Lubrication Performance and Mechanism of Electrostatically Charged Alcohol Aqueous Solvents with Aluminum–Steel Contact
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Lubricants
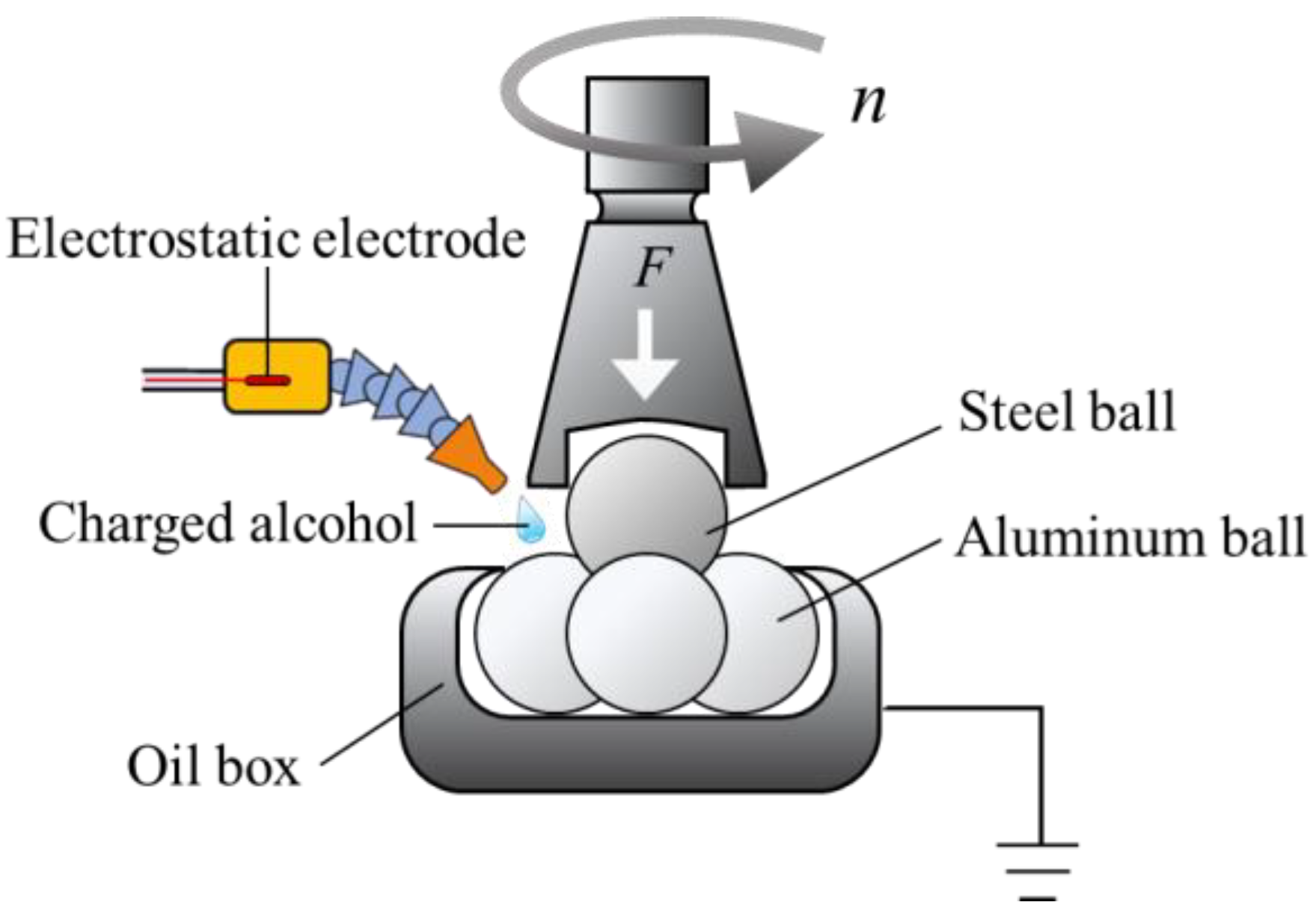
2.2. Tribological Testing
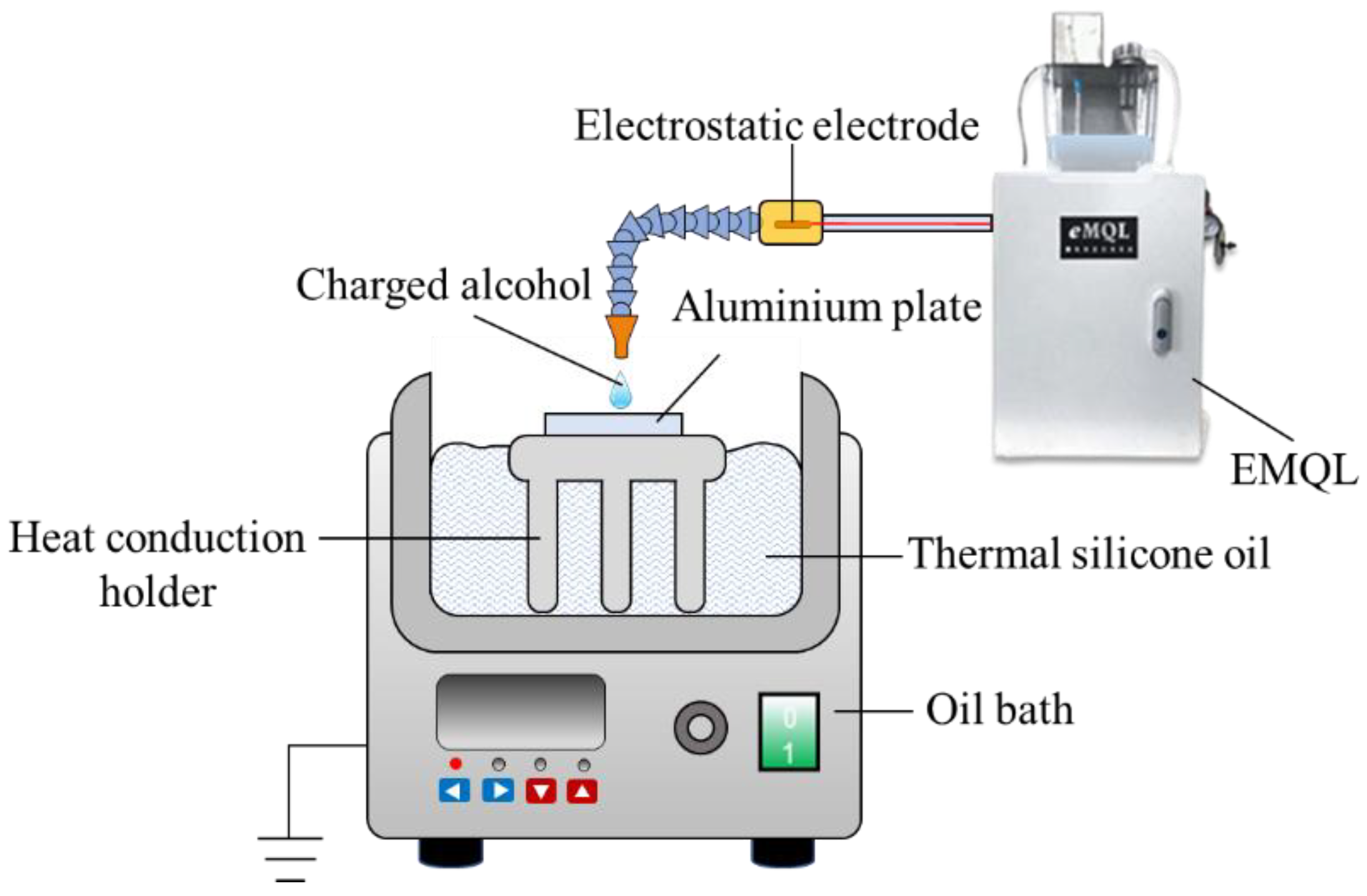
2.3. Static Reaction Experiment
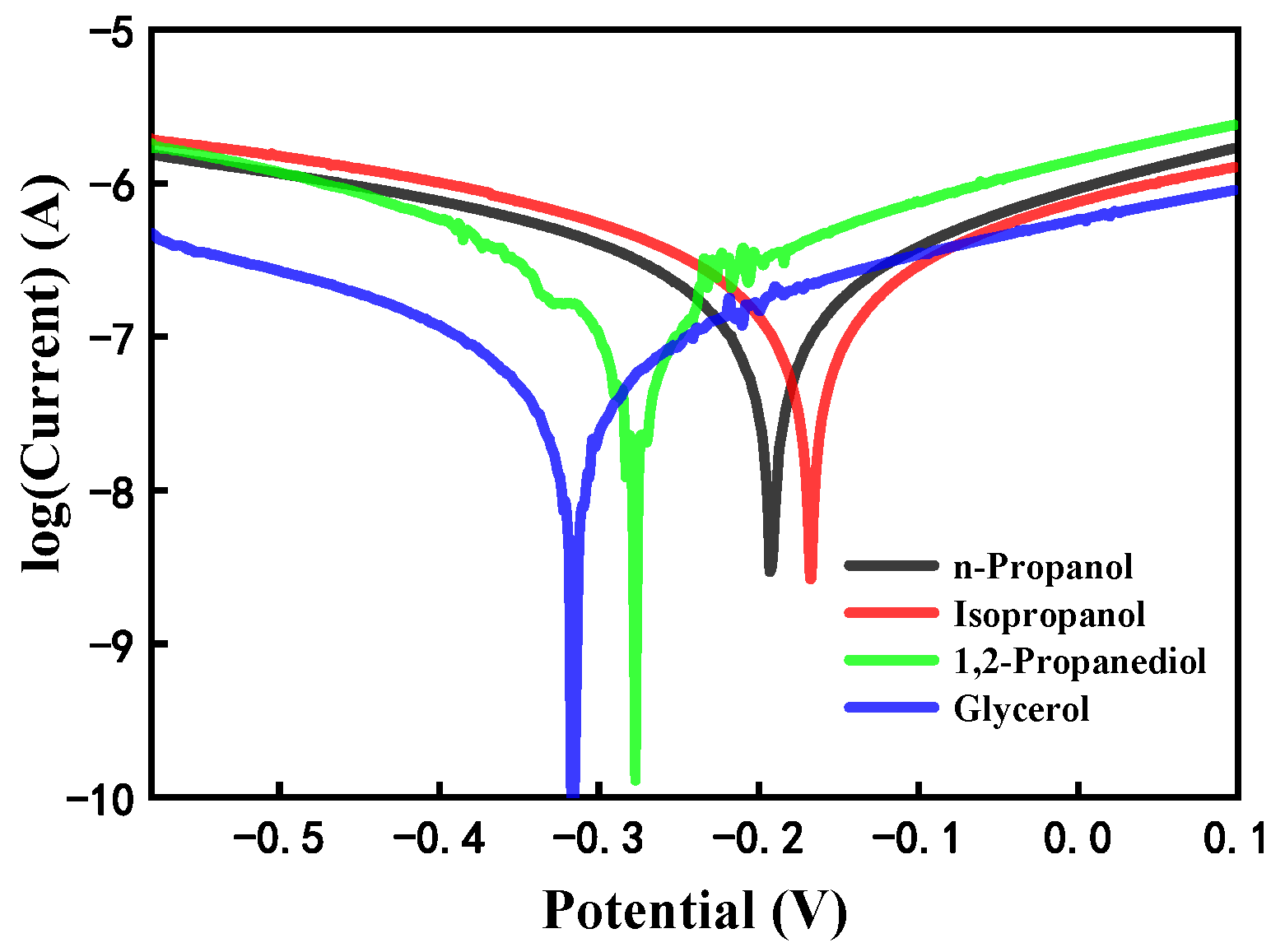
2.4. Electrochemical Polarization Test
3. Results and Discussion
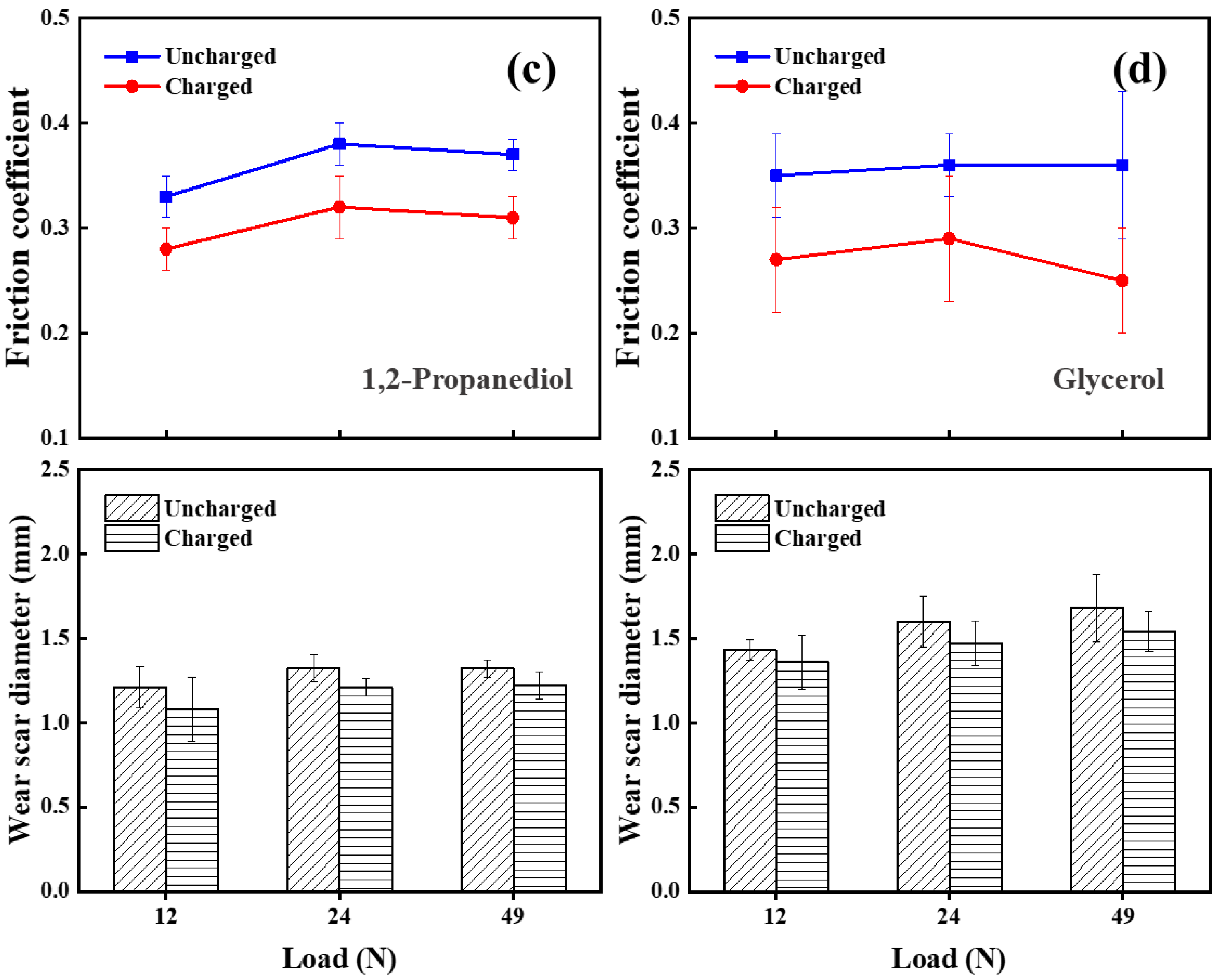
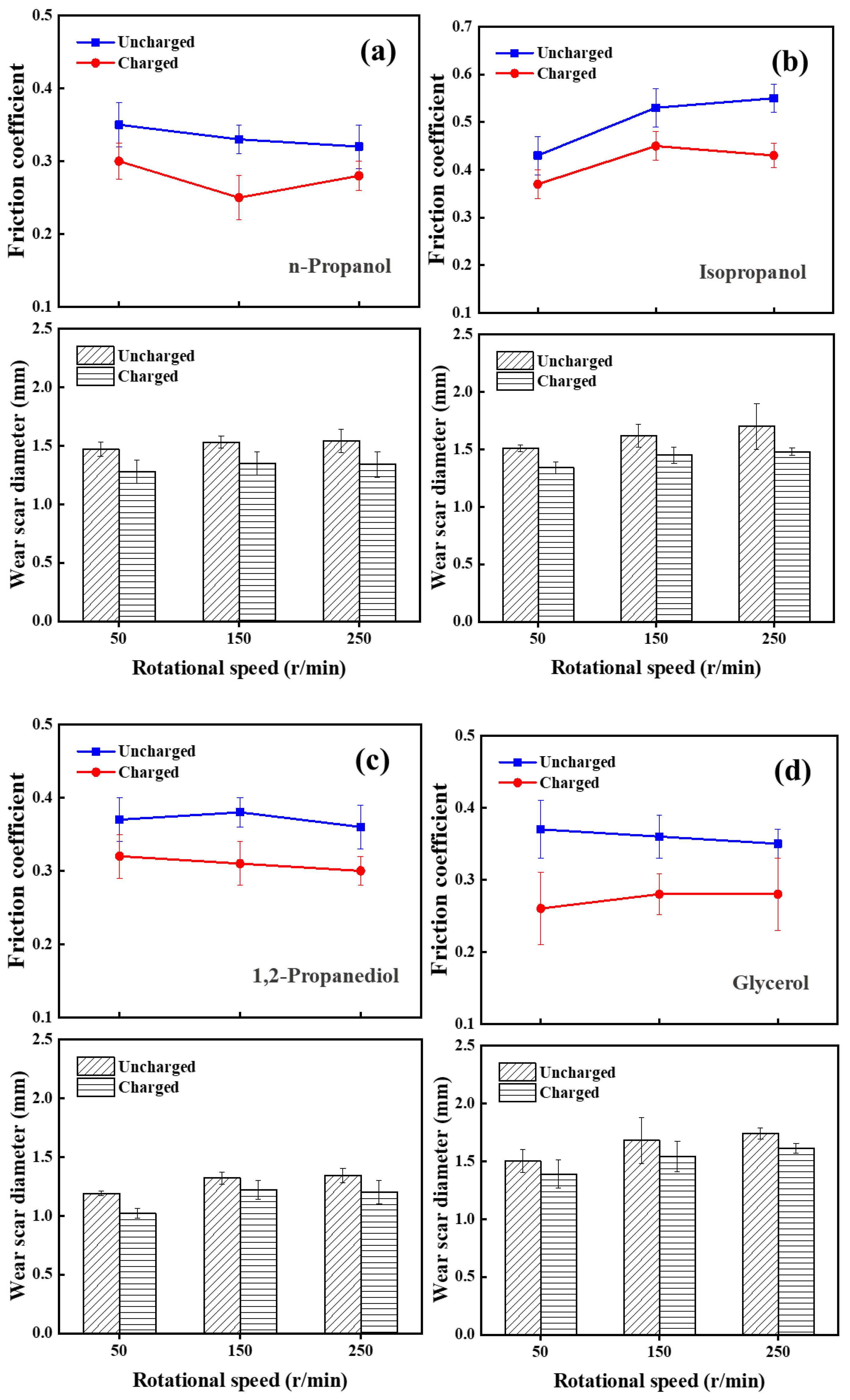
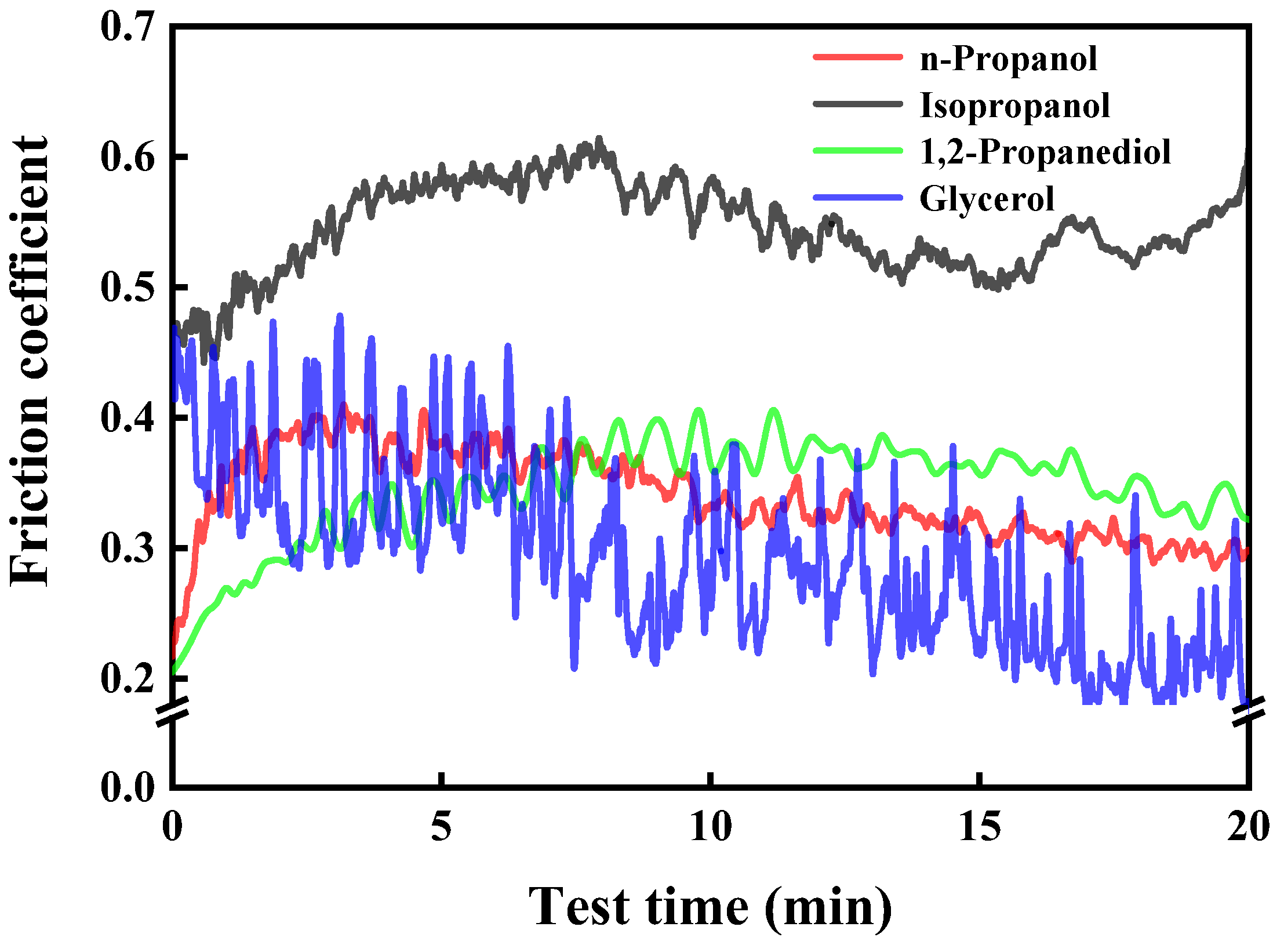
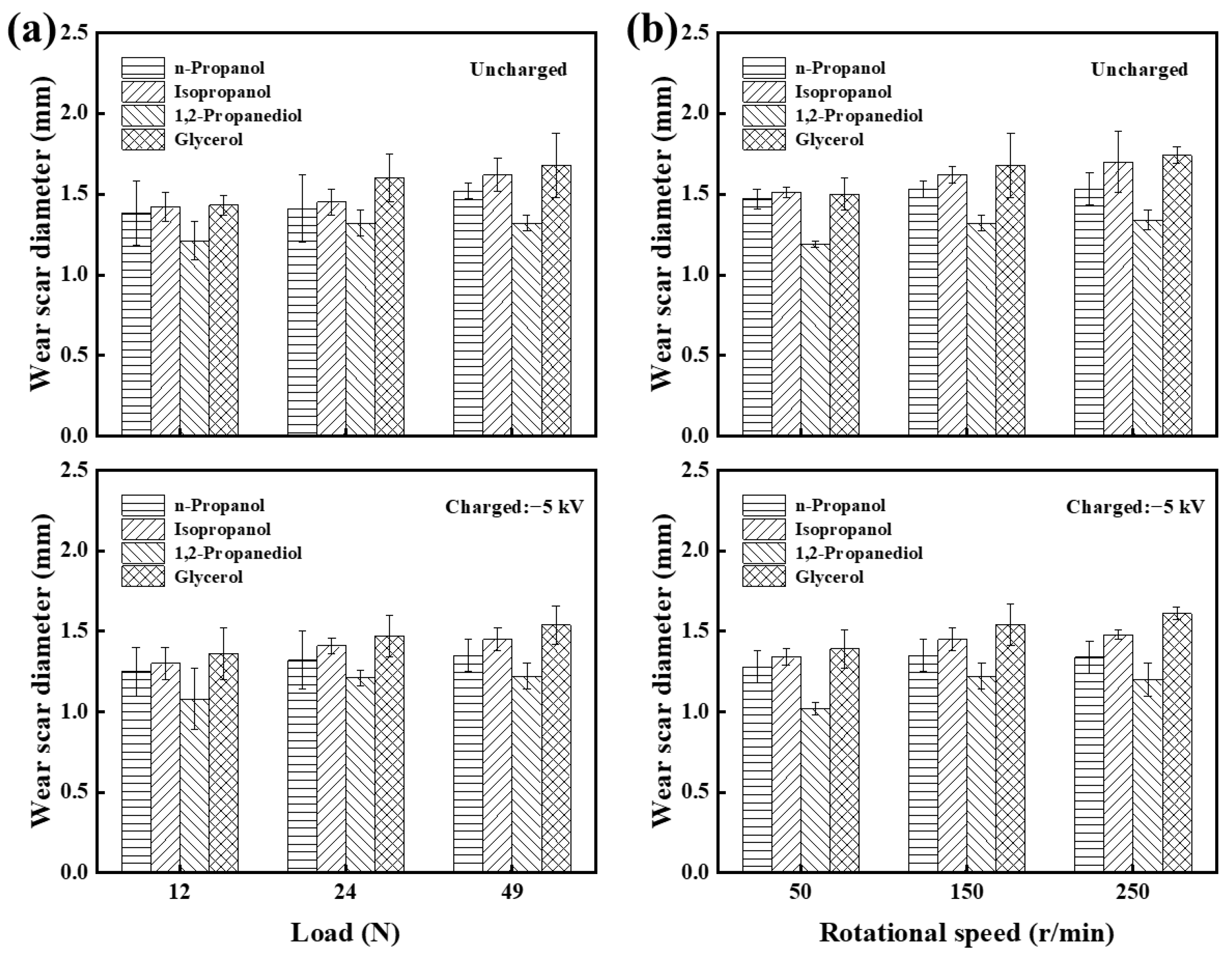
3.1. Tribological Performance
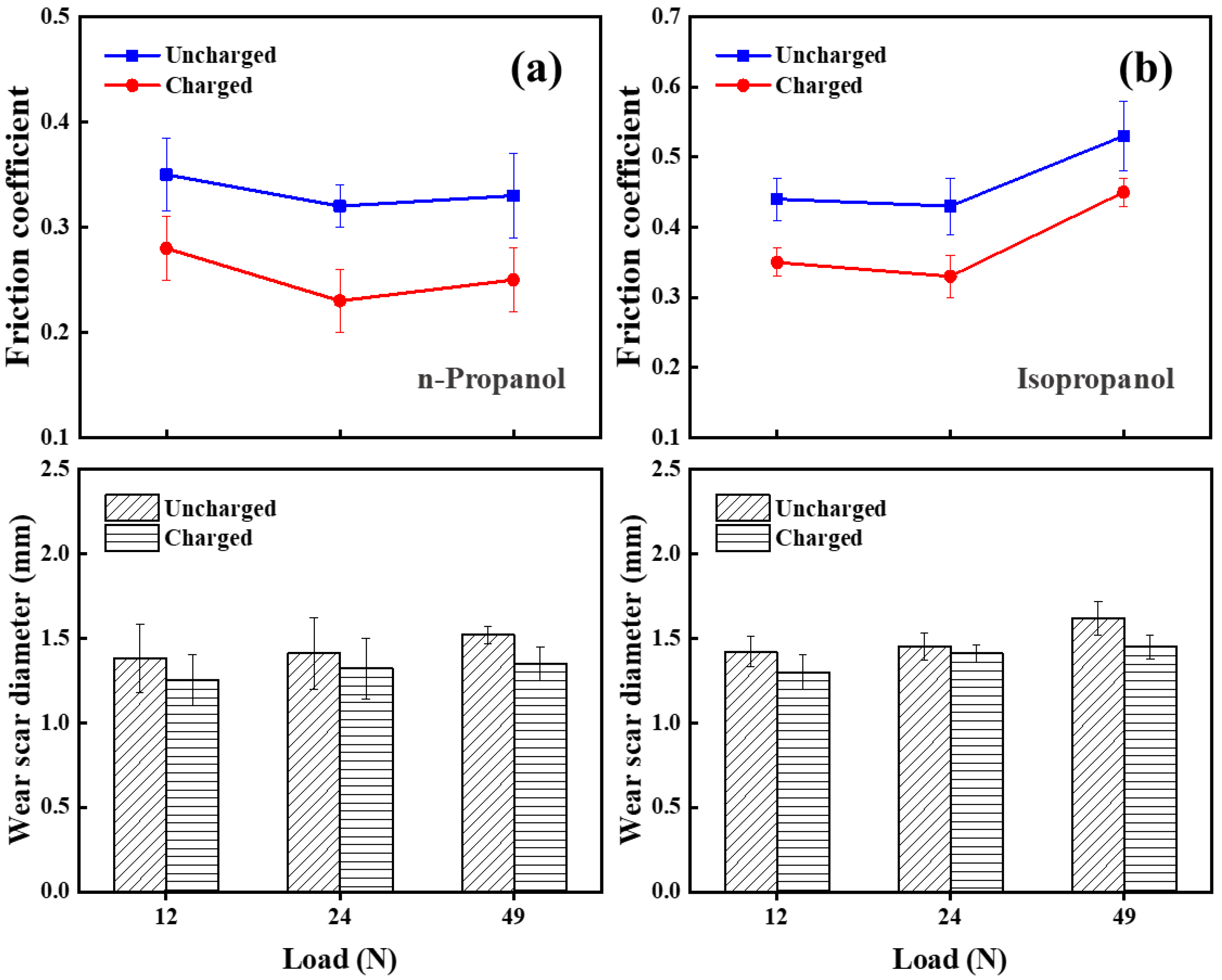
3.1.1. Lubrication Performance of Charged Alcohol Lubricants
3.1.2. Comparison of Four Alcohols’ Lubrication Performance
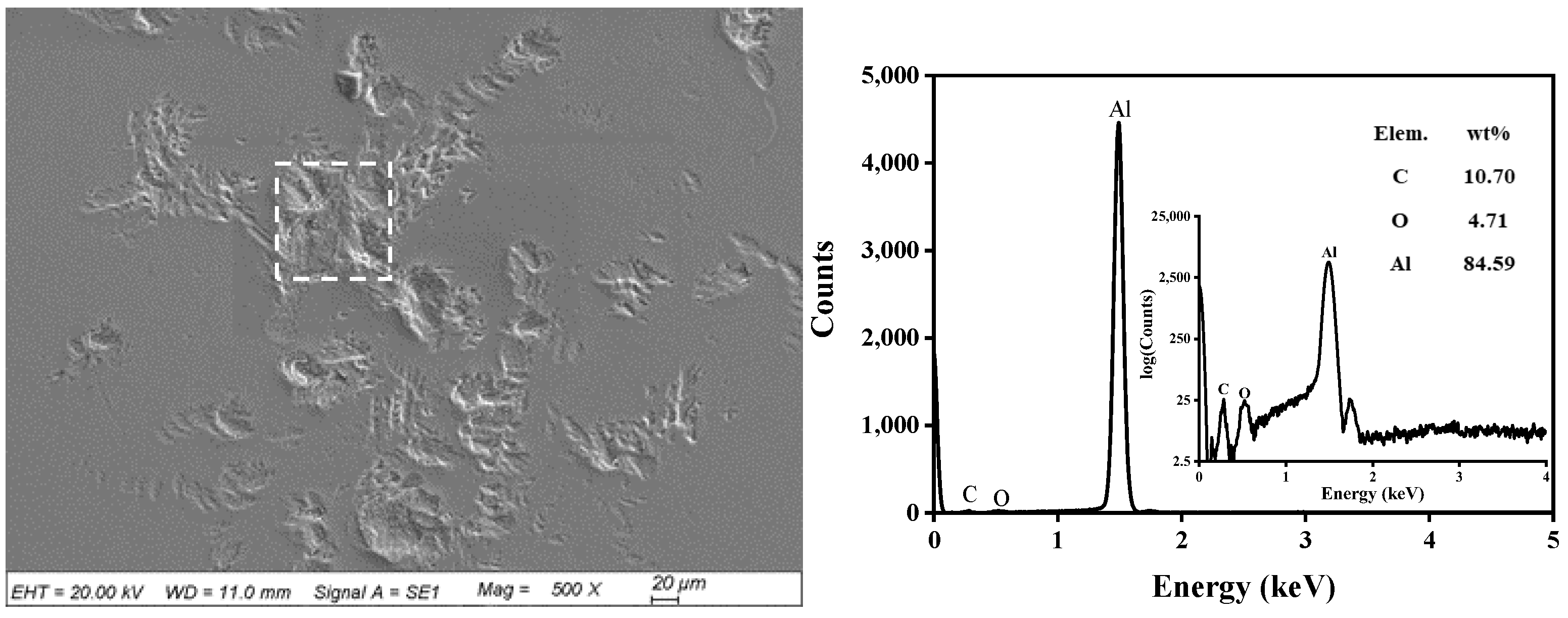
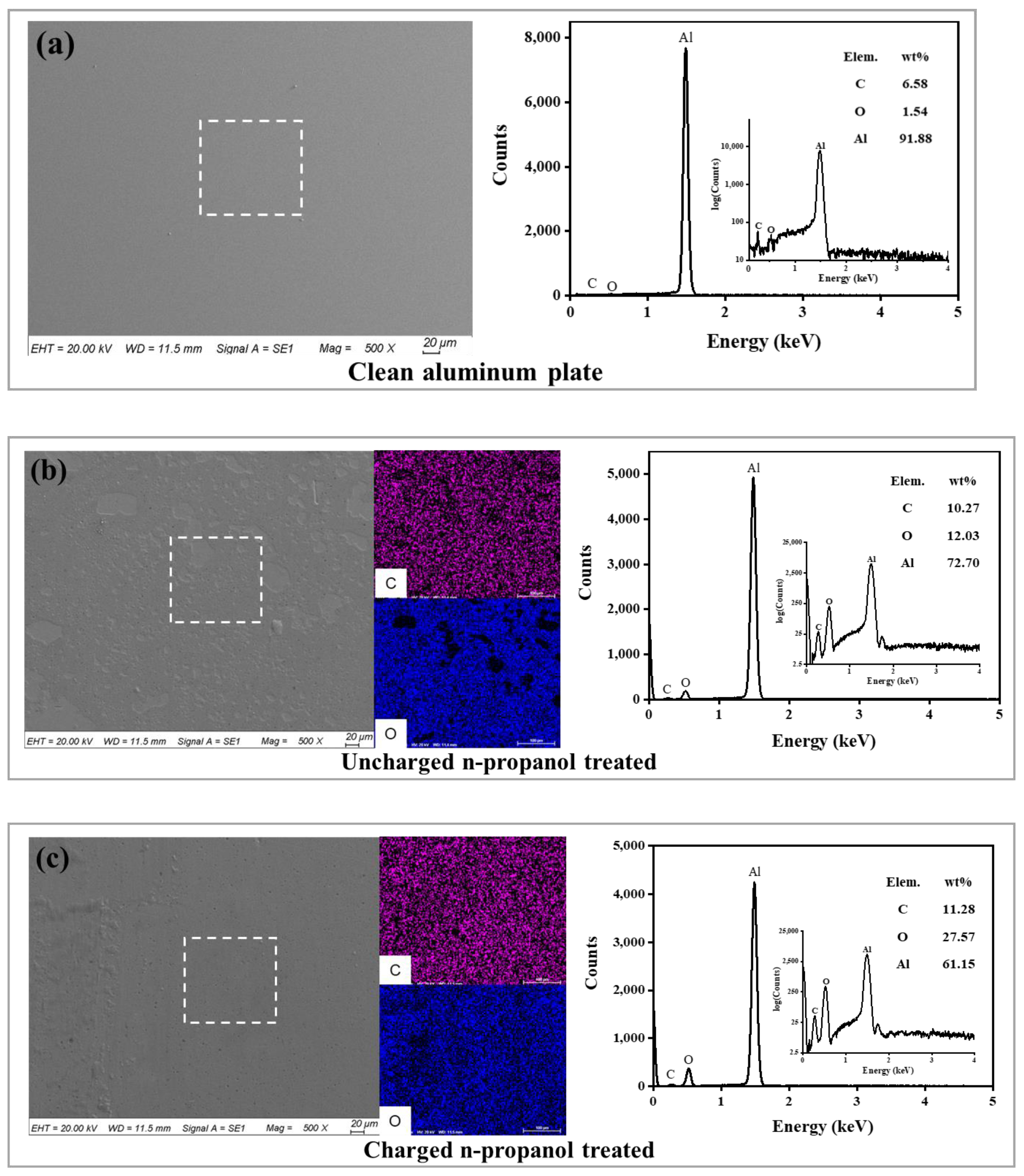
3.2. Static Reaction Experiment Results
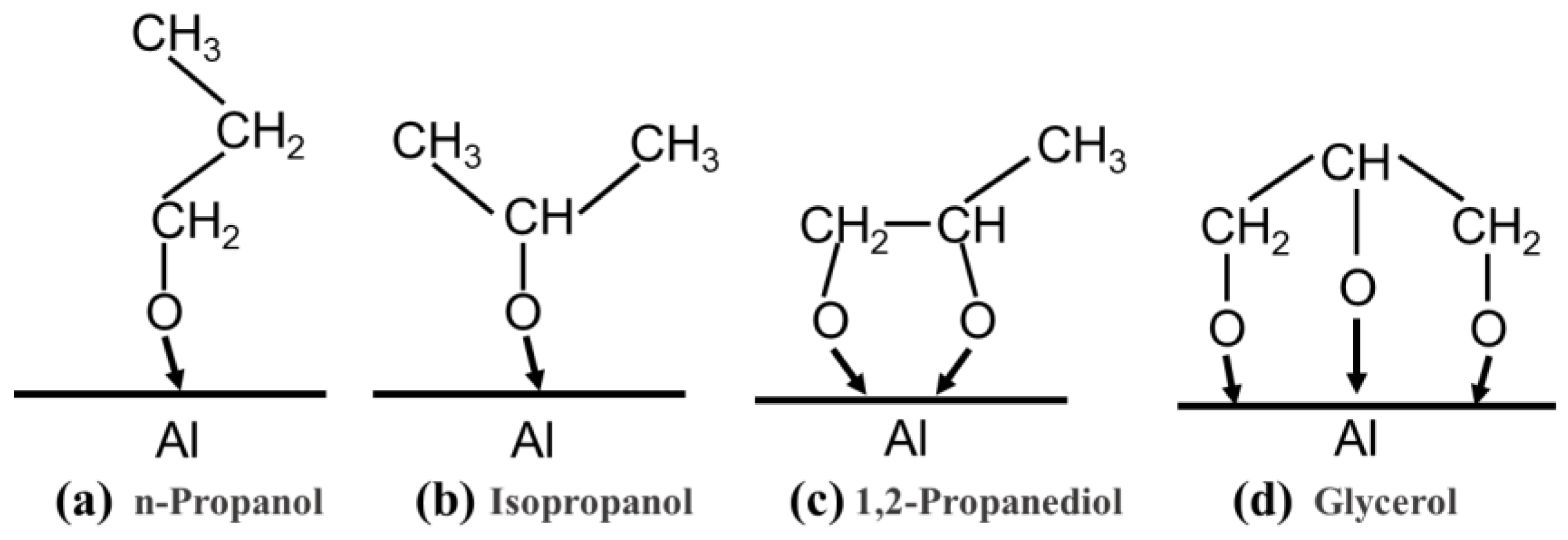
3.3. Lubrication Mechanism of Alcohol with Different Molecular Structures
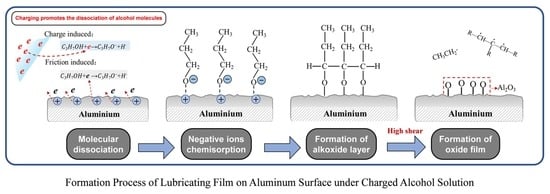
3.4. Lubrication Mechanism of Charged Alcohols
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, M.; Wang, J.; Yang, H.; Shi, J. Research on influence of milling process parameters on residual stress of 7055 aluminum alloy. J. Phys. Conf. Ser. 2022, 2268, 012002. [Google Scholar] [CrossRef]
