Using the Immunophenotype to Predict Response to Biologic Drugs in Rheumatoid Arthritis
Abstract
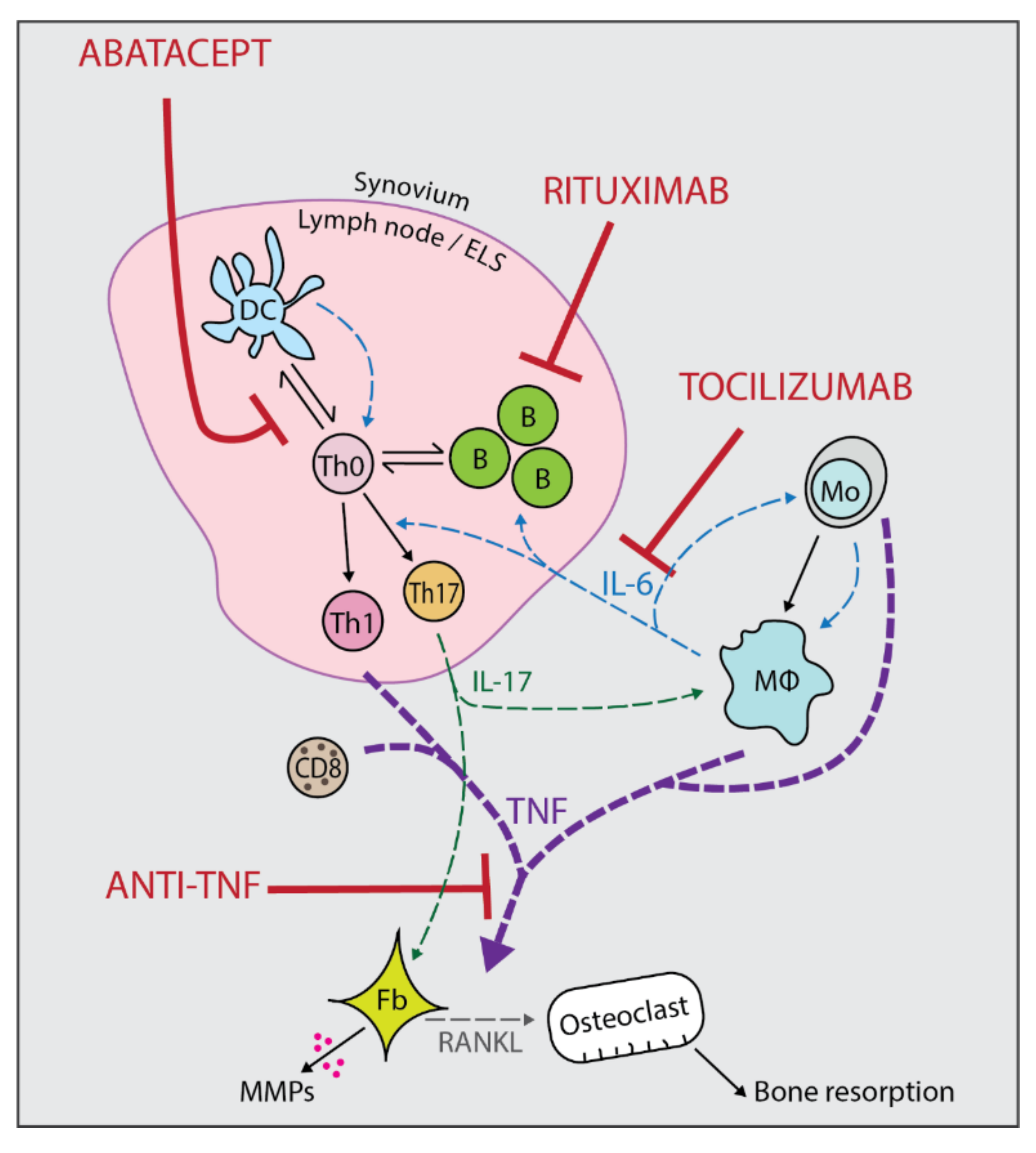
1. Introduction
2. Current Biologic Therapies Available
2.1. Anti-TNFs
2.2. Tocilizumab
2.3. Abatacept
2.4. Rituximab
3. Predicting Response to Biologic Drugs
3.1. Clinical and Demographic Predictors of Response
3.2. Immunological Predictors of Response
3.2.1. Anti-Citrullinated Peptide Antibodies and Rheumatoid Factor
3.2.2. Serum Biomarkers
3.2.3. Adaptive Immune Cells
3.2.4. Innate Immune Cells
3.2.5. Interferon Gene Signatures
3.3. Multiplexed Prediction Models
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alamanos, Y.; Drosos, A.A. Epidemiology of adult rheumatoid arthritis. Autoimmun. Rev. 2005, 4, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Pratt, A.G.; Isaacs, J.D. Seronegative rheumatoid arthritis: Pathogenetic and therapeutic aspects. Best Pract. Res. Clin. Rheumatol. 2014, 28, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.J.; Wells, G.; Verhoeven, A.C.; Felson, D.T. Factors predicting response to treatment in rheumatoid arthritis: The importance of disease duration. Arthritis Rheum. 2000, 43, 22–29. [Google Scholar] [CrossRef]
- Breedveld, F. The value of early intervention in RA—A window of opportunity. Clin. Rheumatol. 2011, 30, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Raza, K.; Buckley, C.E.; Salmon, M.; Buckley, C.D. Treating very early rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 2006, 20, 849–863. [Google Scholar] [CrossRef]
- National Institue for Health and Clinical Excellence. The Management of Rheumatoid Arthritis in Adults. (Clinical Guideline 79); National Institue for Health and Clinical Excellence: London, UK, 2009. [Google Scholar]
- Weinblatt, M.E.; Kremer, J.M.; Bankhurst, A.D.; Bulpitt, K.J.; Fleischmann, R.M.; Fox, R.I.; Jackson, C.G.; Lange, M.; Burge, D.J. A Trial of Etanercept, a Recombinant Tumor Necrosis Factor Receptor: Fc Fusion Protein, in Patients with Rheumatoid Arthritis Receiving Methotrexate. N. Engl. J. Med. 1999, 340, 253–259. [Google Scholar] [CrossRef]
- Wolbink, G.J.; Vis, M.; Lems, W.; Voskuyl, A.E.; de Groot, E.; Nurmohamed, M.T.; Stapel, S.; Tak, P.P.; Aarden, L.; Dijkmans, B. Development of antiinfliximab antibodies and relationship to clinical response in patients with rheumatoid arthritis. Arthritis Rheum. 2006, 54, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Bartelds, G.M.; Wijbrandts, C.A.; Nurmohamed, M.T.; Stapel, S.; Lems, W.F.; Aarden, L.; Dijkmans, B.A.C.; Tak, P.P.; Wolbink, G.J. Clinical response to adalimumab: Relationship to anti-adalimumab antibodies and serum adalimumab concentrations in rheumatoid arthritis. Ann. Rheum. Dis. 2007, 66, 921–926. [Google Scholar] [CrossRef]
- Choy, E.H.S.; Isenberg, D.A.; Garrood, T.; Farrow, S.; Ioannou, Y.; Bird, H.; Cheung, N.; Williams, B.; Hazleman, B.; Price, R.; et al. Therapeutic benefit of blocking interleukin-6 activity with an anti-interleukin-6 receptor monoclonal antibody in rheumatoid arthritis: A randomized, double-blind, placebo-controlled, dose-escalation trial. Arthritis Rheum. 2002, 46, 3143–3150. [Google Scholar] [CrossRef]
- Nishimoto, N.; Hashimoto, J.; Miyasaka, N.; Yamamoto, K.; Kawai, S.; Takeuchi, T.; Murata, N.; van der Heijde, D.; Kishimoto, T. Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): Evidence of clinical and radiographic benefit from an x ray reader-blinded randomised controlled trial of tocilizumab. Ann. Rheum. Dis. 2007, 66, 1162–1167. [Google Scholar] [CrossRef]
