Assessing the High-Temperature Deposit Formation of Paraffinic and Naphthenic Oil Blends Using the Oil Chute Method
Abstract
1. Introduction
- Developing new gas engine oil formulations: As the (thermal) stress levels for gas engine oils continue to rise [5], novel formulations need to be developed that meet the ever-increasing requirements. Both base oils and additives should be investigated in this context. In addition to the high-temperature deposit-formation tendency, the thermo-oxidative stability is one of the most important properties requiring consideration.
- Finding a test strategy: Given the high expenditure of money and time associated with engine [13] or field tests, the establishment of a suitable screening method is advisable. In this context, the implementability of realistic conditions, practical feasibility, and a good correlation of the results with field observations should be provided. Such a laboratory-based test method would enable the evaluation and comparison of various novel formulations before testing or application in the field.
2. Materials and Methods
2.1. Samples
2.2. Methods
2.2.1. Artificial Alteration
2.2.2. Oil Chute
- U-channel temperature: 300 °C;
- Water cooling temperature: 40 °C;
- Sample amount: 85 g;
- Oil flow rate: ~7 mL/min;
- Test duration: 21 h.
3. Results and Discussion
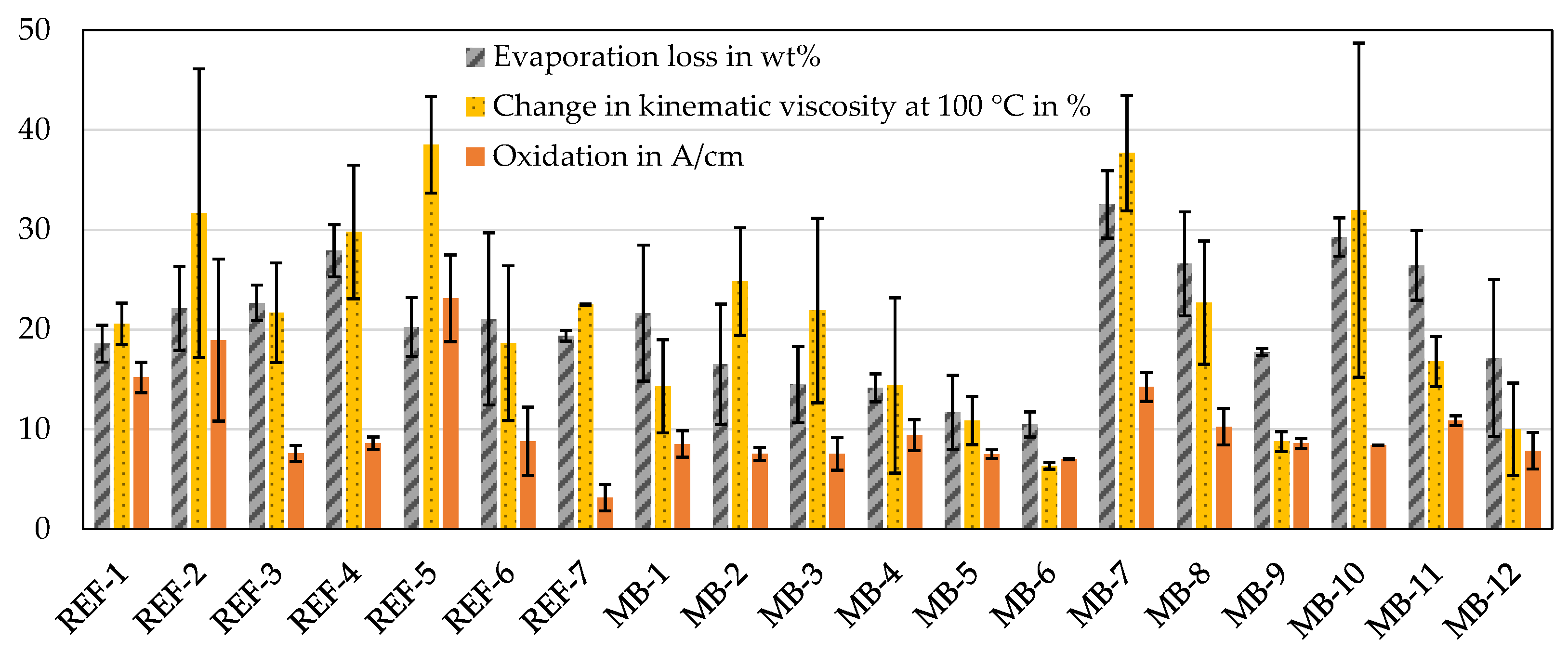
3.1. Oxidation after Artificial Alteration and Oil Chute
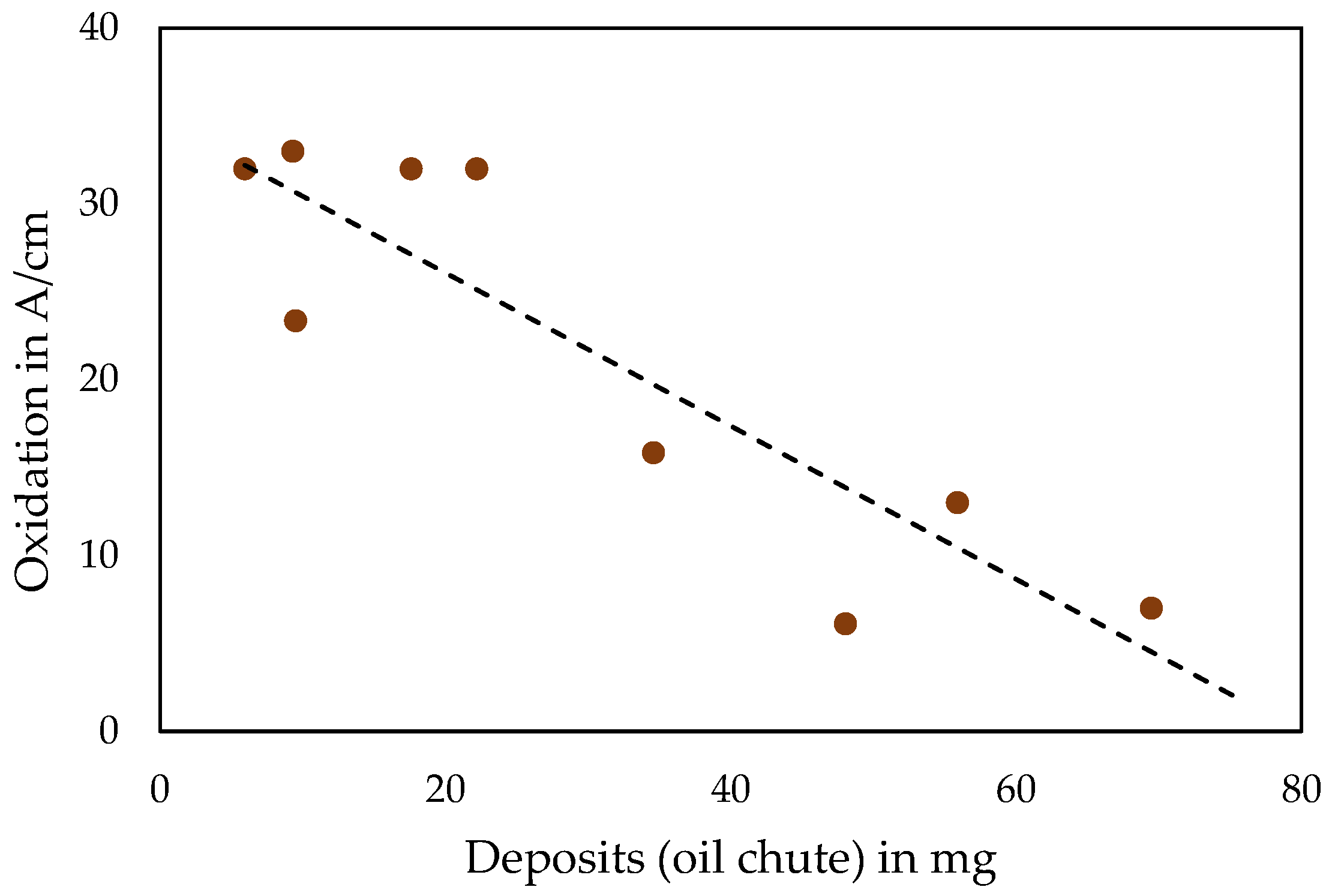
3.2. Deposit Amount and Oil Characterisation after Oil Chute
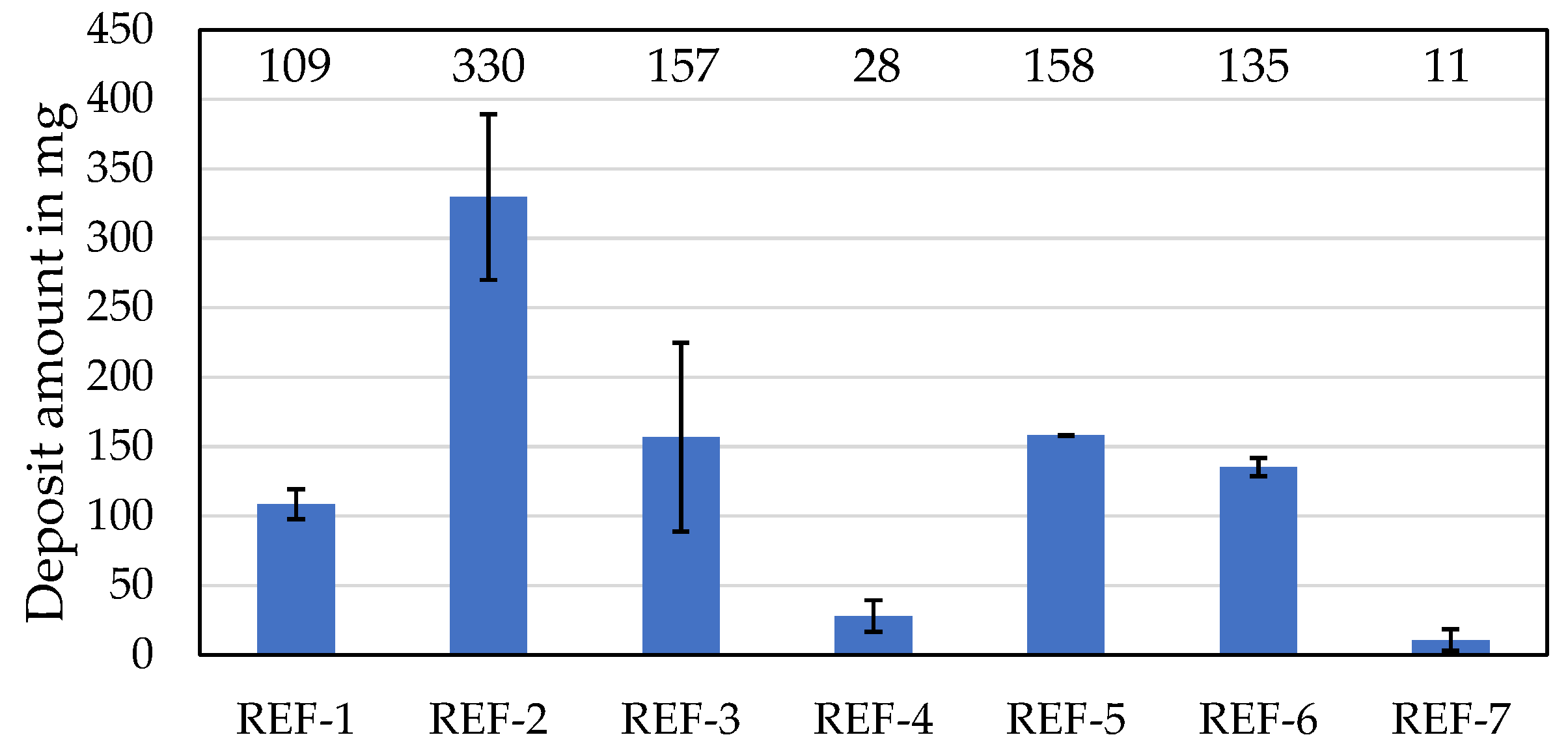
3.2.1. High-Temperature Deposit of Reference Oils
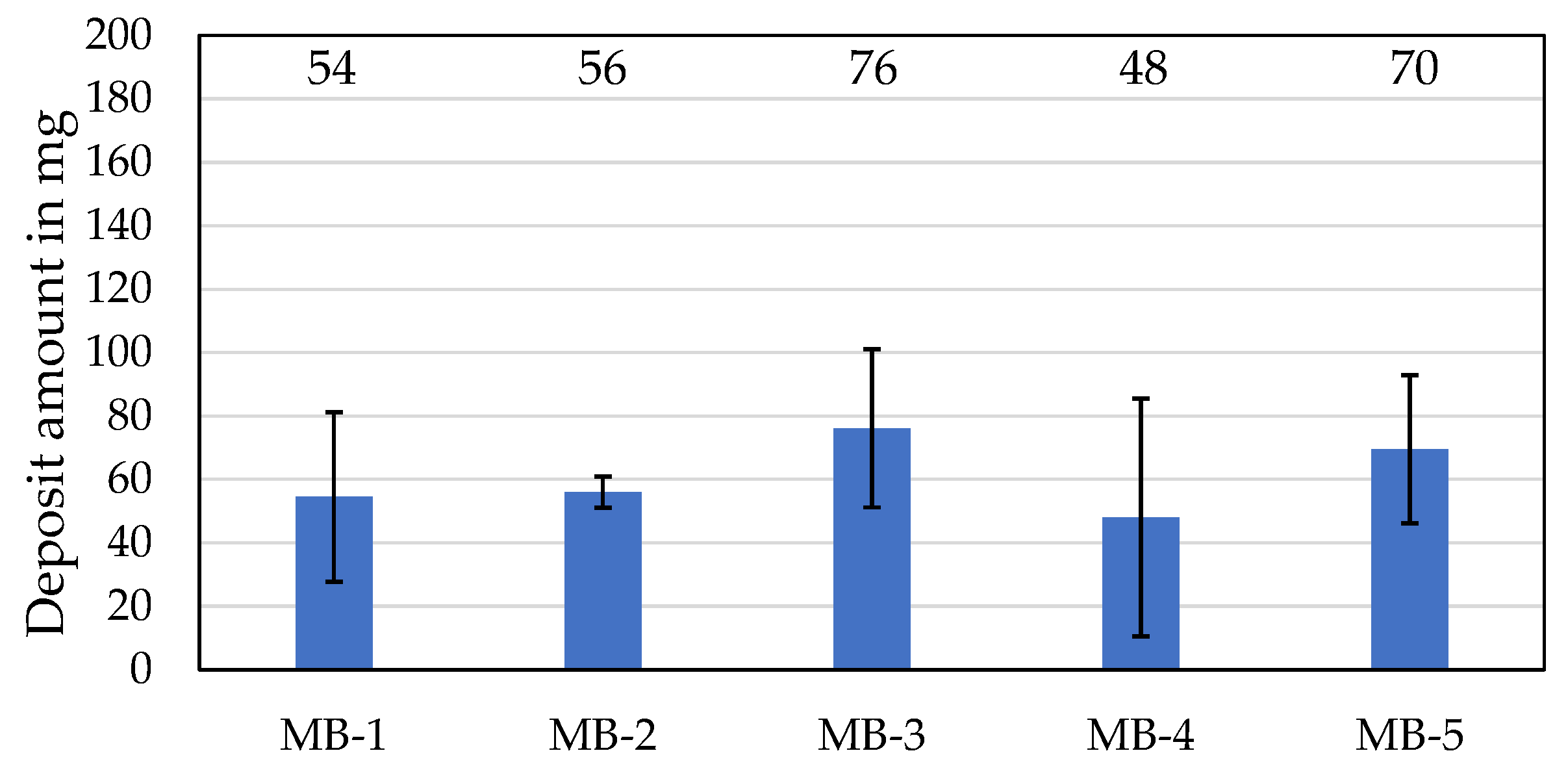
3.2.2. High-Temperature Deposit of Model Blends
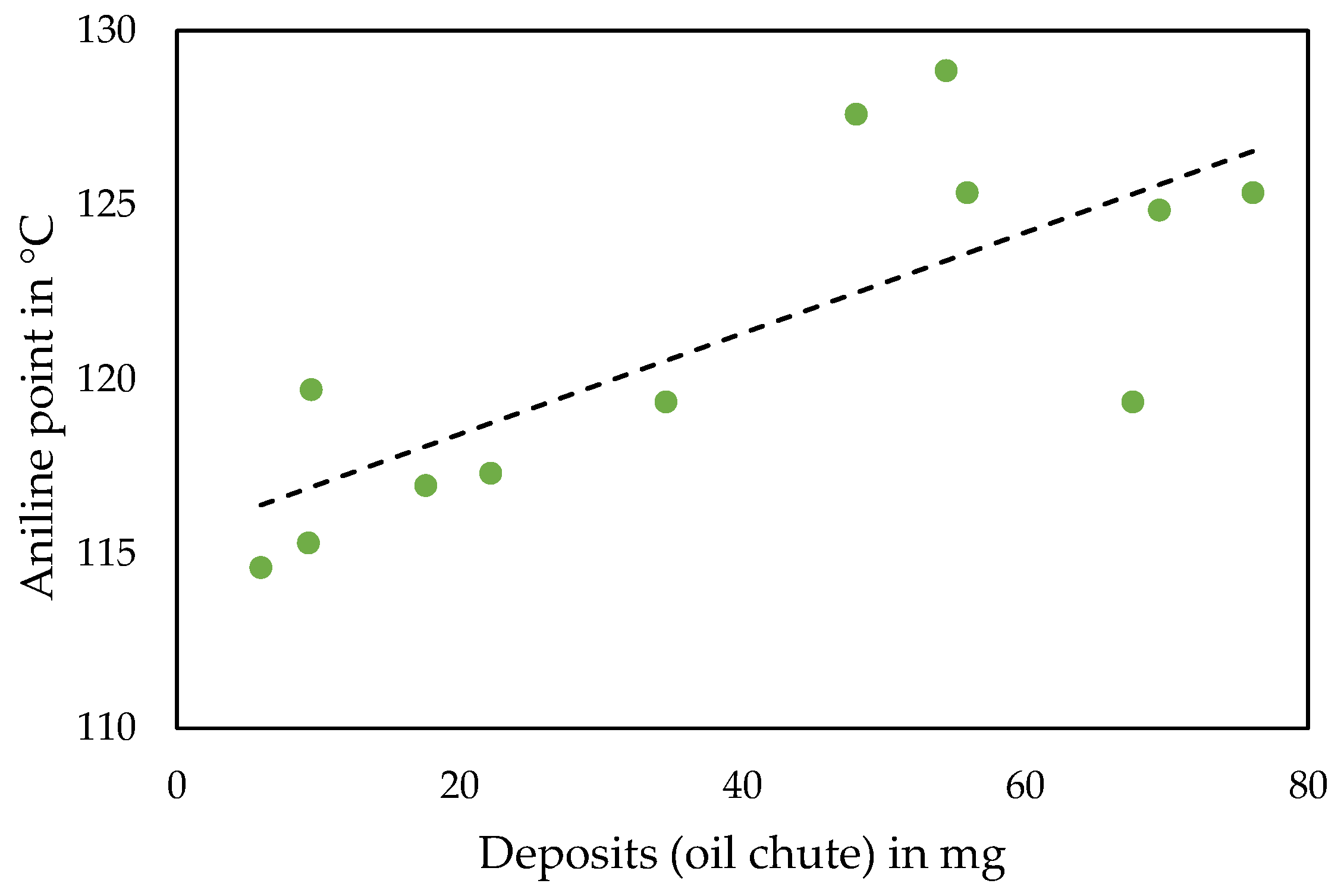
3.3. Formulation Considerations
4. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gharehghani, A.; Fakhari, A.H. Natural Gas as a Clean Fuel for Mobility. In Clean Fuels for Mobility; di Blasio, G., Agarwal, A.K., Belgiorno, G., Shukla, P.C., Eds.; Springer: Singapore, 2022; pp. 215–241. [Google Scholar]
- Divekar, P.; Han, X.; Zhang, X.; Zheng, M.; Tjong, J. Energy Efficiency Improvements and CO2 Emission Reduction by CNG Use in Medium- and Heavy-Duty Spark-Ignition Engines. Energy 2023, 263, 125769. [Google Scholar] [CrossRef]
- Li, F.; Wang, Z.; Wang, Y.; Wang, B. High-Efficiency and Clean Combustion Natural Gas Engines for Vehicles. Automot. Innov. 2019, 2, 284–304. [Google Scholar] [CrossRef]
