Pharmacogenomics of Novel Direct Oral Anticoagulants: Newly Identified Genes and Genetic Variants
Abstract
:1. Introduction
2. Dabigatran
2.1. Pharmacogenomics
2.2. CES1 Gene
2.3. ABCB1
3. Rivaroxaban
3.1. Pharmacogenomics
3.2. ABCB1
3.3. CYP3A
4. Apixaban
4.1. Pharmacogenomics
4.2. SULT1
4.3. ABCB1
5. Edoxaban
5.1. Pharmacogenomics
5.2. ABCB1
5.3. CYP2C9 and VKORC1
6. Conclusions and Future Perspective
7. Executive Summary
7.1. Direct Oral Anticoagulants
- -
- To offset the significant challenges posed by vitamin K antagonists (warfarin) such as narrow therapeutic index, drug interactions, and frequent coagulation monitoring newer direct oral anticoagulants (DOACs) were introduced.
- -
- Four DOACs that are currently in clinical use include dabigatran, rivaroxaban, apixaban, and edoxaban.
- -
- DOACs act on specific coagulation factors inhibiting thrombin generation.
- -
- Currently approved indications of DOACs include prophylaxis against thromboembolism in non-valvular atrial fibrillation, treatment and prevention of deep vein thrombosis and pulmonary embolism, and secondary prevention in chronic coronary artery and peripheral vascular disease.
- -
- DOACs are cost effective and improve quality of life as compared to warfarin in approved clinical conditions.
7.2. Dabigatran
- -
- Dabigatran acts through reversible competitive inhibition of thrombin.
- -
- It is administered as prodrug that is converted into active form dabigatran etelixilate by esterases.
- -
- Principal drug interactions are mediated by p-glycoprotein.
- -
- CES1 and ABCB1 gene loci and their SNPs are implicated in altering plasma peak and trough levels of dabigatran.
7.3. Rivaroxaban
- -
- Rivaroxaban acts through inhibition of factor Xa.
- -
- Principal drug interactions are mediated by CYP3A4 and p-glycoprotein.
- -
- ABCB1 and CYP3A4 gene loci and their SNPs are implicated in altering plasma drug levels of rivaroxaban.
7.4. Apixaban
- -
- Apixaban acts through reversible inhibition of factor Xa.
- -
- Principal drug interactions are mediated by CYP3A4 and p-glycoprotein.
- -
- ABCB1 gene locus and its SNPs are implicated in altering plasma drug levels of apixaban.
- -
- Sulfotransferases polymorphisms may potentially contribute to variability of apixaban metabolism.
7.5. Edoxaban
- -
- Edoxaban acts through inhibition of factor Xa.
- -
- Principal drug interactions are mediated by CYP3A4 and p-glycoprotein.
- -
- Clinical trials failed to show significant association between ABCB1, CYP2C9, and VKORC1 and plasma drug levels of edoxaban.
8. Conclusions
- -
- Drug-gene interactions of DOACs are mainly mediated by genes CES1, ABCB1, and CYP3A4 and their respective SNPs.
- -
- Drug-drug interactions of DOACs are mainly mediated by CYP3A4 and p-glycoprotein.Further research including larger randomized clinical trials should be conducted to uncover other genetic variants and understand their impact on plasma drug levels and clinical efficacy of DOACs.
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Pirmohamed, M. Warfarin: Almost 60 years old and still causing problems. Br. J. Clin. Pharmacol. 2006, 62, 509–511. [Google Scholar] [CrossRef] [PubMed]
- Mekaj, Y.H.; Mekaj, A.Y.; Duci, S.B.; Miftari, E.I. New oral anticoagulants: Their advantages and disadvantages compared with vitamin K antagonists in the prevention and treatment of patients with thromboembolic events. Ther. Clin. Risk Manag. 2015, 11, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.H.; Agnelli, G. Edoxaban: A focused review of its clinical pharmacology. Eur. Heart J. 2014, 35, 1844–1855. [Google Scholar] [CrossRef] [PubMed]
- Burn, J.; Pirmohamed, M. Direct oral anticoagulants versus warfarin: Is new always better than the old? Open Heart 2018, 5, e000712. [Google Scholar] [CrossRef] [PubMed]