- Paul, S.K.; Montvida, O.; Best, J.H.; Gale, S.; Pethoe-Schramm, A.; Sarsour, K. Effectiveness of biologic and non-biologic antirheumatic drugs on anaemia markers in 153,788 patients with rheumatoid arthritis: New evidence from real-world data. Semin. Arthritis Rheum. 2018, 47, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.S.K.; Sansom, D.M. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat. Rev. Immunol. 2011, 11, 852–863. [Google Scholar] [CrossRef] [PubMed]
- Genovese, M.C.; Schiff, M.; Luggen, M.; Becker, J.-C.; Aranda, R.; Teng, J.; Li, T.; Schmidely, N.; Le Bars, M.; Dougados, M. Efficacy and safety of the selective co-stimulation modulator abatacept following 2 years of treatment in patients with rheumatoid arthritis and an inadequate response to anti-tumour necrosis factor therapy. Ann. Rheum. Dis. 2007, 67, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Wells, G.A.; Christensen, R.; Tanjong Ghogomu, E.; Maxwell, L.J.; MacDonald, J.K.; Filippini, G.; Skoetz, N.; Francis, D.K.; Lopes, L.C.; et al. Adverse effects of biologics: A network meta-analysis and Cochrane overview. Cochrane Database Syst. Rev. 2011. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.C.W.; Szczepanski, L.; Szechinski, J.; Filipowicz-Sosnowska, A.; Emery, P.; Close, D.R.; Stevens, R.M.; Shaw, T. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N. Engl. J. Med. 2004, 350, 2572–2581. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.B.; Emery, P.; Greenwald, M.W.; Dougados, M.; Furie, R.A.; Genovese, M.C.; Keystone, E.C.; Loveless, J.E.; Burmester, G.-R.; Cravets, M.W.; et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006, 54, 2793–2806. [Google Scholar] [CrossRef] [PubMed]
- Sellam, J.; Hendel-Chavez, H.; Rouanet, S.; Abbed, K.; Combe, B.; Le Loët, X.; Tebib, J.; Sibilia, J.; Taoufik, Y.; Dougados, M.; et al. B cell activation biomarkers as predictive factors for the response to rituximab in rheumatoid arthritis: A six-month, national, multicenter, open-label study. Arthritis Rheum. 2011, 63, 933–938. [Google Scholar] [CrossRef]
- van Vollenhoven, R.F.; Fleischmann, R.M.; Furst, D.E.; Lacey, S.; Lehane, P.B. Longterm Safety of Rituximab: Final Report of the Rheumatoid Arthritis Global Clinical Trial Program over 11 Years. J. Rheumatol. 2015, 42, 1761–1766. [Google Scholar] [CrossRef]
- Hyrich, K.L.; Watson, K.D.; Silman, A.J.; Symmons, D.P.M. Predictors of response to anti-TNF-alpha therapy among patients with rheumatoid arthritis: Results from the British Society for Rheumatology Biologics Register. Rheumatology (Oxf.) 2006, 45, 1558–1565. [Google Scholar] [CrossRef] [PubMed]
- Vastesaeger, N.; Kutzbach, A.G.; Amital, H.; Pavelka, K.; Lazaro, M.A.; Moots, R.J.; Wollenhaupt, J.; Zerbini, C.A.F.; Louw, I.; Combe, B.; et al. Prediction of remission and low disease activity in disease-modifying anti-rheumatic drug-refractory patients with rheumatoid arthritis treated with golimumab. Rheumatology 2016, 55, 1466–1476. [Google Scholar] [CrossRef]
- Miyoshi, F.; Honne, K.; Minota, S.; Okada, M.; Ogawa, N.; Mimura, T. A novel method predicting clinical response using only background clinical data in RA patients before treatment with infliximab. Mod. Rheumatol. 2016, 26, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Braun-Moscovici, Y.; Markovits, D.; Zinder, O.; Schapira, D.; Rozin, A.; Ehrenburg, M.; Dain, L.; Hoffer, E.; Nahir, A.M.; Balbir-Gurman, A. Anti-cyclic citrullinated protein antibodies as a predictor of response to anti-tumor necrosis factor-alpha therapy in patients with rheumatoid arthritis. J. Rheumatol. 2006, 33, 497–500. [Google Scholar] [PubMed]