- Lang, J.; Schäffert, P.; Böwing, R.; Rivellini, S.; Nota, F.; Klausner, J. Development of a New Generation of GE’s Jenbacher Type 6 Gas Engines. In Heavy-Duty-, On- und Off-Highway-Motoren 2016; Siebenpfeiffer, W., Ed.; Springer: Wiesbaden, Germany, 2016; ISBN 978-3-658-19011-8. [Google Scholar]
- Hughes, J. Development of a New Lubricating Oil for Use in Modern High Efficiency Gas Engines. In Proceedings of the CIMAC Congress, Vancouver, BC, Canada, 10–14 June 2019. [Google Scholar]
- Garcia, L.; Reher, J. Latest Generation of High Performance Gas Engine Oils—Tackling Reliability Challenges and Extending Oil Life in Modern Highly Efficient Gas Engines. In Proceedings of the CIMAC Congress, Vancouver, BC, Canada, 10–14 June 2019. [Google Scholar]
- Dowse, D.G.; Jansten, E.; Maier, K. Deposition in Gas Turbine Oil Systems—Part 1: Analysis and Classification; SAE Technical Paper 851869; Society of Automotive Engineers: Warrendale, PA, USA, 1985. [Google Scholar] [CrossRef]
- Kagaya, M.; Ishikawa, S. An Evaluation and Optimization of Lubricants for Turbocharged Gasoline Engines; SAE Technical Paper 840261; Society of Automotive Engineers: Warrendale, PA, USA, 1984. [Google Scholar] [CrossRef]