- Franco Moreno, A.I.; Martin Diaz, R.M.; Garcia Navarro, M.J. Direct oral anticoagulants: An update. Med. Clin. 2018, 151, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Michalcova, J.; Penka, M.; Bulikova, A.; Zavrelova, J.; Steparova, A. New—Direct oral anticoagulants: Actual review. Vnitr. Lek. 2016, 62, 805–813. [Google Scholar] [PubMed]
- Thachil, J. The newer direct oral anticoagulants: A practical guide. Clin. Med. 2014, 14, 165–175. [Google Scholar] [CrossRef]
- Barnes, G.D.; Lucas, E.; Alexander, G.C.; Goldberger, Z.D. National Trends in Ambulatory Oral Anticoagulant Use. Am. J. Med. 2015, 128, 1300–1305.e2. [Google Scholar] [CrossRef] [Green Version]
- Alalwan, A.A.; Voils, S.A.; Hartzema, A.G. Trends in utilization of warfarin and direct oral anticoagulants in older adult patients with atrial fibrillation. Am. J. Health-Syst. Pharm. Ajhp Off. J. Am. Soc. Health-Syst. Pharm. 2017, 74, 1237–1244. [Google Scholar] [CrossRef] [Green Version]
- Cherubini, A.; Carrieri, B.; Marinelli, P. Advantages and disadvantages of direct oral anticoagulants in older patients. Geriatr. Care 2018, 4, 7227. [Google Scholar] [CrossRef]
- Bauer, K.A. Pros and cons of new oral anticoagulants. Ash Educ. Program Book 2013, 2013, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Milling, T.J.; Frontera, J.A. Exploring Indications for the Use of Direct Oral Anticoagulants and the Associated Risks of Major Bleeding. Am. J. Manag. Care 2017, 23 (Suppl. 4), S67–S80. [Google Scholar]
- Schaefer, J.K.; McBane, R.D.; Wysokinski, W.E. How to choose appropriate direct oral anticoagulant for patient with nonvalvular atrial fibrillation. Ann. Hematol. 2016, 95, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Hinojar, R.; Jiménez-Natcher, J.J.; Fernández-Golfín, C.; Zamorano, J.L. New oral anticoagulants: A practical guide for physicians. Eur. Heart J. Cardiovasc. Pharmacother. 2015, 1, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Dager, W.E.; Banares, L. Reversing the anticoagulation effects of dabigatran. Hosp. Pract. 2017, 45, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Reed, M.; Nicolas, D. Andexanet Alfa; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2018. [Google Scholar]
- Li, J.; Wang, S.; Barone, J.; Malone, B. Warfarin Pharmacogenomics. Pharm. Ther. 2009, 34, 422–427. [Google Scholar]
- Piatkov, I.; Rochester, C.; Jones, T.; Boyages, S. Warfarin Toxicity and Individual Variability—Clinical Case. Toxins 2010, 2, 2584–2592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dean, L. Warfarin Therapy and VKORC1 and CYP Genotype. In Medical Genetics Summaries; Pratt, V., McLeod, H., Rubinstein, W., Dean, L., Kattman, B., Malheiro, A., Eds.; National Center for Biotechnology Information: Bethesda, MD, USA, 2012. [Google Scholar]
- Gulseth, M.P.; Grice, G.R.; Dager, W.E. Pharmacogenomics of warfarin: Uncovering a piece of the warfarin mystery. Am. J. Health-Syst. Pharm. Ajhp Off. J. Am. Soc. Health-Syst. Pharm. 2009, 66, 123–133. [Google Scholar] [CrossRef]
- Anderson, J.L.; Horne, B.D.; Stevens, S.M.; Grove, A.S.; Barton, S.; Nicholas, Z.P.; Kahn, S.F.; May, H.T.; Samuelson, K.M.; Muhlestein, J.B.; et al. Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation. Circulation 2007, 116, 2563–2570. [Google Scholar] [CrossRef]
- Huang, S.-W.; Chen, H.-S.; Wang, X.-Q.; Huang, L.; Xu, D.-L.; Hu, X.-J.; Huang, Z.-H.; He, Y.; Chen, K.-M.; Xiang, D.-K.; et al. Validation of VKORC1 and CYP2C9 genotypes on interindividual warfarin maintenance dose: A prospective study in Chinese patients. Pharm. Genom. 2009, 19, 226–234. [Google Scholar] [CrossRef]
- Berg, R.L.; Yale, S.H.; Rottscheit, C.M.; Glurich, I.E.; Schmelzer, J.R.; Burmester, J.K.; Caldwell, M.D. A randomized controlled trial of genotype-based Coumadin initiation. Genet. Med. Off. J. Am. Coll. Med. Genet. 2011, 13, 509–518. [Google Scholar] [Green Version]