- Potter, C.; Hyrich, K.L.; Tracey, A.; Lunt, M.; Plant, D.; Symmons, D.P.M.; Thomson, W.; Worthington, J.; Emery, P.; Morgan, A.W.; et al. Association of rheumatoid factor and anti-cyclic citrullinated peptide positivity, but not carriage of shared epitope or PTPN22 susceptibility variants, with anti-tumour necrosis factor response in rheumatoid arthritis. Ann. Rheum. Dis. 2009, 68, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, Y.; Nagare, Y.; Hino, S.; Yano, T.; Kishimoto, K.; Shimazu, H.; Ikoma, S.; Kinoshita, K.; Funauchi, M. Therapeutic strategy and significance of serum rheumatoid factor in patients with rheumatoid arthritis during infliximab treatment. Nihon Rinsho Meneki Gakkai Kaishi 2010, 33, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Dejaco, C.; Duftner, C.; Klotz, W.; Schirmer, M.; Herold, M. Third generation anti-cyclic citrullinated peptide antibodies do not predict anti-TNF-α treatment response in rheumatoid arthritis. Rheumatol. Int. 2010, 30, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Vasilopoulos, Y.; Bagiatis, V.; Stamatopoulou, D.; Zisopoulos, D.; Alexiou, I.; Sarafidou, T.; Settas, L.; Sakkas, L.; Mamuris, Z. Association of anti-CCP positivity and carriage of TNFRII susceptibility variant with anti-TNF-?? response in rheumatoid arthritis. Clin. Exp. Rheumatol. 2011, 29, 701–704. [Google Scholar]
- Lv, Q.; Yin, Y.; Li, X.; Shan, G.; Wu, X.; Liang, D.; Li, Y.; Zhang, X. The Status of Rheumatoid Factor and Anti-Cyclic Citrullinated Peptide Antibody Are Not Associated with the Effect of Anti-TNFα Agent Treatment in Patients with Rheumatoid Arthritis: A Meta-Analysis. PLoS ONE 2014, 9, e89442. [Google Scholar] [CrossRef] [PubMed]
- Sokolove, J.; Schiff, M.; Fleischmann, R.; Weinblatt, M.E.; Connolly, S.E.; Johnsen, A.; Zhu, J.; Maldonado, M.A.; Patel, S.; Robinson, W.H. Impact of baseline anti-cyclic citrullinated peptide-2 antibody concentration on efficacy outcomes following treatment with subcutaneous abatacept or adalimumab: 2-year results from the AMPLE trial. Ann. Rheum. Dis. 2015, 75, 709–714. [Google Scholar] [CrossRef]
- Takeuchi, T.; Miyasaka, N.; Inui, T.; Yano, T.; Yoshinari, T.; Abe, T.; Koike, T. High titers of both rheumatoid factor and anti-CCP antibodies at baseline in patients with rheumatoid arthritis are associated with increased circulating baseline TNF level, low drug levels, and reduced clinical responses: A post hoc analysis of the RISING study. Arthritis Res. Ther. 2017, 19, 194. [Google Scholar]
- Harrold, L.R.; Litman, H.J.; Connolly, S.E.; Kelly, S.; Hua, W.; Alemao, E.; Rosenblatt, L.; Rebello, S.; Kremer, J.M. Effect of Anticitrullinated Protein Antibody Status on Response to Abatacept or Antitumor Necrosis Factor-α Therapy in Patients with Rheumatoid Arthritis: A US National Observational Study. J. Rheumatol. 2017, 45, 170007. [Google Scholar] [CrossRef]
- Gottenberg, J.E.; Ravaud, P.; Cantagrel, A.; Combe, B.; Flipo, R.M.; Schaeverbeke, T.; Houvenagel, E.; Gaudin, P.; Loeuille, D.; Rist, S.; et al. Positivity for anti-cyclic citrullinated peptide is associated with a better response to abatacept: Data from the “Orencia and Rheumatoid Arthritis” registry. Ann. Rheum. Dis. 2012, 71, 1815–1819. [Google Scholar] [CrossRef] [PubMed]
- Gottenberg, J.E.; Courvoisier, D.S.; Hernandez, M.V.; Iannone, F.; Lie, E.; Canhão, H.; Pavelka, K.; Hetland, M.L.; Turesson, C.; Mariette, X.; et al. Brief Report: Association of Rheumatoid Factor and Anti-Citrullinated Protein Antibody Positivity With Better Effectiveness of Abatacept: Results From the Pan-European Registry Analysis. Arthritis Rheumatol. (Hoboken, N.J.) 2016, 68, 1346–1352. [Google Scholar] [CrossRef] [PubMed]
- Alten, R.; Mariette, X.; Lorenz, H.-M.; Galeazzi, M.; Cantagrel, A.; Nüßlein, H.G.; Chartier, M.; Elbez, Y.; Rauch, C.; Le Bars, M. Real-world predictors of 12-month intravenous abatacept retention in patients with rheumatoid arthritis in the ACTION observational study. RMD Open 2017, 3, e000538. [Google Scholar] [CrossRef] [PubMed]