- Dahnel, A.N.; Fauzi, M.H.; Raof, N.A.; Mokhtar, S.; Khairussaleh, N.K.M. Tool wear and burr formation during drilling of aluminum alloy 7075 in dry and with cutting fluid. Mater. Today Proc. 2022, 59, 808–813. [Google Scholar] [CrossRef]
- Sharmila, B.; Selvakumar, G.; Prakash, S.R. Investigations on the effect of dielectric medium and wedm parameters on surface characteristics of al 7068 (ordnance aluminium) alloy. Surf. Topogr.-Metrol. 2022, 10, 035031. [Google Scholar]
- Secgin, O.; Sogut, M.Z. Surface roughness optimization in milling operation for aluminum alloy (al 6061-t6) in aviation manufacturing elements. Aircraft Eng. Aerosp. Technol. 2021, 93, 1367–1374. [Google Scholar] [CrossRef]
- Wei, C.; Shao, T.; Dong, Y.; Yang, C. Preparation and application of aluminum alloy semi-synthetic fluids with high performance. Lubr. Eng. 2013, 38, 102–107. [Google Scholar]
- Nautiyal, P.C.; Schey, J.A. Transfer of auminum to steel in sliding contact: Effects of lubricant. J. Tribol. 1990, 112, 282–287. [Google Scholar] [CrossRef]
- Decrozant-Triquenaux, J.; Pelcastre, L.; Courbon, C.; Prakash, B.; Hardell, J. Effect of surface engineered tool steel and lubrication on aluminium transfer at high temperature. Wear 2021, 477, 203879. [Google Scholar] [CrossRef]
