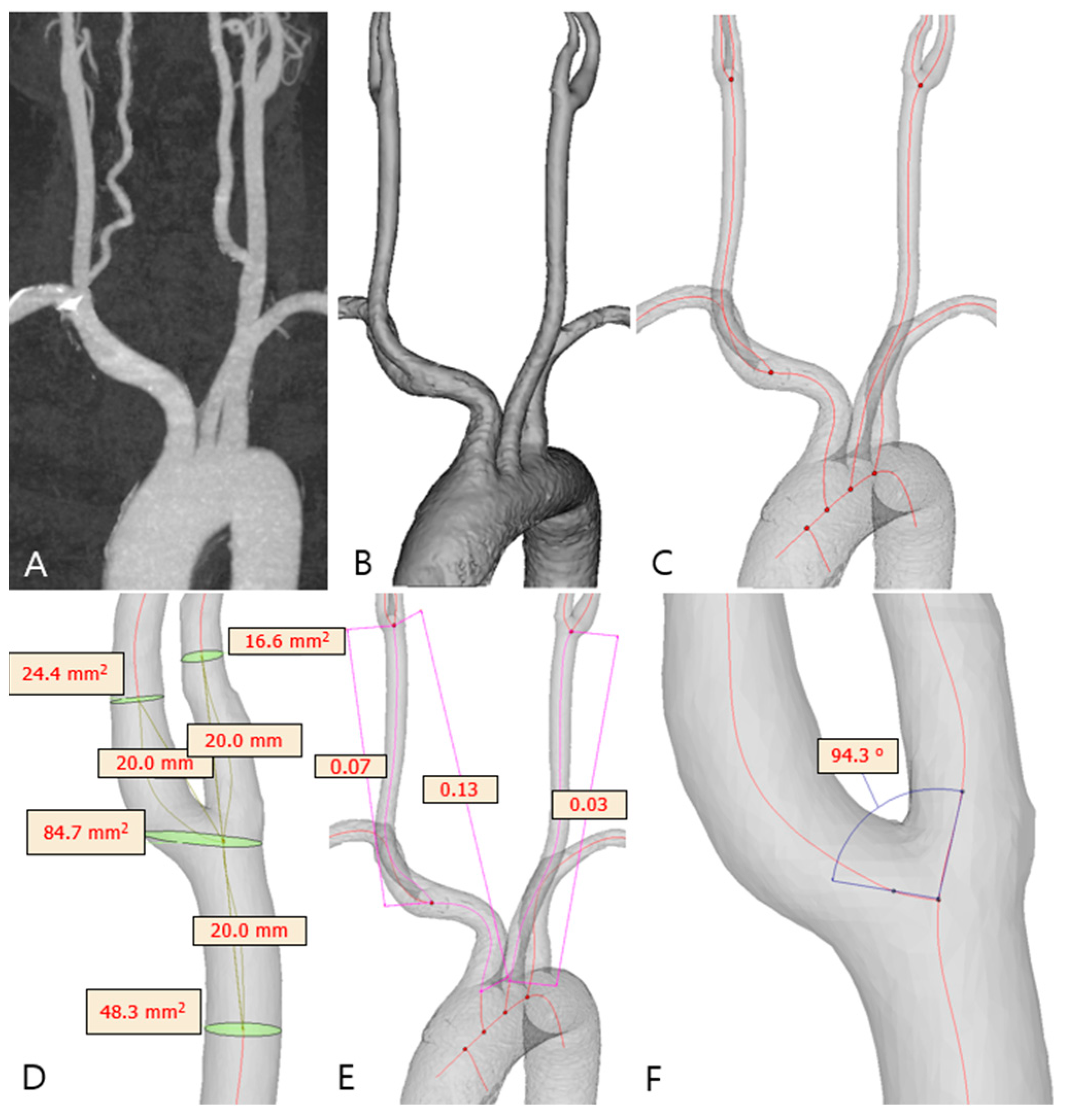
2.3. Carotid Artery 3D Model Reconstruction
2.4. Carotid Geometry Measurement
2.5. Other Clinical Variables
2.6. Statistical Analysis
3. Results
3.1. Analysis of Right Carotid Plaque (Table 1 and Table 2)
3.2. Analysis of Left Carotid Plaque (Table 1 and Table 3)
4. Discussion
4.1. Carotid Plaques Are Associated with Older Age, Hypertension, and Diabetes Mellitus
4.2. Carotid Plaques Are Correlated with a Smaller Carotid Bifurcation and a Larger CCA
4.3. A Left-Sided Carotid Plaque Is Correlated with a Smaller ICA
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| CAB | Carotid artery bifurcation |
| CCA | Common carotid artery |
| CFD | Computational fluid dynamics |
| CTA | Computed tomography angiography |
| DM | Diabetes mellitus |
| ECA | External carotid artery |
| HDL-C | High-density lipoprotein cholesterol |
| ICA | Internal carotid artery |
| LDL-C | Low-density lipoprotein cholesterol |
| NASCET | North American Symptomatic Carotid Endarterectomy Trial |
| OSI | Oscillatory shear index |
| OSS | Oscillatory shear stress |
| ROS | Reactive oxygen species |
| VSMC | Vascular smooth muscle cells |
| WSS | Wall shear stress |
References
- Morrison, A.M.; Sullivan, A.E.; Aday, A.W. Atherosclerotic Disease: Pathogenesis and Approaches to Management. Med. Clin. North. Am. 2023, 107, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Katan, M.; Luft, A. Global Burden of Stroke. Semin. Neurol. 2018, 38, 208–211. [Google Scholar] [CrossRef]
- Petty, G.W.; Brown, R.D., Jr.; Whisnant, J.P.; Sicks, J.D.; O’Fallon, W.M.; Wiebers, D.O. Ischemic stroke subtypes: A population-based study of incidence and risk factors. Stroke 1999, 30, 2513–2516. [Google Scholar] [CrossRef]
- White, H.; Boden-Albala, B.; Wang, C.; Elkind, M.S.; Rundek, T.; Wright, C.B.; Sacco, R.L. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: The Northern Manhattan Study. Circulation 2005, 111, 1327–1331. [Google Scholar] [CrossRef]
- Malekmohammad, K.; Bezsonov, E.E.; Rafieian-Kopaei, M. Role of Lipid Accumulation and Inflammation in Atherosclerosis: Focus on Molecular and Cellular Mechanisms. Front. Cardiovasc. Med. 2021, 8, 707529. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, M.; Wang, C.; Liu, Y.; Naruse, K.; Takahashi, K. The Mechanisms of the Development of Atherosclerosis in Prediabetes. Int. J. Mol. Sci. 2021, 22, 4108. [Google Scholar] [CrossRef] [PubMed]