- Dong, L.; Liang, X.; Shu, G. The Influence of Lubricating Oil on Deposits Formation in a Diesel Engine under the Operation Condition of High Power Density. Lubr. Sci. 2013, 25, 441–451. [Google Scholar] [CrossRef]
- Castro, W.; Weller, D.E.; Cheenkachorn, K.; Perez, J.M. The Effect of Chemical Structure of Basefluids on Antiwear Effectiveness of Additives. Tribol. Int. 2005, 38, 321–326. [Google Scholar] [CrossRef]
- Saini, V.; Bijwe, J.; Seth, S.; Ramakumar, S.S.V. Role of Base Oils in Developing Extreme Pressure Lubricants by Exploring Nano-PTFE Particles. Tribol. Int. 2020, 143, 106071. [Google Scholar] [CrossRef]
- Barman, B.N. Behavioral Differences between Group I and Group II Base Oils during Thermo-Oxidative Degradation. Tribol. Int. 2002, 35, 15–26. [Google Scholar] [CrossRef]
- Lakatos, L.K.; Jones, R.N.; Roby, S.H.; Sukys, D.J. Modeling of ASTM Sequence IIIE Piston Ring Land Deposit Formation; SAE Technical Paper 922293; Society of Automotive Engineers: Warrendale, PA, USA, 1992. [Google Scholar] [CrossRef]
- Josefina, V.C.M.; Armando, I.R. Use of Naphthenic Base Stocks in Engine Oil Formulations; SAE Technical Paper 881585; Society of Automotive Engineers: Warrendale, PA, USA, 1988. [Google Scholar] [CrossRef]