- Udupa, A.; Sugihara, T.; Viswanathan, K.; Chandrasekar, S. Altering the stability of surface plastic flow via mechanochemical effects. Phys. Rev. Appl. 2019, 11, 014021. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, W. Investigation of aliphatic aacohols as lubricating additives for aluminum on steel contact. Tribology 2000, 20, 75–77. [Google Scholar]
- Montgomery, R.S. The effect of alcohols and ethers on the wear behavior of aluminum. Wear 1965, 8, 466–473. [Google Scholar] [CrossRef]
- Hironaka, S.; Sakurai, T. The effect of pentaerythritol partial ester on the wear of aluminum. Wear 1978, 50, 105–114. [Google Scholar] [CrossRef]
- Hotten, B.W. Bidentate organic compounds as boundary lubricants for aluminum. Lubr. Eng. 1974, 30, 398–402. [Google Scholar]
- Wan, Y.; Liu, W.; Xue, Q. Effects of diol compounds on the friction and wear of aluminum alloy in a lubricated aluminum-on-steel contact. Wear 1996, 193, 99–104. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, W. Tribological properties of alcohols as lubricating additives for aluminum-on-steel contact. Wear 1998, 218, 244–249. [Google Scholar]
- Kajdas, C.; Liu, W. Tribochemistry of aluminium and aluminium alloy systems lubricated with liquids containing alcohol or amine additive types and some other lubricants—A review. Lubr. Sci. 2004, 16, 267–292. [Google Scholar] [CrossRef]