- Cavallari, L.H.; Shin, J.; Perera, M.A. Role of pharmacogenomics in the management of traditional and novel oral anticoagulants. Pharmacotherapy 2011, 31, 1192–1207. [Google Scholar] [CrossRef] [PubMed]
- Relling, M.V.; Klein, T.E. CPIC: Clinical Pharmacogenetics Implementation Consortium of the Pharmacogenomics Research Network. Clin. Pharmacol. Ther. 2011, 89, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Cullell, N.; Carrera, C.; Muino, E.; Torres, N.; Krupinski, J.; Fernandez-Cadenas, I. Pharmacogenetic studies with oral anticoagulants. Genome-wide association studies in vitamin K antagonist and direct oral anticoagulants. Oncotarget 2018, 9, 29238–29258. [Google Scholar] [CrossRef]
- Loo, S.Y.; Dell’Aniello, S.; Huiart, L.; Renoux, C. Trends in the prescription of novel oral anticoagulants in UK primary care. Br J Clin Pharm. 2017, 83, 2096–2106. [Google Scholar] [CrossRef]
- Ziakas, P.D.; Kourbeti, I.S.; Poulou, L.S.; Vlachogeorgos, G.S.; Mylonakis, E. Medicare part D prescribing for direct oral anticoagulants in the United States: Cost, use and the “rubber effect”. PLoS ONE 2018, 13, e0198674. [Google Scholar] [CrossRef]
- Pare, G.; Eriksson, N.; Lehr, T.; Connolly, S.; Eikelboom, J.; Ezekowitz, M.D.; Axelsson, T.; Haertter, S.; Oldgren, J.; Reilly, P.; et al. Genetic determinants of dabigatran plasma levels and their relation to bleeding. Circulation 2013, 127, 1404–1412. [Google Scholar] [CrossRef]
- Dimatteo, C.; D’Andrea, G.; Vecchione, G.; Paoletti, O.; Cappucci, F.; Tiscia, G.L.; Buono, M.; Grandone, E.; Testa, S.; Margaglione, M. Pharmacogenetics of dabigatran etexilate interindividual variability. Thromb. Res. 2016, 144, 1–5. [Google Scholar] [CrossRef]
- Sweezy, T.; Mousa, S.A. Genotype-guided use of oral antithrombotic therapy: A pharmacoeconomic perspective. Pers. Med. 2014, 11, 223–235. [Google Scholar] [CrossRef]
- Ganetsky, M.; Babu, K.M.; Salhanick, S.D.; Brown, R.S.; Boyer, E.W. Dabigatran: Review of pharmacology and management of bleeding complications of this novel oral anticoagulant. J. Med. Toxicol. Off. J. Am. Coll. Med. Toxicol. 2011, 7, 281–287. [Google Scholar] [CrossRef]
- Armbruster, A.L.; Buehler, K.S.; Min, S.H.; Riley, M.; Daly, M.W. Evaluation of Dabigatran for Appropriateness of Use and Bleeding Events in a Community Hospital Setting. Am. Health Drug Benefits 2014, 7, 376–384. [Google Scholar] [PubMed]
- Bendel, S.D.; Bona, R.; Baker, W.L. Dabigatran: An oral direct thrombin inhibitor for use in atrial fibrillation. Adv. Ther. 2011, 28, 460–472. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Levin, V.; Malacoff, R.; Martinez, M.W. Dabigatran: A new chapter in anticoagulation. Cardiovasc. Hematol. Agents Med. Chem. 2012, 10, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.W.; Vu, H. Dabigatran etexilate: An oral direct thrombin inhibitor for the management of thromboembolic disorders. Clin. Ther. 2012, 34, 766–787. [Google Scholar] [CrossRef]
- Schulman, S.; Majeed, A. A benefit-risk assessment of dabigatran in the prevention of venous thromboembolism in orthopaedic surgery. Drug Saf. 2011, 34, 449–463. [Google Scholar] [CrossRef]
- Nagarakanti, R.; Ellis, C.R. Dabigatran in clinical practice. Clin. Ther. 2012, 34, 2051–2060. [Google Scholar] [CrossRef]
- Kyrle, P.A.; Binder, K.; Eichinger, S.; Függer, R.; Gollackner, B.; Hiesmayr, J.M.; Huber, K.; Lang, W.; Perger, P.; Quehenberger, P.; et al. Dabigatran: Patient management in specific clinical settings. Wien. Klin. Wochenschr. 2014, 126, 503–508. [Google Scholar] [CrossRef]
- Stangier, J.; Rathgen, K.; Stähle, H.; Gansser, D.; Roth, W. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br. J. Clin. Pharmacol. 2007, 64, 292–303. [Google Scholar] [CrossRef] [Green Version]