- Nosalski, R.; Guzik, T.J. Perivascular adipose tissue inflammation in vascular disease. Br. J. Pharmacol. 2017, 174, 3496–3513. [Google Scholar] [CrossRef] [PubMed]
- Lobato, N.S.; Filgueira, F.P.; Akamine, E.H.; Tostes, R.C.; Carvalho, M.H.; Fortes, Z.B. Mechanisms of endothelial dysfunction in obesity-associated hypertension. Braz. J. Med. Biol. Res. Rev. 2012, 45, 392–400. [Google Scholar] [CrossRef]
- Durham, A.L.; Speer, M.Y.; Scatena, M.; Giachelli, C.M.; Shanahan, C.M. Role of smooth muscle cells in vascular calcification: Implications in atherosclerosis and arterial stiffness. Cardiovasc. Res. 2018, 114, 590–600. [Google Scholar] [CrossRef]
- Song, B.; Bie, Y.; Feng, H.; Xie, B.; Liu, M.; Zhao, F. Inflammatory Factors Driving Atherosclerotic Plaque Progression New Insights. J. Transl. Intern. Med. 2022, 10, 36–47. [Google Scholar] [CrossRef]
- Hou, P.; Fang, J.; Liu, Z.; Shi, Y.; Agostini, M.; Bernassola, F.; Bove, P.; Candi, E.; Rovella, V.; Sica, G.; et al. Macrophage polarization and metabolism in atherosclerosis. Cell Death Dis. 2023, 14, 691. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Luo, X.; Yu, J.; Jia, H.; Yu, B. Ferroptosis: A Potential Target in Cardiovascular Disease. Front. Cell Dev. Biol. 2021, 9, 813668. [Google Scholar] [CrossRef]
- Sakamoto, A.; Suwa, K.; Kawakami, R.; Finn, A.V.; Maekawa, Y.; Virmani, R.; Finn, A.V. Significance of Intra-plaque Hemorrhage for the Development of High-Risk Vulnerable Plaque: Current Understanding from Basic to Clinical Points of View. Int. J. Mol. Sci. 2023, 24, 13298. [Google Scholar] [CrossRef]
- Miceli, G.; Basso, M.G.; Pintus, C.; Pennacchio, A.R.; Cocciola, E.; Cuffaro, M.; Profita, M.; Rizzo, G.; Tuttolomondo, A. Molecular Pathways of Vulnerable Carotid Plaques at Risk of Ischemic Stroke: A Narrative Review. Int. J. Mol. Sci. 2024, 25, 4351. [Google Scholar] [CrossRef] [PubMed]
- Oyejide, A.J.; Awonusi, A.A.; Ige, E.O. Fluid-structure interaction study of hemodynamics and its biomechanical influence on carotid artery atherosclerotic plaque deposits. Med. Eng. Phys. 2023, 117, 103998. [Google Scholar] [CrossRef]
- Gnasso, A.; Irace, C.; Carallo, C.; De Franceschi, M.S.; Motti, C.; Mattioli, P.L.; Pujia, A. In vivo association between low wall shear stress and plaque in subjects with asymmetrical carotid atherosclerosis. Stroke 1997, 28, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, A.M.; Duerinckx, A.J. Wall shear stress and early atherosclerosis: A review. AJR. Am. J. Roentgenol. 2000, 174, 1657–1665. [Google Scholar] [CrossRef]