- Kajdas, C. About an anionic-radical concept of the lubrication mechanism of alcohols. Wear 1987, 116, 167–180. [Google Scholar] [CrossRef]
- Kajdas, C. On a negative-ion concept of ep action of organo-sulfur compounds. ASLE Trans. 1985, 28, 21–30. [Google Scholar] [CrossRef]
- Kajdas, C. Importance of anionic reactive intermediates for lubricant component reactions with friction surfaces. Lubr. Sci. 1994, 6, 203–228. [Google Scholar] [CrossRef]
- Hiratsuka, K.; Kajdas, C. Mechanochemistry as a key to understand the mechanisms of boundary lubrication, mechanolysis and gas evolution during friction. Proc. Inst. Mech. Eng. Part J-J. Eng. Tribol. 2013, 227, 1191–1203. [Google Scholar] [CrossRef]
- Lv, T.; Huang, S.Q.; Hu, X.D.; Feng, B.H.; Xu, X.F. Study on aerosol characteristics of electrostatic minimum quantity lubrication and its turning performance. J. Mech. Eng. Sci. 2019, 55, 129–138. [Google Scholar] [CrossRef]
- Xu, X.; Huang, S.; Wang, M.; Yao, W. A study on process parameters in end milling of aisi-304 stainless steel under electrostatic minimum quantity lubrication conditions. Int. J. Adv. Manuf. Technol. 2017, 90, 979–989. [Google Scholar] [CrossRef]
- Huang, S.; Yao, W.; Hu, J.; Xu, X. Tribological performance and lubrication mechanism of contact-charged electrostatic spray lubrication technique. Tribol. Lett. 2015, 59, 28. [Google Scholar] [CrossRef]