- Maneiro, R.J.; Salgado, E.; Carmona, L.; Gomez-Reino, J.J. Rheumatoid factor as predictor of response to abatacept, rituximab and tocilizumab in rheumatoid arthritis: Systematic review and meta-analysis. Semin. Arthritis Rheum. 2013, 43, 9–17. [Google Scholar] [CrossRef]
- Isaacs, J.D.; Cohen, S.B.; Emery, P.; Tak, P.P.; Wang, J.; Lei, G.; Williams, S.; Lal, P.; Read, S.J. Effect of baseline rheumatoid factor and anticitrullinated peptide antibody serotype on rituximab clinical response: A meta-analysis. Ann. Rheum. Dis. 2013, 72, 329–336. [Google Scholar] [CrossRef]
- Gardette, A.; Ottaviani, S.; Tubach, F.; Roy, C.; Nicaise-Roland, P.; Palazzo, E.; Gill, G.; Meyer, O.; Dieudé, P. High anti-CCP antibody titres predict good response to rituximab in patients with active rheumatoid arthritis. Joint. Bone Spine 2014, 81, 416–420. [Google Scholar] [CrossRef]
- Shi, R.; Chen, M.; Litifu, B. Serum interleukin-6 and survivin levels predict clinical response to etanercept treatment in patients with established rheumatoid arthritis. Mod. Rheumatol. 2018, 28, 126–132. [Google Scholar] [CrossRef]
- Diaz-Torne, C.; Ortiz, M.D.A.; Moya, P.; Hernandez, M.V.; Reina, D.; Castellvi, I.; De Agustin, J.J.; de la Fuente, D.; Corominas, H.; Sanmarti, R.; et al. The combination of IL-6 and its soluble receptor is associated with the response of rheumatoid arthritis patients to tocilizumab. Semin. Arthritis Rheum. 2017, 47, 757–764. [Google Scholar] [CrossRef]
- Sellam, J.; Rivière, E.; Courties, A.; Rouzaire, P.-O.; Tolusso, B.; Vital, E.M.; Emery, P.; Ferraccioli, G.; Soubrier, M.; Ly, B.; et al. Serum IL-33, a new marker predicting response to rituximab in rheumatoid arthritis. Arthritis Res. Ther. 2016, 18, 294. [Google Scholar] [CrossRef]
- Han, B.K.; Kuzin, I.; Gaughan, J.P.; Olsen, N.J.; Bottaro, A. Baseline CXCL10 and CXCL13 levels are predictive biomarkers for tumor necrosis factor inhibitor therapy in patients with moderate to severe rheumatoid arthritis: A pilot, prospective study. Arthritis Res. Ther. 2016, 18, 93. [Google Scholar] [CrossRef]
- Dennis, G.; Holweg, C.T.J.; Kummerfeld, S.K.; Choy, D.F.; Setiadi, A.F.; Hackney, J.A.; Haverty, P.M.; Gilbert, H.; Lin, W.Y.; Diehl, L.; et al. Synovial phenotypes in rheumatoid arthritis correlate with response to biologic therapeutics. Arthritis Res. Ther. 2014, 16, R90. [Google Scholar] [CrossRef] [PubMed]
- Sellam, J.; Rouanet, S.; Hendel-Chavez, H.; Miceli-Richard, C.; Combe, B.; Sibilia, J.; Le Loët, X.; Tebib, J.; Jourdan, R.; Dougados, M.; et al. CCL19, a B Cell Chemokine, Is Related to the Decrease of Blood Memory B Cells and Predicts the Clinical Response to Rituximab in Patients With Rheumatoid Arthritis. Arthritis Rheum. 2013, 65, 2253–2261. [Google Scholar] [CrossRef] [PubMed]
- Daien, C.I.; Gailhac, S.; Mura, T.; Combe, B.; Hahne, M.; Morel, J. High levels of memory B cells are associated with response to a first tumor necrosis factor inhibitor in patients with rheumatoid arthritis in a longitudinal prospective study. Arthritis Res. Ther. 2014, 16, R95. [Google Scholar] [CrossRef] [PubMed]
- Gazeau, P.; Alegria, G.C.; Devauchelle-Pensec, V.; Jamin, C.; Lemerle, J.; Bendaoud, B.; Brooks, W.H.; Saraux, A.; Cornec, D.; Renaudineau, Y. Memory B Cells and Response to Abatacept in Rheumatoid Arthritis. Clin. Rev. Allergy Immunol. 2017, 53, 166–176. [Google Scholar] [CrossRef]
- Tony, H.-P.; Roll, P.; Mei, H.E.; Blümner, E.; Straka, A.; Gnuegge, L.; Dörner, T.; FIRST/ReFIRST study teams. Combination of B cell biomarkers as independent predictors of response in patients with rheumatoid arthritis treated with rituximab. Clin. Exp. Rheumatol. 2015, 33, 887–894. [Google Scholar] [PubMed]