- Nguyen, K.T.; Clark, C.D.; Chancellor, T.J.; Papavassiliou, D.V. Carotid geometry effects on blood flow and on risk for vascular disease. J. Biomech. 2008, 41, 11–19. [Google Scholar] [CrossRef]
- Larson, A.S.; Brinjikji, W.; Savastano, L.; Scharf, E.; Huston, J.; Benson, J.C. Left-sided carotid arteries have a higher prevalence of intraplaque hemorrhage than right-sided: An asymmetric conundrum. Neuroradiol. J. 2020, 33, 494–500. [Google Scholar] [CrossRef]
- Koch, S.; Nelson, D.; Rundek, T.; Mandrekar, J.; Rabinstein, A. Race-ethnic variation in carotid bifurcation geometry. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2009, 18, 349–353. [Google Scholar] [CrossRef]
- Schulz, U.G.; Rothwell, P.M. Major variation in carotid bifurcation anatomy: A possible risk factor for plaque development? Stroke 2001, 32, 2522–2529. [Google Scholar] [CrossRef]
- Gregg, S.; Li, T.Y.; Hétu, M.F.; Pang, S.C.; Ewart, P.; Johri, A.M. Relationship between carotid artery atherosclerosis and bulb geometry. Int. J. Cardiovasc. Imaging 2018, 34, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Chen, Z.; Hippe, D.S.; Watase, H.; Sun, B.; Lin, R.; Yang, Z.; Xue, Y.; Zhao, X.; Yuan, C. Association Between Carotid Bifurcation Geometry and Atherosclerotic Plaque Vulnerability: A Chinese Atherosclerosis Risk Evaluation Study. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1383–1391. [Google Scholar] [CrossRef]
- Gallo, D.; Bijari, P.B.; Morbiducci, U.; Qiao, Y.; Xie, Y.J.; Etesami, M.; Habets, D.; Lakatta, E.G.; Wasserman, B.A.; Steinman, D.A. Segment-specific associations between local haemodynamic and imaging markers of early atherosclerosis at the carotid artery: An in vivo human study. J. R. Soc. Interface 2018, 15, 1520180352. [Google Scholar] [CrossRef] [PubMed]
- Bijari, P.B.; Wasserman, B.A.; Steinman, D.A. Carotid bifurcation geometry is an independent predictor of early wall thickening at the carotid bulb. Stroke 2014, 45, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, G.G.; Eliasziw, M.; Barr, H.W.K.; Clagett, G.P.; Barnes, R.W.; Wallace, M.C.; Taylor, D.W.; Haynes, R.B.; Finan, J.W.; Hachinski, V.C.; et al. The North American Symptomatic Carotid Endarterectomy Trial. Stroke 1999, 30, 1751–1758. [Google Scholar] [CrossRef]