- Lv, T.; Xu, X.; Yu, A.; Hu, X. Oil mist concentration and machining characteristics of sio2 water-based nano-lubricants in electrostatic minimum quantity lubrication-emql milling. J. Mater. Process. Technol. 2021, 290, 116964. [Google Scholar] [CrossRef]
- Huang, S.; Lv, T.; Wang, M.; Xu, X. Enhanced machining performance and lubrication mechanism of electrostatic minimum quantity lubrication-emql milling process. Int. J. Adv. Manuf. Techol. 2018, 94, 655–666. [Google Scholar] [CrossRef]
- Huang, S.; Wu, H.; Jiang, Z.; Huang, H. Water-based nanosuspensions: Formulation, tribological property, lubrication mechanism, and applications. J. Manuf. Process. 2021, 71, 625–644. [Google Scholar] [CrossRef]
- Minami, I.; Sugibuchi, A. Surface chemistry of aluminium alloy slid against steel lubricated by organic friction modifier in hydrocarbon oil. Adv. Tribol. 2012, 2012, 926870. [Google Scholar] [CrossRef]
- Huang, G.; Fan, S.; Li, T.; Sun, T.; Fan, H.; Su, Y.; Song, J. Construction of graphite-copper 3-d composite lubricating layer on cu663 alloy surface and its tribological performances. Tribology 2021, 41, 304–315. [Google Scholar]
- Wang, L.; Ge, X.; Liu, Z.; He, M. Effect of particles on oil film characteristics of journal bearing. Lubr. Eng. 2021, 46, 51–57. [Google Scholar]