- Citro, A.; Scrivo, R.; Martini, H.; Martire, C.; De Marzio, P.; Vestri, A.R.; Sidney, J.; Sette, A.; Barnaba, V.; Valesini, G. CD8+ T Cells Specific to Apoptosis-Associated Antigens Predict the Response to Tumor Necrosis Factor Inhibitor Therapy in Rheumatoid Arthritis. PLoS ONE 2015, 10, e0128607. [Google Scholar] [CrossRef]
- Scarsi, M.; Ziglioli, T.; Airo, P. Baseline numbers of circulating CD28-negative T cells may predict clinical response to abatacept in patients with rheumatoid arthritis. J. Rheumatol. 2011, 38, 2105–2111. [Google Scholar] [CrossRef]
- Daien, C.I.; Gailhac, S.; Audo, R.; Mura, T.; Hahne, M.; Combe, B.; Morel, J.; Daïen, C.I.; Gailhac, S.; Audo, R.; et al. High levels of natural killer cells are associated with response to tocilizumab in patients with severe rheumatoid arthritis. Rheumatology (Oxf.) 2014, 54, 1–8. [Google Scholar] [CrossRef]
- Mavragani, C.P.; La, D.T.; Stohl, W.; Crow, M.K. Association of the response to tumor necrosis factor antagonists with plasma type I interferon activity and interferon-beta/alpha ratios in rheumatoid arthritis patients: A post hoc analysis of a predominantly Hispanic cohort. Arthritis Rheum. 2010, 62, 392–401. [Google Scholar] [CrossRef]
- Thurlings, R.M.; Boumans, M.; Tekstra, J.; van Roon, J.A.; Vos, K.; van Westing, D.M.; van Baarsen, L.G.; Bos, C.; Kirou, K.A.; Gerlag, D.M.; et al. Relationship between the type I interferon signature and the response to rituximab in rheumatoid arthritis patients. Arthritis Rheum. 2010, 62, 3607–3614. [Google Scholar] [CrossRef]
- Raterman, H.G.; Vosslamber, S.; de Ridder, S.; Nurmohamed, M.T.; Lems, W.F.; Boers, M.; van de Wiel, M.; Dijkmans, B.A.C.; Verweij, C.L.; Voskuyl, A.E. The interferon type I signature towards prediction of non-response to rituximab in rheumatoid arthritis patients. Arthritis Res. Ther. 2012, 14, R95. [Google Scholar] [CrossRef] [PubMed]
- Felson, D.T.; Anderson, J.J.; Boers, M.; Bombardier, C.; Furst, D.; Goldsmith, C.; Katz, L.M.; Lightfoot, R.; Paulus, H.; Strand, V. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum. 1995, 38, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Prevoo, M.L.; van ’t Hof, M.A.; Kuper, H.H.; van Leeuwen, M.A.; van de Putte, L.B.; van Riel, P.L. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995, 38, 44–48. [Google Scholar] [CrossRef] [PubMed]
- van Gestel, A.M.; Prevoo, M.L.; van’t Hof, M.A.; van Rijswijk, M.H.; van de Putte, L.B.; van Riel, P.L. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Cri. Arthritis Rheum. 1996, 39, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Fabre, S.; Guisset, C.; Tatem, L.; Dossat, N.; Dupuy, A.M.; Cohen, J.D.; Cristol, J.P.; Daures, J.P.; Jorgensen, C. Protein biochip array technology to monitor rituximab in rheumatoid arthritis. Clin. Exp. Immunol. 2009, 155, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Ortea, I.; Roschitzki, B.; López-Rodríguez, R.; Tomero, E.G.; Ovalles, J.G.; López-Longo, J.; de la Torre, I.; González-Alvaro, I.; Gómez-Reino, J.J.; González, A. Independent Candidate Serum Protein Biomarkers of Response to Adalimumab and to Infliximab in Rheumatoid Arthritis: An Exploratory Study. PLoS ONE 2016, 11, e0153140. [Google Scholar] [CrossRef]