- Lineback, C.M.; Stamm, B.; Sorond, F.; Caprio, F.Z. Carotid disease, cognition, and aging: Time to redefine asymptomatic disease? GeroScience 2023, 45, 719–725. [Google Scholar] [CrossRef]
- Lu, S.X.; Wu, T.W.; Chou, C.L.; Cheng, C.F.; Wang, L.Y. Combined effects of hypertension, hyperlipidemia, and diabetes mellitus on the presence and severity of carotid atherosclerosis in community-dwelling elders: A community-based study. J. Chin. Med. Assoc. JCMA 2023, 86, 220–226. [Google Scholar] [CrossRef]
- Sung, M.; Jung, Y.H.; Yoon, Y.H.; Lee, K.-Y. Association between Coronary Artery Calcification and Carotid Plaque Using Health Check-Up Data. J. Neurosonol. Neuroimag. 2024, 16, 86–92. [Google Scholar] [CrossRef]
- Sung, M.; Jung, Y.H.; Youn, Y.H.; Lee, K.-Y. Correlation Between Elevated Lipoprotein(a) and Carotid Plaque in Asymptomatic Individuals. J. Neurosonol. Neuroimag. 2024, 16, 1–7. [Google Scholar] [CrossRef]
- Mashaba, G.R.; Phoswa, W.N.; Lebelo, S.L.; Choma, S.S.R.; Maimela, E.; Mokgalaboni, K. A Longitudinal Cohort Assessing the Carotid Intima-Media Thickness Progression and Cardiovascular Risk Factors in a Rural Black South African Community. J. Clin. Med. 2025, 14, 1033. [Google Scholar] [CrossRef]
- Mashaba, R.G.; Phoswa, W.; Maimela, E.; Lebelo, S.; Modjadji, P.; Mokgalaboni, K. Systematic review and meta-analysis assessing the status of carotid intima-media thickness and lipid profiles in type 2 diabetes mellitus. BMJ Open 2024, 14, e087496. [Google Scholar] [CrossRef] [PubMed]
- White, R.P.; Markus, H.S. Impaired Dynamic Cerebral Autoregulation in Carotid Artery Stenosis. Stroke 1997, 28, 1340–1344. [Google Scholar] [CrossRef]
- Ballotta, E.; Meneghetti, G.; Manara, R.; Baracchini, C. Long-term survival and stroke-free survival after eversion carotid endarterectomy for asymptomatic severe carotid stenosis. J. Vasc. Surg. 2007, 46, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Sutton-Tyrrell, K.; Alcorn, H.G.; Wolfson, S.K., Jr.; Kelsey, S.F.; Kuller, L.H. Predictors of carotid stenosis in older adults with and without isolated systolic hypertension. Stroke 1993, 24, 355–416. [Google Scholar] [CrossRef]