- Liang, X.; Yang, X.; Wu, J.; Tu, Z.; Wang, Z.; Yuan, Z. Effect of alcohol additives on the plate-out oil film in cold rolling and its molecular dynamics simulations. Tribol. Trans. 2019, 62, 504–511. [Google Scholar] [CrossRef]
- Igari, S.; Mori, S.; Takikawa, Y. Effects of molecular structure of aliphatic diols and polyalkylene glycol as lubricants on the wear of aluminum. Wear 2000, 244, 180–184. [Google Scholar] [CrossRef]
- Hsu, S.M.; Gates, R.S. Boundary lubricating films: Formation and lubrication mechanism. Tribol. Int. 2005, 38, 305–312. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.; Luo, J. Superlubricity achieved with mixtures of polyhydroxy alcohols and acids. Langmuir 2013, 29, 5239–5245. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, C.; Yu, Y.; Ma, X. Study on the effects of additives on the lubricity for 7075. Lubr. Eng. 2010, 35, 52–57. [Google Scholar]
- Pearson, R.G. Hard and soft acids and bases—The evolution of a chemical concept. Coord. Chem. Rev. 1990, 100, 403–425. [Google Scholar] [CrossRef]
- Carlton, H.; Huitink, D.; Liang, H. Tribochemistry as an alternative synthesis pathway. Lubricants 2020, 8, 87. [Google Scholar] [CrossRef]
- Podrabinnik, P.; Gershman, I.; Mironov, A.; Kuznetsova, E.; Peretyagin, P. Tribochemical interaction of multicomponent aluminum alloys during sliding friction with steel. Lubricants 2020, 8, 24. [Google Scholar] [CrossRef]