- Cuppen, B.; Fritsch-Stork, R.; Eekhout, I.; de Jager, W.; Marijnissen, A.; Bijlsma, J.; Custers, M.; van Laar, J.; Lafeber, F.; Welsing, P.; et al. Proteomics to predict the response to tumour necrosis factor-α inhibitors in rheumatoid arthritis using a supervised cluster-analysis based protein score. Scand. J. Rheumatol. 2017, 47, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Obry, A.; Hardouin, J.; Lequerré, T.; Jarnier, F.; Boyer, O.; Fardellone, P.; Philippe, P.; Marcelli, C.; Loët, X.L.; Vittecoq, O.; et al. Identification of 7 Proteins in Sera of RA Patients with Potential to Predict ETA/MTX Treatment Response. Theranostics 2015, 5, 1214–1224. [Google Scholar] [CrossRef][Green Version]
- Ziegler-Heitbrock, L.; Ancuta, P.; Crowe, S.; Dalod, M.; Grau, V.; Hart, D.N.; Leenen, P.J.M.; Liu, Y.-J.Y.-J.; MacPherson, G.; Randolph, G.J.; et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010, 116, e74–e80. [Google Scholar] [CrossRef]
- Baeten, D.; Boots, A.M.H.; Steenbakkers, P.G.A.; Elewaut, D.; Bos, E.; Verheijden, G.F.M.; Verbruggen, G.; Miltenburg, A.M.M.; Rijnders, A.W.M.; Veys, E.M.; et al. Human cartilage gp-39, CD16+ monocytes in peripheral blood and synovium: Correlation with joint destruction in rheumatoid arthritis. Arthritis Rheum. 2000, 43, 1233–1243. [Google Scholar] [CrossRef]
- Chara, L.; Sánchez-Atrio, A.; Pérez, A.; Cuende, E.; Albarrán, F.; Turrión, A.; Chevarria, J.; Del Barco, A.; Sánchez, M.A.; Monserrat, J.; et al. The number of circulating monocytes as biomarkers of the clinical response to methotrexate in untreated patients with rheumatoid arthritis. J. Transl. Med. 2015, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Chara, L.; Sánchez-Atrio, A.; Pérez, A.; Cuende, E.; Albarrán, F.; Turrión, A.; Chevarria, J.; Sánchez, M.A.; Monserrat, J.; de la Hera, A.; et al. Monocyte populations as markers of response to adalimumab plus MTX in rheumatoid arthritis. Arthritis Res. Ther. 2012, 14, R175. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A.; Fehniger, T.A.; Turner, S.C.; Chen, K.S.; Ghaheri, B.A.; Ghayur, T.; Carson, W.E.; Caligiuri, M.A. Human natural killer cells: A unique innate immunoregulatory role for the CD56(bright) subset. Blood 2001, 97, 3146–3151. [Google Scholar] [CrossRef] [PubMed]
- van der Pouw Kraan, T.C.T.M.; Wijbrandts, C.A.; van Baarsen, L.G.M.; Voskuyl, A.E.; Rustenburg, F.; Baggen, J.M.; Ibrahim, S.M.; Fero, M.; Dijkmans, B.A.C.; Tak, P.P.; et al. Rheumatoid arthritis subtypes identified by genomic profiling of peripheral blood cells: Assignment of a type I interferon signature in a subpopulation of patients. Ann. Rheum. Dis. 2007, 66, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- de Jong, T.D.; Vosslamber, S.; Blits, M.; Wolbink, G.; Nurmohamed, M.T.; van der Laken, C.J.; Jansen, G.; Voskuyl, A.E.; Verweij, C.L. Effect of prednisone on type I interferon signature in rheumatoid arthritis: Consequences for response prediction to rituximab. Arthritis Res. Ther. 2015, 17, 78. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carrio, J.; Alperi-López, M.; López, P.; Ballina-García, F.J.; Suárez, A. Heterogeneity of the Type I Interferon Signature in Rheumatoid Arthritis: A Potential Limitation for Its Use As a Clinical Biomarker. Front. Immunol. 2018, 8, 2007. [Google Scholar] [CrossRef] [PubMed]
- Hueber, W.; Tomooka, B.H.; Batliwalla, F.; Li, W.; Monach, P.A.; Tibshirani, R.J.; Van Vollenhoven, R.F.; Lampa, J.; Saito, K.; Tanaka, Y.; et al. Blood autoantibody and cytokine profiles predict response to anti-tumor necrosis factor therapy in rheumatoid arthritis. Arthritis Res. Ther. 2009, 11, R76. [Google Scholar] [CrossRef]
- Ally, M.M.T.M.; Hodkinson, B.; Meyer, P.W.A.; Musenge, E.; Tintinger, G.R.; Tikly, M.; Anderson, R. Circulating anti-citrullinated peptide antibodies, cytokines and genotype as biomarkers of response to disease-modifying antirheumatic drug therapy in early rheumatoid arthritis. BMC Musculoskelet. Disord. 2015, 16, 130. [Google Scholar] [CrossRef][Green Version]