- Stangier, J.; Stähle, H.; Rathgen, K.; Fuhr, R. Pharmacokinetics and Pharmacodynamics of the Direct Oral Thrombin Inhibitor Dabigatran in Healthy Elderly Subjects. Clin. Pharmacokinet. 2008, 47, 47–59. [Google Scholar] [CrossRef]
- Blech, S.; Ebner, T.; Ludwig-Schwellinger, E.; Stangier, J.; Roth, W. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab. Dispos. Biol. Fate Chem. 2008, 36, 386–399. [Google Scholar] [CrossRef]
- Reilly, P.A.; Lehr, T.; Haertter, S.; Connolly, S.J.; Yusuf, S.; Eikelboom, J.W.; Ezekowitz, M.D.; Nehmiz, G.; Wang, S.; Wallentin, L.; et al. The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: The RE-LY Trial (Randomized Evaluation of Long-Term Anticoagulation Therapy). J. Am. Coll. Cardiol. 2014, 63, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Knauf, F.; Chaknos, C.M.; Berns, J.S.; Perazella, M.A. Dabigatran and kidney disease: A bad combination. Clin. J. Am. Soc. Nephrol. CJASN 2013, 8, 1591–1597. [Google Scholar] [CrossRef]
- Gosselin, R.C.; Adcock, D.M.; Bates, S.M.; Douxfils, J.; Favaloro, E.J.; Gouin-Thibault, I.; Guillermo, C.; Kawai, Y.; Lindhoff-Last, E.; Kitchen, S.; et al. International Council for Standardization in Haematology (ICSH) Recommendations for Laboratory Measurement of Direct Oral Anticoagulants. Thromb. Haemost. 2018, 118, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Hapgood, G.; Butler, J.; Malan, E.; Chunilal, S.; Tran, H. The effect of dabigatran on the activated partial thromboplastin time and thrombin time as determined by the Hemoclot thrombin inhibitor assay in patient plasma samples. Thromb. Haemost. 2013, 110, 308–315. [Google Scholar] [PubMed]
- Ciurus, T.; Sobczak, S.; Cichocka-Radwan, A.; Lelonek, M. New oral anticoagulants—A practical guide. Pol. J. Cardio-Thorac. Surg. 2015, 12, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Dabigatran and atrial fibrillation: The alternative to warfarin for selected patients. Prescrire Int. 2012, 21, 33–36.
- Hellwig, T.; Gulseth, M. Pharmacokinetic and pharmacodynamic drug interactions with new oral anticoagulants: What do they mean for patients with atrial fibrillation? Ann. Pharmacother. 2013, 47, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Wessler, J.D.; Grip, L.T.; Mendell, J.; Giugliano, R.P. The P-Glycoprotein Transport System and Cardiovascular Drugs. J. Am. Coll. Cardiol. 2013, 61, 2495–2502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.; Wang, X.; Nguyen, J.H.; Bleske, B.E.; Liang, Y.; Liu, L.; Zhu, H.J. Dabigatran etexilate activation is affected by the CES1 genetic polymorphism G143E (rs71647871) and gender. Biochem. Pharmacol. 2016, 119, 76–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sychev, D.A.; Levanov, A.N.; Shelekhova, T.V.; Bochkov, P.O.; Denisenko, N.P.; Ryzhikova, K.A.; Mirzaev, K.B.; Grishina, E.A.; Gavrilov, M.A.; Ramenskaya, G.V.; et al. The impact of ABCB1 (rs1045642 and rs4148738) and CES1 (rs2244613) gene polymorphisms on dabigatran equilibrium peak concentration in patients after total knee arthroplasty. Pharm. Pers. Med. 2018, 11, 127–137. [Google Scholar]
- Ross, S.; Pare, G. Pharmacogenetics of antiplatelets and anticoagulants: A report on clopidogrel, warfarin and dabigatran. Pharmacogenomics 2013, 14, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Asic, A.; Marjanovic, D.; Mirat, J.; Primorac, D. Pharmacogenetics of novel oral anticoagulants: A review of identified gene variants & future perspectives. Per Med. 2018, 15, 209–221. [Google Scholar] [PubMed]
- Gouin-Thibault, I.; Delavenne, X.; Blanchard, A.; Siguret, V.; Salem, J.E.; Narjoz, C.; Gaussem, P.; Beaune, P.; Funck-Brentano, C.; Azizi, M.; et al. Interindividual variability in dabigatran and rivaroxaban exposure: Contribution of ABCB1 genetic polymorphisms and interaction with clarithromycin. J. Thromb. Haemost. 2017, 15, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, T.; Dobesh, P.P. Clinical use of rivaroxaban: Pharmacokinetic and pharmacodynamic rationale for dosing regimens in different indications. Drugs 2014, 74, 1587–1603. [Google Scholar] [CrossRef]