- Torbacke, M.; Rudolphi, Å.K.; Kassfeldt, E. Lubricants: Introduction to Properties and Performance; Wiley: Chichester, UK, 2014; ISBN 9781118799741. [Google Scholar]
- Mortier, R.M.; Fox, M.F.; Orszulik, S.T. (Eds.) Chemistry and Technology of Lubricants; Springer: Dordrecht, The Netherlands, 2010; ISBN 978-1-4020-8661-8. [Google Scholar]
- ASTM D611; Standard Test Methods for Aniline Point and Mixed Aniline Point of Petroleum Products and Hydrocarbon Solvents. ASTM International: West Conshohocken, PA, USA, 2016.
- Norrby, T.; Jonsson, A.-L. Base Oil and Antioxidant Selection—The Role of Secondary Antioxidants and Base Oil Sulfur Content. In Proceedings of the 72nd STLE Annual Meeting, Atlanta, GA, USA, 21–25 May 2017. [Google Scholar]
- le Pera, M.E.; Oswald, G.E. Heat Transfer Characteristics of Lubricants for Internal-Combustion Engines. Ind. Eng. Chem. Prod. Res. Dev. 1974, 13, 209–213. [Google Scholar] [CrossRef]
- GFC-LU-29-A-15; Panel Coker Test. Groupement Français de Coordination pour le développement des essais de performance des Carburants, des Lubrifiants et autres fluides dans le Transport: Rueil-Malmaison, France, 2015.