- Rimmele, D.L.; Borof, K.; Jensen, M.; Behrendt, C.A.; Cheng, B.; Debus, E.S.; Gerloff, C.; Thomalla, G. Association Between Carotid Atherosclerosis and Atrial Fibrillation, Cardiac, and Renal Function. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2022, 63, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Gusev, E.; Sarapultsev, A. Atherosclerosis and Inflammation: Insights from the Theory of General Pathological Processes. Int. J. Mol. Sci. 2023, 24, 7910. [Google Scholar] [CrossRef]
- Hayıroğlu, M.; Şaylık, F.; Çınar, T.; Tokgözoğlu, L. Meta-analysis of the Current Research on the Relationship Between Blood Lipid Levels and the Occurrence of Atrial Fibrillation. Heart Lung Circ. 2023, 32, 1158–1166. [Google Scholar] [CrossRef]
- Lage, J.G.B.; Bortolotto, A.L.; Scanavacca, M.I.; Bortolotto, L.A.; Darrieux, F. Arterial stiffness and atrial fibrillation: A review. Clinics 2022, 77, 100014. [Google Scholar] [CrossRef]
- Dhawan, S.S.; Avati Nanjundappa, R.P.; Branch, J.R.; Taylor, W.R.; Quyyumi, A.A.; Jo, H.; McDaniel, M.C.; Suo, J.; Giddens, D.; Samady, H. Shear stress and plaque development. Expert. Rev. Cardiovasc. Ther. 2010, 8, 545–556. [Google Scholar] [CrossRef]
- Peiffer, V.; Sherwin, S.J.; Weinberg, P.D. Does low and oscillatory wall shear stress correlate spatially with early atherosclerosis? A systematic review. Cardiovasc. Res. 2013, 99, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Chiu, J.-J.; Chien, S. Effects of Disturbed Flow on Vascular Endothelium: Pathophysiological Basis and Clinical Perspectives. Physiol. Rev. 2011, 91, 327–387. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.G.; Beare, R.J.; Jolley, D.; Das, G.; Ren, M.; Wong, K.; Chong, W.; Sinnott, M.D.; Hilton, J.E.; Srikanth, V. Carotid Artery Anatomy and Geometry as Risk Factors for Carotid Atherosclerotic Disease. Stroke 2012, 43, 1596–1601. [Google Scholar] [CrossRef]
- Sai, S.; Honglu, S.; Guangbin, W.; Rui, L.; Qinjian, S.; Bin, Y.; Hiroko, W.; Daniel, S.H.; Chun, Y.; Xihai, Z. Differences in left and right carotid plaque vulnerability in patients with bilateral carotid plaques: A CARE-II study. Stroke Vasc. Neurol. 2023, 8, 284–291. [Google Scholar] [CrossRef]
- Saba, L.; Maindarkar, M.; Johri, A.M.; Mantella, L.; Laird, J.R.; Khanna, N.N.; Paraskevas, K.I.; Ruzsa, Z.; Kalra, M.K.; Fernandes, J.F.E.; et al. UltraAIGenomics: Artificial Intelligence-Based Cardiovascular Disease Risk Assessment by Fusion of Ultrasound-Based Radiomics and Genomics Features for Preventive, Personalized and Precision Medicine: A Narrative Review. Rev. Cardiovasc. Med. 2024, 25, 184. [Google Scholar] [CrossRef]