- Kajdas, C.; Hiratsuka, K. Tribochemistry, tribocatalysis, and the negative-ion-radical action mechanism. Proc. Inst. Mech. Eng. Part J-J. Eng. Tribol. 2009, 223, 827–848. [Google Scholar] [CrossRef]
- Nakayama, K. Triboemission of charged particles and resistivity of solids. Tribol. Lett. 1999, 6, 37–40. [Google Scholar] [CrossRef]
- Molina, G.J.; Furey, M.J.; Ritter, A.L.; Kajdas, C. Triboemission from alumina, single crystal sapphire, and aluminum. Wear 2001, 249, 214–219. [Google Scholar] [CrossRef]






| Alcohol | Formula | Molecular Mass | Boiling Point (°C) | Viscosity at 20 °C (mPa·s) |
|---|---|---|---|---|
| n-Propanol | 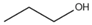 | 60.1 | 97 | 2.256 |
| Isopropanol | 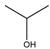 | 60.1 | 83 | 2.038 |
| 1,2-Propanediol | 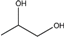 | 76.1 | 195 | 60.5 |
| Glycerol |  | 92.1 | 290 | 1412 |
| 25 °C | 75 °C | 140 °C | ||||
|---|---|---|---|---|---|---|
| Uncharged | Charged | Uncharged | Charged | Uncharged | Charged | |
| n-Propanol |  |  |  |  | ||
| Isopropanol |  |  |  |  | ||
| 1,2-Propanediol |  |  |  |  | ||
| Glycerol |  |  |  |  |  |  |
| Alcohols | n-Propanol | Isopropanol | 1,2-Propanediol | Glycerol | |||||
|---|---|---|---|---|---|---|---|---|---|
| Content % | Uncharged | Charged | Uncharged | Charged | Uncharged | Charged | Uncharged | Charged | |
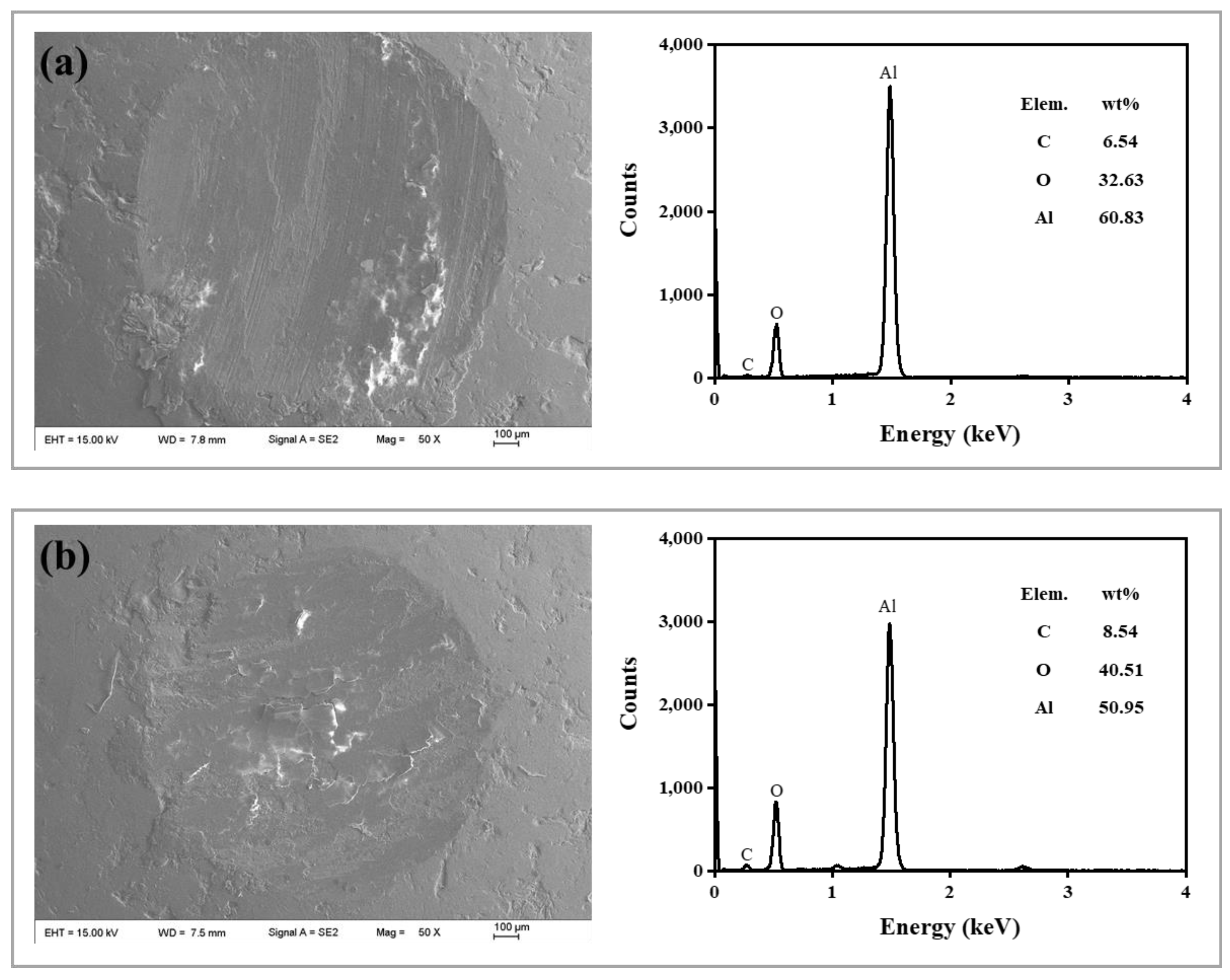