- ASTM D7097; Standard Test Method for Determination of Moderately High Temperature Piston Deposits by Thermo-Oxidation Engine Oil Simulation Test—TEOST MHT. ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM D6335; Standard Test Method for Determination of High Temperature Deposits by Thermo-Oxidation Engine Oil Simulation Test. ASTM International: West Conshohocken, PA, USA, 2019.
- Brown, G.; Barr, D.; Calder, R.; Durham, J.; McAtee, R.; Sutton, M. A New Screen Test for the Thermal Oxidative Stability of Engine Oils—The Glass Panel Coker; SAE Technical Paper 2004-01-2024; Society of Automotive Engineers: Warrendale, PA, USA, 2004. [Google Scholar] [CrossRef]
- Kouame, S.D.B. Application of Temperature-Programmed Oxidation (TPO) in Studying the Mechanism of Deposits Formation from the Degradation of Lubricants on Metal Surfaces. Lubr. Sci. 2014, 26, 117–130. [Google Scholar] [CrossRef]
- Kouame, S.D.; Liu, E. Characterization of Fully and Partially Additized Lubricant Deposits by Temperature Programmed Oxidation. Tribol. Int. 2014, 72, 58–64. [Google Scholar] [CrossRef]
- ASTM D189; Standard Test Method for Conradson Carbon Residue of Petroleum Products. ASTM International: West Conshohocken, PA, USA, 2006.
- ASTM D524; Standard Test Method for Ramsbottom Carbon Residue of Petroleum Products. ASTM International: West Conshohocken, PA, USA, 2015.