- Thomas, T.F.; Ganetsky, V.; Spinler, S.A. Rivaroxaban: An Oral Factor Xa Inhibitor. Clin. Ther. 2013, 35, 4–27. [Google Scholar] [CrossRef]
- Samama, M.M. The mechanism of action of rivaroxaban—An oral, direct Factor Xa inhibitor—Compared with other anticoagulants. Thromb. Res. 2011, 127, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Vimalesvaran, K.; Dockrill, S.J.; Gorog, D.A. Role of rivaroxaban in the management of atrial fibrillation: Insights from clinical practice. Vasc. Health Risk Manag. 2018, 14, 13–21. [Google Scholar] [CrossRef]
- Kreutz, R. Pharmacodynamic and pharmacokinetic basics of rivaroxaban. Fundam. Clin. Pharmacol. 2012, 26, 27–32. [Google Scholar] [CrossRef]
- Mueck, W.; Stampfuss, J.; Kubitza, D.; Becka, M. Clinical pharmacokinetic and pharmacodynamic profile of rivaroxaban. Clin. Pharmacokinet. 2014, 53, 1–16. [Google Scholar] [CrossRef]
- Stampfuss, J.; Kubitza, D.; Becka, M.; Mueck, W. The effect of food on the absorption and pharmacokinetics of rivaroxaban. Int. J. Clin. Pharmacol. Ther. 2013, 51, 549–561. [Google Scholar] [CrossRef]
- Jiang, J.; Hu, Y.; Zhang, J.; Yang, J.; Mueck, W.; Kubitza, D.; Bauer, R.; Meng, L.; Hu, P.; Bauer, R.J. Safety, pharmacokinetics and pharmacodynamics of single doses of rivaroxaban—An oral, direct factor Xa inhibitor—In elderly Chinese subjects. Thromb. Haemost. 2010, 103, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Korber, M.K.; Langer, E.; Kaufner, L.; Sander, M.; Von Heymann, C. In vitro reversal of supratherapeutic rivaroxaban levels with coagulation factor concentrates. Blood Transfus. 2016, 14, 481–486. [Google Scholar] [PubMed]
- Ghadimi, K.; Dombrowski, K.E.; Levy, J.H.; Welsby, I.J. Andexanet alfa for the reversal of Factor Xa inhibitor related anticoagulation. Expert Rev. Hematol. 2016, 9, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Derogis, P.B.; Sanches, L.R.; de Aranda, V.F.; Colombini, M.P.; Mangueira, C.L.P.; Katz, M.; Faulhaber, A.C.L.; Mendes, C.E.A.; Ferreira, C.E.D.S.; França, C.N.; et al. Determination of rivaroxaban in patient’s plasma samples by anti-Xa chromogenic test associated to High Performance Liquid Chromatography tandem Mass Spectrometry (HPLC-MS/MS). PLoS ONE 2017, 12, e0171272. [Google Scholar] [CrossRef] [PubMed]
- Ing Lorenzini, K.; Daali, Y.; Fontana, P.; Desmeules, J.; Samer, C. Rivaroxaban-Induced Hemorrhage Associated with ABCB1 Genetic Defect. Front. Pharmacol. 2016, 7, 494. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, F. New Oral Anticoagulants: Comparative Pharmacology with Vitamin K Antagonists. Clin. Pharmacokinet. 2013, 52, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Mueck, W.; Kubitza, D.; Becka, M. Co-administration of rivaroxaban with drugs that share its elimination pathways: Pharmacokinetic effects in healthy subjects. Br. J. Clin. Pharmacol. 2013, 76, 455–466. [Google Scholar] [CrossRef]
- Sychev, D.A.; Vardanyan, A.; Rozhkov, A.; Hachatryan, E.; Badanyan, A.; Smirnov, V.; Ananichuk, A.; Denisenko, N. CYP3A Activity and Rivaroxaban Serum Concentrations in Russian Patients with Deep Vein Thrombosis. Genet. Test. Mol. Biomark. 2018, 22, 51–54. [Google Scholar] [CrossRef]
- Deedwania, P.; Huang, G.W. An evidence-based review of apixaban and its potential in the prevention of stroke in patients with atrial fibrillation. Core Evid. 2012, 7, 49–59. [Google Scholar] [CrossRef]
- Pinyol, C.; Cepeda, J.M.; Roldan, I.; Roldan, V.; Jimenez, S.; Gonzalez, P.; Soto, J. A Systematic Literature Review on the Cost-Effectiveness of Apixaban for Stroke Prevention in Non-valvular Atrial Fibrillation. Cardiol. Ther. 2016, 5, 171–186. [Google Scholar] [CrossRef] [Green Version]