| C | 10.27 | 11.28 | 6.13 | 12.88 | 11.07 | 16.28 | 7.89 | 15.02 | |
| O | 17.03 | 127.57 | 10.82 | 9.67 | 4.28 | 17.91 | 2.86 | 5.89 | |
| Al | 72.70 | 61.15 | 83.05 | 77.45 | 84.65 | 65.81 | 89.25 | 79.09 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Wang, Y.; Tang, H.; Xia, Y.; Huang, S.; Xu, X.; Zhang, R. Lubrication Performance and Mechanism of Electrostatically Charged Alcohol Aqueous Solvents with Aluminum–Steel Contact. Lubricants 2022, 10, 322. https://doi.org/10.3390/lubricants10110322
Hu X, Wang Y, Tang H, Xia Y, Huang S, Xu X, Zhang R. Lubrication Performance and Mechanism of Electrostatically Charged Alcohol Aqueous Solvents with Aluminum–Steel Contact. Lubricants. 2022; 10(11):322. https://doi.org/10.3390/lubricants10110322
Chicago/Turabian StyleHu, Xiaodong, Ying Wang, Hongmei Tang, Yu Xia, Shuiquan Huang, Xuefeng Xu, and Ruochong Zhang. 2022. "Lubrication Performance and Mechanism of Electrostatically Charged Alcohol Aqueous Solvents with Aluminum–Steel Contact" Lubricants 10, no. 11: 322. https://doi.org/10.3390/lubricants10110322
APA StyleHu, X., Wang, Y., Tang, H., Xia, Y., Huang, S., Xu, X., & Zhang, R. (2022). Lubrication Performance and Mechanism of Electrostatically Charged Alcohol Aqueous Solvents with Aluminum–Steel Contact. Lubricants, 10(11), 322. https://doi.org/10.3390/lubricants10110322