- Folkersen, L.; Brynedal, B.; Diaz-Gallo, L.M.; Ramsköld, D.; Shchetynsky, K.; Westerlind, H.; Sundström, Y.; Schepis, D.; Hensvold, A.; Vivar, N.; et al. Integration of known DNA, RNA and protein biomarkers provides prediction of anti-TNF response in rheumatoid arthritis: Results from the COMBINE study. Mol. Med. 2016, 22, 322–328. [Google Scholar] [CrossRef]
- Mandelin, A.M.; Homan, P.J.; Shaffer, A.M.; Cuda, C.M.; Dominguez, S.T.; Bacalao, E.; Carns, M.; Hinchcliff, M.; Lee, J.; Aren, K.; et al. Transcriptional Profiling of Synovial Macrophages using Minimally Invasive Ultrasound-Guided Synovial Biopsies in Rheumatoid Arthritis. Arthritis Rheumatol. (Hoboken, N.J.) 2018, 70, 841–854. [Google Scholar] [CrossRef]
- Barton, A.; Pitzalis, C. Stratified medicine in rheumatoid arthritis-the MATURA programme. Rheumatology (Oxf.) 2017, 56, 1247–1250. [Google Scholar] [CrossRef] [PubMed]
- Donlin, L.T.; Rao, D.A.; Wei, K.; Slowikowski, K.; McGeachy, M.J.; Turner, J.D.; Meednu, N.; Mizoguchi, F.; Gutierrez-Arcelus, M.; Lieb, D.J.; et al. Methods for high-dimensonal analysis of cells dissociated from cyropreserved synovial tissue. Arthritis Res. Ther. 2018, 20, 139. [Google Scholar] [CrossRef] [PubMed]
- Gadina, M.; Gazaniga, N.; Vian, L.; Furumoto, Y. Small molecules to the rescue: Inhibition of cytokine signaling in immune-mediated diseases. J. Autoimmun. 2017, 85, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Burska, A.; Boissinot, M.; Ponchel, F. Cytokines as biomarkers in rheumatoid arthritis. Mediat. Inflamm. 2014, 2014, 545493. [Google Scholar] [CrossRef]
- HPA Tuberculosis Programme Board. Health Protection Agency Position Statement on the Use of Interferon Gamma Release Assay (IGRA) Tests for Tuberculosis (TB) Introduction and Background; Health Protection Agency: London, UK, 2012. [Google Scholar]
- Singh, U.; Cui, Y.; Dimaano, N.; Mehta, S.; Pruitt, S.; Yearley, J.; Laterza, O.; Juco, J.; Dogdas, B. Analytical validation of quantitative immunohistochemical assays of tumor infiltrating lymphocyte biomarkers. Biotech. Histochem. 2018, 93, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Brück, O.; Blom, S.; Dufva, O.; Turkki, R.; Chheda, H.; Ribeiro, A.; Kovanen, P.; Aittokallio, T.; Koskenvesa, P.; Kallioniemi, O.; et al. Immune cell contexture in the bone marrow tumor microenvironment impacts therapy response in CML. Leukemia 2018, 32, 1643. [Google Scholar] [CrossRef]
- Ermann, J.; Rao, D.A.; Teslovich, N.C.; Brenner, M.B.; Raychaudhuri, S. Immune cell profiling to guide therapeutic decisions in rheumatic diseases. Nat. Rev. Rheumatol. 2015, 11, 541–551. [Google Scholar] [CrossRef]
- Yabu, J.M.; Siebert, J.C.; Maecker, H.T. Immune Profiles to Predict Response to Desensitization Therapy in Highly HLA-Sensitized Kidney Transplant Candidates. PLoS ONE 2016, 11, e0153355. [Google Scholar] [CrossRef]
| Biomarker | Drug | Ethnicity | Concurrent DMARDs | Response Criteria | Predictor of Response | N Cases | Reference |
|---|---|---|---|---|---|---|---|
| Seropositive status | Infliximab | Caucasian | 100% | DAS28 ≥ 1.2 | Low ACPA titre predictive of response (PPV 0.95) | 30 | [23] |
| Caucasian | 73% | EULAR | Low RF/ACPA titre | 1195 | [24] | ||
| Infliximab | Asian | 100% | ΔCRP | Low RF titre | 62 | [25] | |
| Caucasian | 100% | EULAR | ACPA not associated with response | 42 | [26] | ||
| Caucasian | 100% | DAS28 | ACPA not associated with response | 31 | [27] | ||
| Mixed | n/a | DAS28 ACR20 EULAR | Meta-analysis found no association between seropositive status and anti-TNF response | 5561 | [28] | ||
| Adalimumab | Caucasian | 100% | EULAR DAS28 ACR20 | ACPA+ | 245 | [29] | |
| Infliximab | Asian | 100% | DAS28 | High RF/ACPA titre | 307 | [30] | |