- Hurst, K.V.; O’Callaghan, J.M.; Handa, A. Quick reference guide to apixaban. Vasc. Health Risk Manag. 2017, 13, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Greig, S.L.; Garnock-Jones, K.P. Apixaban: A Review in Venous Thromboembolism. Drugs 2016, 76, 1493–1504. [Google Scholar] [CrossRef] [PubMed]
- Fazeel, Z. Apixaban: An oral anticoagulant having unique mechanism of action with better safety and efficacy profile. Mamc J. Med. Sci. 2016, 2, 63–68. [Google Scholar] [CrossRef]
- Mueck, W.; Schwers, S.; Stampfuss, J. Rivaroxaban and other novel oral anticoagulants: Pharmacokinetics in healthy subjects, specific patient populations and relevance of coagulation monitoring. Thromb. J. 2013, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, N.; Frost, C.E.; Yu, Z.; He, K.; Zhang, H.; Humphreys, W.G.; Pinto, D.; Chen, S.; Bonacorsi, S.; Wong, P.C.; et al. Apixaban Metabolism and Pharmacokinetics after Oral Administration to Humans. Drug Metab. Dispos. 2009, 37, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Siegal, D.M.; Curnutte, J.T.; Connolly, S.J.; Lu, G.; Conley, P.B.; Wiens, B.L.; Mathur, V.S.; Castillo, J.; Bronson, M.D.; Leeds, J.M.; et al. Andexanet Alfa for the Reversal of Factor Xa Inhibitor Activity. N. Engl. J. Med. 2015, 373, 2413–2424. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Raghavan, N.; He, K.; Luettgen, J.M.; Humphreys, W.G.; Knabb, R.M.; Pinto, D.J.; Zhang, D. Sulfation of o-Demethyl Apixaban: Enzyme Identification and Species Comparison. Drug Metab. Dispos. 2009, 37, 802–808. [Google Scholar] [CrossRef]
- Carlini, E.J.; Raftogianis, R.B.; Wood, T.C.; Jin, F.; Zheng, W.; Rebbeck, T.R.; Weinshilboum, R.M. Sulfation pharmacogenetics: SULT1A1 and SULT1A2 allele frequencies in Caucasian, Chinese and African-American subjects. Pharmacogenetics 2001, 11, 57–68. [Google Scholar] [CrossRef]
- Nagar, S.; Walther, S.; Blanchard, R.L. Sulfotransferase (SULT) 1A1 polymorphic variants *1, *2, and *3 are associated with altered enzymatic activity, cellular phenotype, and protein degradation. Mol. Pharmacol. 2006, 69, 2084–2092. [Google Scholar] [CrossRef]
- Raftogianis, R.B.; Wood, T.C.; Otterness, D.M.; Van Loon, J.A.; Weinshilboum, R.M. Phenol Sulfotransferase Pharmacogenetics in Humans: Association of Common SULT1A1 Alleles with TS PST Phenotype. Biochem. Biophys. Res. Commun. 1997, 239, 298–304. [Google Scholar] [CrossRef]
- Dimatteo, C.; D’Andrea, G.; Vecchione, G.; Paoletti, O.; Tiscia, G.L.; Santacroce, R.; Correale, M.; Brunetti, N.; Grandone, E.; Testa, S.; et al. ABCB1 SNP rs4148738 modulation of apixaban interindividual variability. Thromb. Res. 2016, 145, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Kryukov, A.V.; Sychev, D.A.; Andreev, D.A.; Ryzhikova, K.A.; Grishina, E.A.; Ryabova, A.V.; Loskutnikov, M.A.; Smirnov, V.V.; Konova, O.D.; Matsneva, I.A.; et al. Influence of ABCB1 and CYP3A5 gene polymorphisms on pharmacokinetics of apixaban in patients with atrial fibrillation and acute stroke. Pharm. Pers. Med. 2018, 11, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Poulakos, M.; Walker, J.N.; Baig, U.; David, T. Edoxaban: A direct oral anticoagulant. Am. J. Health-Syst. Pharm. AJHP Off. J. Am. Soc. Health-Syst. Pharm. 2017, 74, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.J.; Hilas, O. Edoxaban: An Investigational Factor Xa Inhibitor. Pharm. Ther. 2014, 39, 686–715. [Google Scholar]
- Stacy, Z.A.; Call, W.B.; Hartmann, A.P.; Peters, G.L.; Richter, S.K. Edoxaban: A Comprehensive Review of the Pharmacology and Clinical Data for the Management of Atrial Fibrillation and Venous Thromboembolism. Cardiol. Ther. 2016, 5, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furugohri, T.; Isobe, K.; Honda, Y.; Kamisato-Matsumoto, C.; Sugiyama, N.; Nagahara, T.; Morishima, Y.; Shibano, T. DU-176b, a potent and orally active factor Xa inhibitor: In vitro and in vivo pharmacological profiles. J. Thromb. Haemost. 2008, 6, 1542–1549. [Google Scholar]