- Tang, D.; Yang, C.; Zheng, J.; Canton, G.; Bach, R.G.; Hatsukami, T.S.; Wang, L.; Yang, D.; Billiar, K.L.; Yuan, C. Image-based modeling and precision medicine: Patient-specific carotid and coronary plaque assessment and predictions. IEEE Trans. Bio-Med. Eng. 2013, 60, 643–694. [Google Scholar] [CrossRef]
- Miceli, G.; Rizzo, G.; Basso, M.G.; Cocciola, E.; Pennacchio, A.R.; Pintus, C.; Tuttolomondo, A. Artificial Intelligence in Symptomatic Carotid Plaque Detection: A Narrative Review. App. Sci. 2023, 13, 4321. [Google Scholar] [CrossRef]
| Right Carotids | Left Carotids | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Total (N = 983, 100%) | Normal (N = 685, 69.7%) | Group 1 (N = 190, 19.3%) | Group 2 (N = 108, 11%) | p | Total (N = 954, 100%) | Normal (N = 685, 71.8%) | Group 1 (N = 170, 17.8%) | Group 2 (N = 99, 10.4%) | p |
| Age, years | 72.02 ± 0.34 | 69.99 ± 0.41 | 77.25 ± 0.66 | 75.69 ± 0.89 | <0.001 | 71.75 ± 0.35 | 69.99 ± 0.41 | 76.51 ± 0.73 | 75.71 ± 0.87 | <0.001 |
| Sex, male | 451 (45.9) | 310 (45.3) | 78 (41.1) | 63 (58.3) | 0.013 | 449 (47.1) | 310 (45.3) | 74 (43.5) | 65 (65.7) | <0.001 |
| Alcohol | 156 (15.9) | 122 (17.8) | 15 (7.9) | 19 (17.6) | 0.004 | 158 (16.6) | 122 (17.8) | 17 (10) | 19 (19.2) | 0.038 |
| Smoking | 109 (11.1) | 81 (11.8) | 22 (11.6) | 6 (5.6) | 0.151 | 107 (11.2) | 81 (11.8) | 20 (11.8) | 6 (6.1) | 0.229 |
| HTN | 565 (57.5) | 353 (51.5) | 141 (74.2) | 71 (65.7) | <0.001 | 543 (56.9) | 353 (51.5) | 121 (71.2) | 69 (69.7) | <0.001 |
| Diabetes | 250 (25.4) | 150 (21.9) | 62 (32.6) | 38 (35.2) | <0.001 | 235 (24.6) | 150 (21.9) | 50 (29.4) | 35 (35.4) | 0.004 |
| Dyslipidemia | 118 (12) | 75 (10.9) | 26 (13.7) | 17 (15.7) | 0.265 | 106 (11.1) | 75 (10.9) | 19 (11.2) | 12 (12.1) | 0.941 |
| Atrial fibrillation | 164 (16.7) | 101 (14.7) | 46 (24.2) | 17 (15.7) | 0.008 | 155 (16.2) | 101 (14.7) | 41 (24.1) | 13 (13.1) | 0.008 |
| Variables | Total (N = 983, 100%) | Normal (N = 685, 69.7%) | Group 1 (N = 190, 19.3%) | Group 2 (N = 108, 11%) | p | p [1] | p [2] | p [3] |
|---|---|---|---|---|---|---|---|---|
| Vascular Tortuosity | ||||||||
| Brachiocephalic to Bifurcation | 0.16 (0.11) | 0.16 (0.11) | 0.17 (0.12) | 0.15 (0.12) | 0.197 | 0.399 | >0.99 | 0.301 |
| CCA to Bifurcation | 0.10 (0.11) | 0.10 (0.11) | 0.12 (0.11) | 0.09 (0.12) | 0.186 | 0.293 | >0.99 | 0.366 |
| Vascular Diameter | ||||||||