| Caucasian | n/a | CDAI | No association | 1715 | [31] | ||
| Abatacept | Caucasian | 64.8% | EULAR | ACPA+ (OR 1.9; 1.2–2.9) | 558 | [32] | |
| Caucasian | 100% | EULAR DAS28 ACR20 | High ACPA titre | 252 | [29] | ||
| Caucasian | n/a | Higher continuation of abatacept in seropositive cohorts | 1357 | [33] | |||
| Caucasian | n/a | CDAI | ACPA+ | 566 | [31] | ||
| Caucasian | 75% | Retention rate | Double RF+/ACPA+ | 2350 | [34] | ||
| Rituximab | Mixed | n/a | ACR20 EULAR | Meta-analysis found RF+ associated with treatment response | 2103 | [35] | |
| Caucasian | n/a | DAS28 | Meta-analysis showing seropositive patients respond better to rituximab than seronegative patients | 2177 | [36] | ||
| Caucasian | 74.6% | EULAR DAS28 | High ACPA titre | 114 | [37] | ||
| Tocilizumab | Mixed | n/a | ACR20 EULAR | Meta-analysis found RF+ associated with treatment response | [35] | ||
| IL-6 | Etanercept | Asian | n/a | n/a | Increased IL-6 (with low survivin) associated with response (OR 19.7, CI 4.1–94.8) | 73 | [38] |
| Tocilizumab | Caucasian | 48.6% | EULAR | Increased IL-6 (with low IL-6R) associated with response | 63 | [39] | |
| IL-33 | Rituximab | Caucasian | 100% | EULAR | High IL-33 (and ACPA+) associated with response (OR 29.61, CI 1.3–674.8) | 74 | [40] |
| CXCL13 | Anti-TNFs | Caucasian | 100% | EULAR | High CXCL13 (and high CXCL10) associated with response (AUC 0.83) | 29 | [41] |
| Tocilizumab | Caucasian | 0% | ACR | High CXCL13 (with low sICAM1) (AUC 0.65) | 198 | [42] | |
| CCL19 | Rituximab | Caucasian | 100% | EULAR | High CCL19 associated with response (OR 1.43, CI 1.08–1.90) | 208 | [43] |
| B cells | Anti-TNFs | Caucasian | 69% | EULAR | High CD27+ B cells associated with response (RR 4.9, CI 1.3–18.6) | 21 | [44] |
| Abatacept | Caucasian | 51.2% | EULAR | High CD27+ and/or CD38+ B cells associated with response | 43 | [45] | |
| Rituximab | Caucasian | 100% | EULAR | High CD27− B cells are associated with response | 154 | [46] | |
| CD8+ T cells | Etanercept | Caucasian | n/a | EULAR | High apoptotic epitope-specific CD8+ T cells associated with response (AUC 0.82) | 16 | [47] |
| Abatacept | Caucasian | n/a | DAS28 | Low CD28− CD8+ T cells is associated with response | 32 | [48] | |
| NK cells | Tocilizumab | Caucasian | 60% | DAS28 | Low CD56brightCD16− NK cells associated with response | 20 | [49] |
| Type I interferon signature | Anti-TNF | Hispanic | 71–100% | EULAR | High type I IFN activity associated with response (OR 1.36, CI 1.05–3.29) | 35 | [50] |
| Rituximab | Caucasian | 55% | EULAR | High type I IFN signature negatively associated with response | 20 | [51] | |
| Caucasian | 77% | DAS28 | High type I IFN signature negatively associated with response (AUC 0.87) | 26 | [52] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulhearn, B.; Barton, A.; Viatte, S. Using the Immunophenotype to Predict Response to Biologic Drugs in Rheumatoid Arthritis. J. Pers. Med. 2019, 9, 46. https://doi.org/10.3390/jpm9040046
Mulhearn B, Barton A, Viatte S. Using the Immunophenotype to Predict Response to Biologic Drugs in Rheumatoid Arthritis. Journal of Personalized Medicine. 2019; 9(4):46. https://doi.org/10.3390/jpm9040046
Chicago/Turabian StyleMulhearn, Ben, Anne Barton, and Sebastien Viatte. 2019. "Using the Immunophenotype to Predict Response to Biologic Drugs in Rheumatoid Arthritis" Journal of Personalized Medicine 9, no. 4: 46. https://doi.org/10.3390/jpm9040046
APA StyleMulhearn, B., Barton, A., & Viatte, S. (2019). Using the Immunophenotype to Predict Response to Biologic Drugs in Rheumatoid Arthritis. Journal of Personalized Medicine, 9(4), 46. https://doi.org/10.3390/jpm9040046