- Morishima, Y.; Honda, Y.; Kamisato, C. Prevention of Stent Thrombosis in Rats by a Direct Oral Factor Xa Inhibitor Edoxaban. Pharmacology 2018, 103, 17–22. [Google Scholar] [CrossRef]
- Honda, Y.; Kamisato, C.; Morishima, Y. Prevention of arterial thrombosis by edoxaban, an oral factor Xa inhibitor in rats: Monotherapy and in combination with antiplatelet agents. Eur. J. Pharmacol. 2016, 786, 246–252. [Google Scholar] [CrossRef]
- Parasrampuria, D.A.; Truitt, K.E. Pharmacokinetics and Pharmacodynamics of Edoxaban, a Non-Vitamin K Antagonist Oral Anticoagulant that Inhibits Clotting Factor Xa. Clin. Pharm. 2016, 55, 641–655. [Google Scholar] [CrossRef]
- Mendell, J.; Tachibana, M.; Shi, M.; Kunitada, S. Effects of food on the pharmacokinetics of edoxaban, an oral direct factor Xa inhibitor, in healthy volunteers. J. Clin. Pharmacol. 2011, 51, 687–694. [Google Scholar] [CrossRef]
- Vandell, A.G.; Lee, J.; Shi, M.; Rubets, I.; Brown, K.S.; Walker, J.R. An integrated pharmacokinetic/pharmacogenomic analysis of ABCB1 and SLCO1B1 polymorphisms on edoxaban exposure. Pharm. J. 2018, 18, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Vandell, A.G.; Walker, J.; Brown, K.S.; Zhang, G.; Lin, M.; Grosso, M.A.; Mercuri, M.F. Genetics and clinical response to warfarin and edoxaban in patients with venous thromboembolism. Heart 2017, 103, 1800–1805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mega, J.L.; Walker, J.R.; Ruff, C.T.; Vandell, A.G.; Nordio, F.; Deenadayalu, N.; Murphy, S.A.; Lee, J.; Mercuri, M.F.; Giugliano, R.P.; et al. Genetics and the clinical response to warfarin and edoxaban: Findings from the randomised, double-blind ENGAGE AF-TIMI 48 trial. Lancet (Lond. Engl.) 2015, 385, 2280–2287. [Google Scholar] [CrossRef]
- Sennesael, A.-L.; Panin, N.; Vancraeynest, C.; Pochet, L.; Spinewine, A.; Haufroid, V.; Elens, L. Effect of ABCB1 genetic polymorphisms on the transport of rivaroxaban in HEK293 recombinant cell lines. Sci. Rep. 2018, 8, 10514. [Google Scholar] [CrossRef] [PubMed]

| Peak Levels | SNP | Locus | Function | p-Value | Change (Concentration) | Clinical Outcome | Ref. | Year |
| rs8192935 | CES1 | Intron | 3.2 × 10−8 | 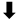 | No significant association with clinical events | [29] | 2013 | |
| rs4148738 | ABCB1 | Intron | 8.2 × 10−8 |  | No significant association with clinical events | [29] | 2013 | |
| rs1045642 | ABCB1 | Intron | 0.008 |  | No significant difference | [52] | 2018 | |
| rs71647871 (G143E) | CES1 | Intron | 0.018 | 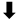 | Not tested | [51] | 2016 | |
| 2677–3455 | ABCB1 | Intron | 0.58 |  | Not tested | [55] | 2016 | |
| Trough Levels | SNP | Locus | Function | p-Value | Change | Clinical Outcome | Ref. | Year |
| rs2244613 | CES1 | Intron | 1.2 × 10−8 | 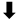 | 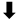 Bleeding Bleeding | [29] | 2013 | |
| G14E | CES1 | Intron | 0.018 | 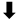 | Not tested | [51] | 2016 | |
| rs8192935 | CES1 | Intron | 0.023 | 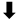 | Not tested | [30] | 2016 | |
| rs2244613 | CES1 | Intron | 0.04 | 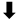 | Not tested | [30] | 2016 |
| Gene | Exon | SNP | DNA Polymorphism | Changes in Peak Plasma Levels | Study Population | Reference | Year |
|---|---|---|---|---|---|---|---|
| ABCB1 | 21 | rs2032582 | C.2677G>T | Increased | Case report | [67] | 2016 |
| ABCB1 | 21 26 | rs2032582 rs1045642 Combined haplotype (2677–3435) | C.2677G>T C.3435C>T | Non-significant increase | Healthy volunteers | [55] | 2016 |