- DIN EN ISO 10370; Mineralölerzeugnisse—Bestimmung Des Koksrückstandes—Mikroverfahren. DIN (Deutsches Institut für Normung): Berlin, Germany, 2015.
- ASTM D7042; Standard Test Method for Dynamic Viscosity and Density of Liquids by Stabinger Viscometer (and the Calculation of Kinematic Viscosity). ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM D5800; Standard Test Method for Evaporation Loss of Lubricating Oils by the Noack Method. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM D2622; Standard Test Method for Sulfur in Petroleum Products by Wavelength Dispersive X-Ray Fluorescence Spectrometry. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM D4629; Standard Test Method for Trace Nitrogen in Liquid Hydrocarbons by Syringe/Inlet Oxidative Combustion and Chemiluminescence Detection. ASTM International: West Conshohocken, PA, USA, 2017.
- SAE J300; Engine Oil Viscosity Classification. SAE International: Warrendale, PA, USA, 2015.
- CEC L-48-A00; Oxidation Stability of Lubricating Oils Used in Automotive Transmissions by Artificial Ageing. CEC (Co-ordinating European Council for the Development of Performance Tests for Fuels Lubricants and Other Fluids): Brussels, Belgium, 2007.
- DIN 51453; Prüfung von Schmierstoffen—Bestimmung Der Oxidation Und Nitration von Gebrauchten Motorenölen—Infrarotspektrometrisches Verfahren. DIN (Deutsches Institut für Normung): Berlin, Germany, 2004.
- Novotny-Farkas, F.; (Lubex Consulting e.U., Schwechat, Austria). Personal communication, 2021.
- Gracia, N.; Thomas, S.; Bazin, P.; Duponchel, L.; Thibault-Starzyk, F.; Lerasle, O. Combination of Mid-Infrared Spectroscopy and Chemometric Factorization Tools to Study the Oxidation of Lubricating Base Oils. Catal Today 2010, 155, 255–260. [Google Scholar] [CrossRef]
- Besser, C.; Agocs, A.; Ronai, B.; Ristic, A.; Repka, M.; Jankes, E.; McAleese, C.; Dörr, N. Generation of Engine Oils with Defined Degree of Degradation by Means of a Large Scale Artificial Alteration Method. Tribol. Int. 2019, 132, 39–49. [Google Scholar] [CrossRef]
- Diaby, M.; Sablier, M.; le Negrate, A.; el Fassi, M.; Bocquet, J. Understanding Carbonaceous Deposit Formation Resulting from Engine Oil Degradation. Carbon 2009, 47, 355–366. [Google Scholar] [CrossRef]
- Yokoyama, F.; Iwama, Y. Mechanism of Carbonaceous Deposit Formation Caused by Lubricating Oil on High Temperature Metal Surfaces. Tribol. Online 2014, 9, 71–79. [Google Scholar] [CrossRef][Green Version]




| Designation | Base Oil Type | API Base Oil Classification | Kinematic Viscosity at 40 °C in mm²/s | Kinematic Viscosity at 100 °C in mm²/s | Viscosity Index | Sulphur Content in mg/kg | Nitrogen Content in mg/kg | Aniline Point in °C | NOACK-Volatility in wt% |
|---|---|---|---|---|---|---|---|---|---|
| P-1 | paraffinic | Group I | 339.8 | 24.3 | 91.2 | 3130 | 465 | 118 | 3.8 |
| P-2 | paraffinic | Group II | 42.2 | 6.6 | 107.3 | 12 | 0.2 | 116 | 6.8 |
| P-3 | paraffinic | Group II | 104.1 | 12.1 | 106.2 | 7 | 0.6 | 127 | 2.6 |
| P-4 | paraffinic | Group III | 48.2 | 8.0 | 135.5 | 0 | 0.2 | 129 | 1.5 |
| N-1 | naphthenic | Group V | 358.5 | 19.0 | 39.2 | 1230 | 625 | 98 | 1.9 |
| N-2 | naphthenic | Group V | 607.1 | 21.2 | −20.4 | 788 | 750 | 92 | 4 |