| ICA (mm) | 5.19 ± 0.03 | 5.17 ± 0.03 | 5.27 ± 0.06 | 5.19 ± 0.1 | 0.388 | 0.506 | >0.99 | >0.99 |
| ECA (mm) | 4.17 ± 0.03 | 4.17 ± 0.03 | 4.18 ± 0.06 | 4.14 ± 0.08 | 0.9 | >0.99 | >0.99 | >0.99 |
| CCA (mm) | 7.45 ± 0.03 | 7.35 ± 0.03 | 7.65 ± 0.07 | 7.69 ± 0.1 | <0.001 | <0.001 | 0.001 | >0.99 |
| Bifurcation (mm) | 10.45 (2.30) | 10.57 (2.27) | 10.38 (1.99) | 10.14 (3.04) | 0.002 | 0.31 | 0.002 | 0.192 |
| Cross-Sectional Area | ||||||||
| ICA (mm2) | 20.40 (9.10) | 20.29 (9.21) | 20.92 (9.51) | 20.57 (8.73) | 0.958 | >0.99 | >0.99 | >0.99 |
| ECA (mm2) | 14 ± 0.17 | 13.97 ± 0.19 | 13.91 ± 0.4 | 14.3 ± 0.52 | 0.808 | >0.99 | >0.99 | >0.99 |
| CCA (mm2) | 42.61 (14.10) | 41.73 (13.39) | 44.28 (15.29) | 44.80 (15.98) | <0.001 | 0.004 | 0.031 | >0.99 |
| Bifurcation (mm2) | 87.03 ± 0.93 | 89.19 ± 1.11 | 82.09 ± 2.07 | 82.03 ± 2.74 | 0.002 | 0.009 | 0.051 | >0.99 |
| ICA Angle (degree) | 18.61 (17.73) | 18.45 (17.77) | 20.19 (17.89) | 18.35 (17.95) | 0.737 | >0.99 | >0.99 | >0.99 |
| Bifurcation Angle (degree) | 39.05 ± 0.5 | 39.16 ± 0.6 | 38.9 ± 1.19 | 38.63 ± 1.53 | 0.938 | >0.99 | >0.99 | >0.99 |
| Variables | Total (N = 954, 100%) | Normal (N = 685, 71.8%) | Group 1 (N = 170, 17.8%) | Group 2 (N = 99, 10.4%) | p | p [1] | p [2] | p [3] |
|---|---|---|---|---|---|---|---|---|
| Vascular Tortuosity | ||||||||
| CCA to Bifurcation | 0.10 (0.09) | 0.1 (0.09) | 0.11 (0.09) | 0.08 (0.08) | 0.084 | 0.502 | 0.397 | 0.08 |
| Vascular Diameter | ||||||||
| ICA (mm) | 5.30 (1.15) | 5.32 (1.13) | 5.33 (1.04) | 5.06 (1.32) | 0.017 | >0.99 | 0.025 | 0.022 |
| ECA (mm) | 4.14 ± 0.03 | 4.13 ± 0.03 | 4.19 ± 0.07 | 4.18 ± 0.08 | 0.602 | >0.99 | >0.99 | >0.99 |
| CCA (mm) | 7.29 ± 0.03 | 7.23 ± 0.03 | 7.41 ± 0.07 | 7.46 ± 0.1 | 0.006 | 0.044 | 0.041 | > 0.99 |
| Bifurcation (mm) | 11.02 ± 0.06 | 11.13 ± 0.07 | 10.93 ± 0.15 | 10.4 ± 0.25 | 0.002 | 0.719 | 0.001 | 0.09 |
| Cross-Sectional Area | ||||||||
| ICA (mm2) | 23.19 ± 0.27 | 23.32 ± 0.31 | 23.88 ± 0.67 | 21.07 ± 0.89 | 0.019 | >0.99 | 0.033 | 0.021 |
| ECA (mm2) | 13.56 (6.98) | 13.64 (7.20) | 13.50 (6.34) | 13.48 (6.26) | 0.872 | >0.99 | >0.99 | >0.99 |
| CCA (mm2) | 42.27 ± 0.34 | 41.65 ± 0.39 | 43.51 ± 0.8 | 44.39 ± 1.29 | 0.014 | 0.125 | 0.049 | > 0.99 |
| Bifurcation (mm2) | 92.63 ± 1 | 94.16 ± 1.15 | 91.68 ± 2.25 | 83.66 ± 3.75 | 0.006 | > 0.99 | 0.005 | 0.117 |