| ABCB1 | 26 | rs1045642 | C.3435C>T | Case report | [67] | 2016 | |
| ABCB1 | --- | rs1128503 Combined haplotype (1236–2677–3455) | C.1236C>T | No Change | Healthy volunteers | [55] | 2016 |
| SULT1A | Allelic Variants | Substitution | Whites | Blacks | Chinese | Reference |
|---|---|---|---|---|---|---|
| SULT1A1*1 | Wild type | 65.6% | 47.7% | 91.4% | [80,81,82,83] | |
| SULT1A1*2 | G to A change at nucleotide 638 | 33.2% | 29.4% | 8% | [80,81,82,83] | |
| SULT1A1*3 | A to G change at nucleotide 667 | 1.2% | 22.9% | 0.6% | [80,81,82,83] |
| Apixaban Levels | SNP | Genotype | Locus | Function | Change | Clinical Outcome | Ref. | Year |
|---|---|---|---|---|---|---|---|---|
| Peak levels | rs4148738 | G>A | ABCB1 | Intron | Increase | Not tested | [83] | 2016 |
| Peak levels and AUC | rs1045642 | CC, CT, TT | ABCB1 | Intron | No significant difference | Not tested | [84] | 2018 |
| Peak levels and AUC | rs4148738 | CC, CT, TT | ABCB1 | Intron | No significant difference | Not tested | [84] | 2018 |
| Peak levels and AUC | rs776746 | CC, CG, GG | CYP3A5 | Intron | No significant difference | Not tested | [84] | 2018 |
| Clinical Trial | Gene | SNP | DNA Polymorphism | Study Population | Effect on Edoxaban Levels | Clinical Outcomes | Ref. | Year |
|---|---|---|---|---|---|---|---|---|
| Integrated analysis of 14 phase I studies | ABCB1 | rs1045642 | C3435T | Healthy population | No effect | Not tested | [93] | 2018 |
| Integrated analysis of 14 phase I studies | SLCO1B1 | rs4149056 | T521C | Healthy population | Slight increase in M4 metabolite | Not tested | [93] | 2018 |
| Randomized Double blind | CYP2C9 | rs1799853 | --- | Venous Thromboembolism | Not tested | No effect | [94] | 2017 |
| Randomized Double blind | CYP2C9 | rs1057910 | --- | Venous Thromboembolism | Not tested | No effect | [94] | 2017 |
| Randomized Double blind | VKORC1 | rs9923231 | --- | Venous Thromboembolism | Not tested- | No effect | [94] | 2017 |
| Randomized Double blind | CYP2C9 | rs1799853 | --- | Atrial Fibrillation | Not tested | No effect | [95] | 2015 |
| Randomized Double blind | CYP2C9 | rs1057910 | --- | Atrial Fibrillation | Not tested | No effect | [95] | 2015 |
| Randomized Double blind | VKORC1 | rs9923231 | --- | Atrial Fibrillation | Not tested | No effect | [95] | 2015 |
| Gene | SNP | Genotype | Ethnic Group | N % | Minor Allele | MAF% | p-Value | Reference |
|---|---|---|---|---|---|---|---|---|
| CES1 | rs2244613 | CC | Caucasian | 2 | C | 22.3 | 0.230 | Dimatteo et al. [30] |
| CA | 37 | |||||||
| AA | 53 | |||||||
| ABCB1 | rs1045642 | CC | Russian | 15 | T | 50.8 | 0.49 | Sychev et al. [52] |
| CT | 29 | |||||||
| TT | 16 | |||||||
| ABCB1 | rs4148738 | GG | Caucasian | 27 | A | 47.3 | 0.678 | Dimatteo et al. [30] |
| AG | 43 | |||||||
| AA | 22 | |||||||
| ABCB1 | rs8192935 | CC | Caucasian | 43 | T | 31.5 | 0.956 | Dimatteo et al. [30] |
| CT | 40 | |||||||
| TT | 9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanuri, S.H.; Kreutz, R.P. Pharmacogenomics of Novel Direct Oral Anticoagulants: Newly Identified Genes and Genetic Variants. J. Pers. Med. 2019, 9, 7. https://doi.org/10.3390/jpm9010007
Kanuri SH, Kreutz RP. Pharmacogenomics of Novel Direct Oral Anticoagulants: Newly Identified Genes and Genetic Variants. Journal of Personalized Medicine. 2019; 9(1):7. https://doi.org/10.3390/jpm9010007
Chicago/Turabian StyleKanuri, Sri H., and Rolf P. Kreutz. 2019. "Pharmacogenomics of Novel Direct Oral Anticoagulants: Newly Identified Genes and Genetic Variants" Journal of Personalized Medicine 9, no. 1: 7. https://doi.org/10.3390/jpm9010007
APA StyleKanuri, S. H., & Kreutz, R. P. (2019). Pharmacogenomics of Novel Direct Oral Anticoagulants: Newly Identified Genes and Genetic Variants. Journal of Personalized Medicine, 9(1), 7. https://doi.org/10.3390/jpm9010007