| Designation | Comp. 1 | Concentration of Comp. 1 in wt% | Comp. 2 | Concentration of Comp. 2 in wt% | Concentration of Additive Package in wt% | Kinematic Viscosity at 100 °C in mm²/s | Viscosity Index | Aniline Point of Base Oil Blend in °C |
|---|---|---|---|---|---|---|---|---|
| MB-1 | P-4 | 89.5 | - | - | 10.5 | 9.8 | 133 | 129 |
| MB-2 | P-4 | 60.0 | P-1 | 29.5 | 10.5 | 13.2 | 122 | 125 |
| MB-3 | P-4 | 62.5 | P-1 | 30.5 | 7.0 | 12.2 | 120 | 125 |
| MB-4 | P-4 | 60.0 | P-3 | 29.5 | 10.5 | 11.1 | 128 | 128 |
| MB-5 | P-3 | 69.5 | P-2 | 20.0 | 10.5 | 13.0 | 110 | 125 |
| MB-6 | P-4 | 60.0 | N-1 | 29.5 | 10.5 | 12.2 | 113 | 120 |
| MB-7 | P-4 | 50.0 | N-1 | 39.5 | 10.5 | 13.2 | 104 | 117 |
| MB-8 | P-4 | 50.0 | N-1 | 40.8 | 9.2 | 12.9 | 103 | 117 |
| MB-9 | P-4 | 60.0 | N-2 | 29.5 | 10.5 | 12.1 | 106 | 119 |
| MB-10 | P-4 | 62.0 | N-2 | 31.0 | 7.0 | 11.2 | 104 | 119 |
| MB-11 | P-4 | 50.0 | N-2 | 39.5 | 10.5 | 13.1 | 96 | 115 |
| MB-12 | P-4 | 50.0 | N-2 | 40.8 | 9.2 | 12.9 | 95 | 115 |
| Designation | API Base Oil Classification | Performance Expectations in Field |
|---|---|---|
| REF-1 | Group I | good/medium |
| REF-2 | Group II | poor |
| REF-3 | Group II | good/medium |
| REF-4 | Group I/II/III | high |
| REF-5 | Group I | medium |
| REF-6 | Group II | good |
| REF-7 | Group III + Group V | very high |
| Designation | Deposit Amount in mg | Evaporation Loss in wt% | Change in Kinematic Viscosity at 100 °C in % | Oxidation in A/cm |
|---|---|---|---|---|
| REF-1 | 109 | 19 | 21 | 15 |
| REF-2 | 330 | 22 | 32 | 19 |
| REF-3 | 157 | 23 | 22 | 8 |
| REF-4 | 28 | 28 | 30 | 9 |
| REF-5 | 158 | 20 | 39 | 23 |
| REF-6 | 135 | 21 | 19 | 9 |
| REF-7 | 11 | 19 | 22 | 3 |
| MB-1 | 54 | 22 | 14 | 9 |
| MB-2 | 56 | 17 | 25 | 8 |
| MB-3 | 76 | 14 | 22 | 8 |
| MB-4 | 48 | 14 | 14 | 9 |
| MB-5 | 70 | 12 | 11 | 8 |
| MB-6 | 10 | 10 | 6 | 7 |
| MB-7 | 18 | 33 | 38 | 14 |
| MB-8 | 22 | 27 | 23 | 10 |
| MB-9 | 35 | 18 | 9 | 9 |
| MB-10 | 68 | 29 | 32 | 8 |
| MB-11 | 9 | 26 | 17 | 11 |
| MB-12 | 6 | 17 | 10 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ronai, B.; Schneidhofer, C.; Novotny-Farkas, F.; Norrby, T.; Li, J.; Pichler, J.; Frauscher, M. Assessing the High-Temperature Deposit Formation of Paraffinic and Naphthenic Oil Blends Using the Oil Chute Method. Lubricants 2022, 10, 327. https://doi.org/10.3390/lubricants10120327
Ronai B, Schneidhofer C, Novotny-Farkas F, Norrby T, Li J, Pichler J, Frauscher M. Assessing the High-Temperature Deposit Formation of Paraffinic and Naphthenic Oil Blends Using the Oil Chute Method. Lubricants. 2022; 10(12):327. https://doi.org/10.3390/lubricants10120327
Chicago/Turabian StyleRonai, Bettina, Christoph Schneidhofer, Franz Novotny-Farkas, Thomas Norrby, Jinxia Li, Jasmin Pichler, and Marcella Frauscher. 2022. "Assessing the High-Temperature Deposit Formation of Paraffinic and Naphthenic Oil Blends Using the Oil Chute Method" Lubricants 10, no. 12: 327. https://doi.org/10.3390/lubricants10120327
APA StyleRonai, B., Schneidhofer, C., Novotny-Farkas, F., Norrby, T., Li, J., Pichler, J., & Frauscher, M. (2022). Assessing the High-Temperature Deposit Formation of Paraffinic and Naphthenic Oil Blends Using the Oil Chute Method. Lubricants, 10(12), 327. https://doi.org/10.3390/lubricants10120327