| ICA Angle (degree) | 24.31 (22.98) | 24.62 (22.45) | 25.2 (25.14) | 21.71 (67.86) | 0.115 | 0.545 | 0.52 | 0.117 |
| Bifurcation Angle (degree) | 45.78 ± 0.57 | 45.65 ± 0.65 | 47.36 ± 1.55 | 43.95 ± 1.63 | 0.288 | 0.769 | > 0.99 | 0.374 |
| Right Carotids | Left Carotids | |||
|---|---|---|---|---|
| Variables | Spearman’s rho | p | Spearman’s rho | p |
| Age | 0.275 | <0.001 | 0.254 | <0.001 |
| Alcohol | −0.067 | 0.035 | −0.043 | 0.186 |
| Smoking | −0.044 | 0.170 | −0.038 | 0.244 |
| HTN | 0.172 | <0.001 | 0.170 | <0.001 |
| Diabetes | 0.124 | <0.001 | 0.106 | 0.001 |
| Atrial Fibrillation | 0.067 | 0.035 | 0.052 | 0.108 |
| Dyslipidemia | 0.051 | 0.107 | 0.009 | 0.773 |
| Brachiocephalic to Bifurcation Tortuosity | <0.001 | 0.980 | Not measured | |
| CCA to Bifurcation Tortuosity | 0.008 | 0.812 | −0.005 | 0.889 |
| ICA Diameter (mm) | 0.028 | 0.374 | −0.066 | 0.042 |
| ECA Diameter (mm) | −0.001 | 0.992 | 0.021 | 0.522 |
| CCA Diameter (mm) | 0.133 | <0.001 | 0.077 | 0.017 |
| Bifurcation Diameter (mm) | −0.092 | 0.004 | −0.106 | 0.001 |
| ICA Sectional Area (mm2) | −0.001 | 0.988 | −0.070 | 0.029 |
| ECA Sectional Area (mm2) | 0.011 | 0.723 | 0.010 | 0.755 |
| CCA Sectional Area (mm2) | 0.115 | <0.001 | 0.071 | 0.028 |
| Bifurcation Sectional Area (mm2) | −0.102 | 0.001 | −0.106 | 0.001 |
| ICA Angle (degree) | Not measured | −0.022 | 0.500 | |
| Bifurcation Angle (degree) | −0.023 | 0.467 | 0.015 | 0.647 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngo, D.H.A.; Hwang, S.B.; Kwak, H.S. Impact of Carotid Artery Geometry and Clinical Risk Factors on Carotid Atherosclerotic Plaque Prevalence. J. Pers. Med. 2025, 15, 152. https://doi.org/10.3390/jpm15040152
Ngo DHA, Hwang SB, Kwak HS. Impact of Carotid Artery Geometry and Clinical Risk Factors on Carotid Atherosclerotic Plaque Prevalence. Journal of Personalized Medicine. 2025; 15(4):152. https://doi.org/10.3390/jpm15040152
Chicago/Turabian StyleNgo, Dac Hong An, Seung Bae Hwang, and Hyo Sung Kwak. 2025. "Impact of Carotid Artery Geometry and Clinical Risk Factors on Carotid Atherosclerotic Plaque Prevalence" Journal of Personalized Medicine 15, no. 4: 152. https://doi.org/10.3390/jpm15040152
APA StyleNgo, D. H. A., Hwang, S. B., & Kwak, H. S. (2025). Impact of Carotid Artery Geometry and Clinical Risk Factors on Carotid Atherosclerotic Plaque Prevalence. Journal of Personalized Medicine, 15(4), 152. https://doi.org/10.3390/jpm15040152






