Microstructure and Performance of Fe50Mn30Cr10Ni10 High-Entropy Alloy Produced by High-Efficiency and Low-Cost Wire Arc Additive Manufacturing
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Welding Wire and Additive Manufactured High-Entropy Alloy Preparation
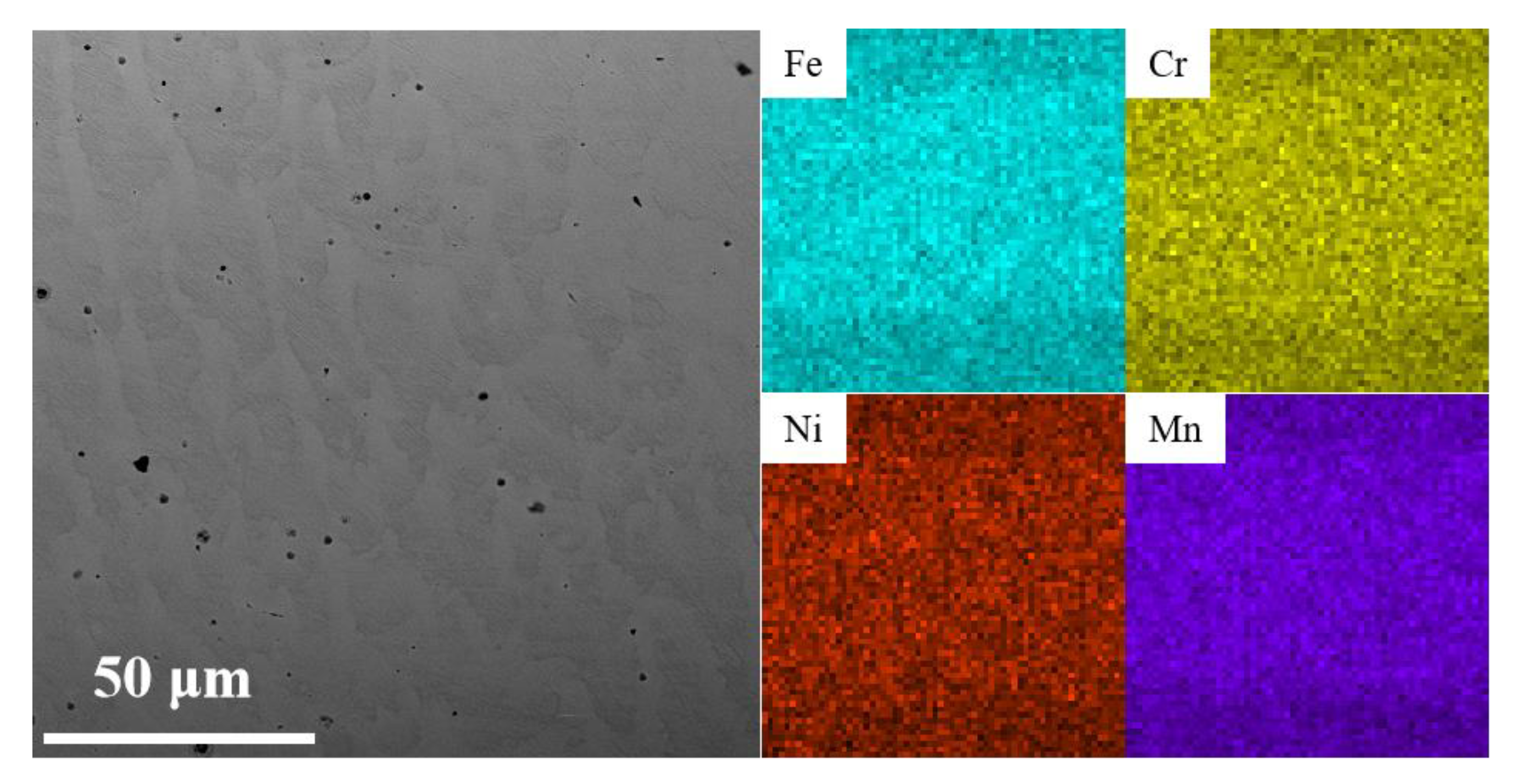
2.2. Microstructure and Composition Characterization
2.3. Performance Testing
3. Experimental Results and Discussion
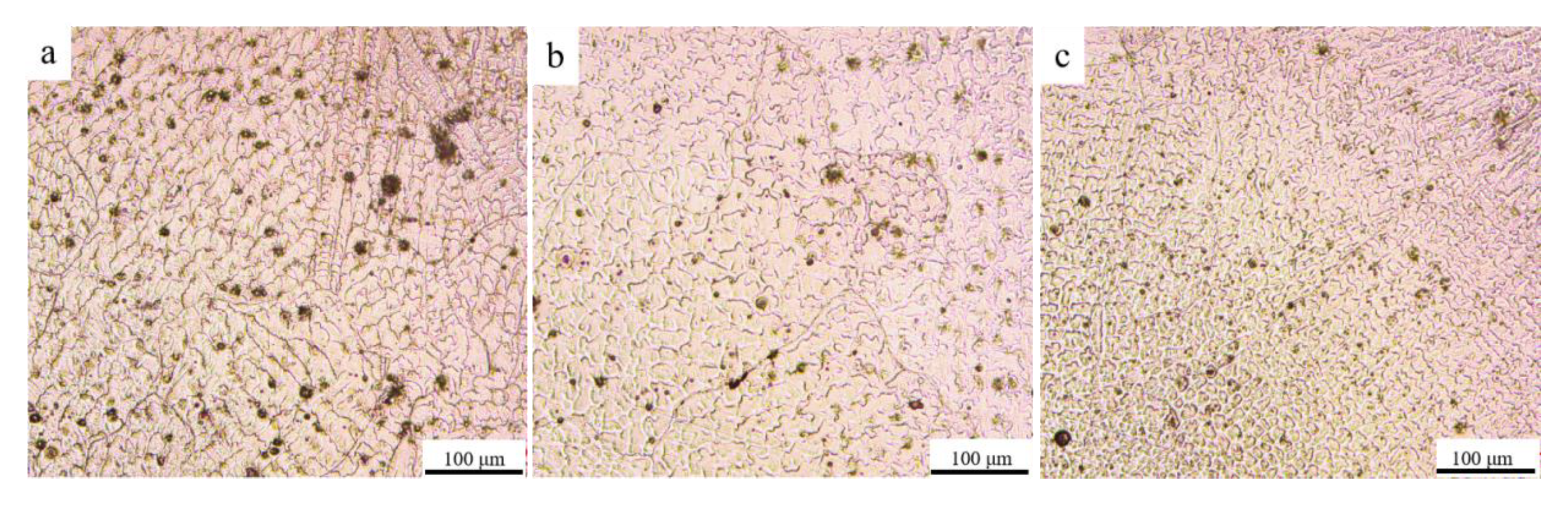
3.1. Phase Composition and Microstructure of the AM HEA
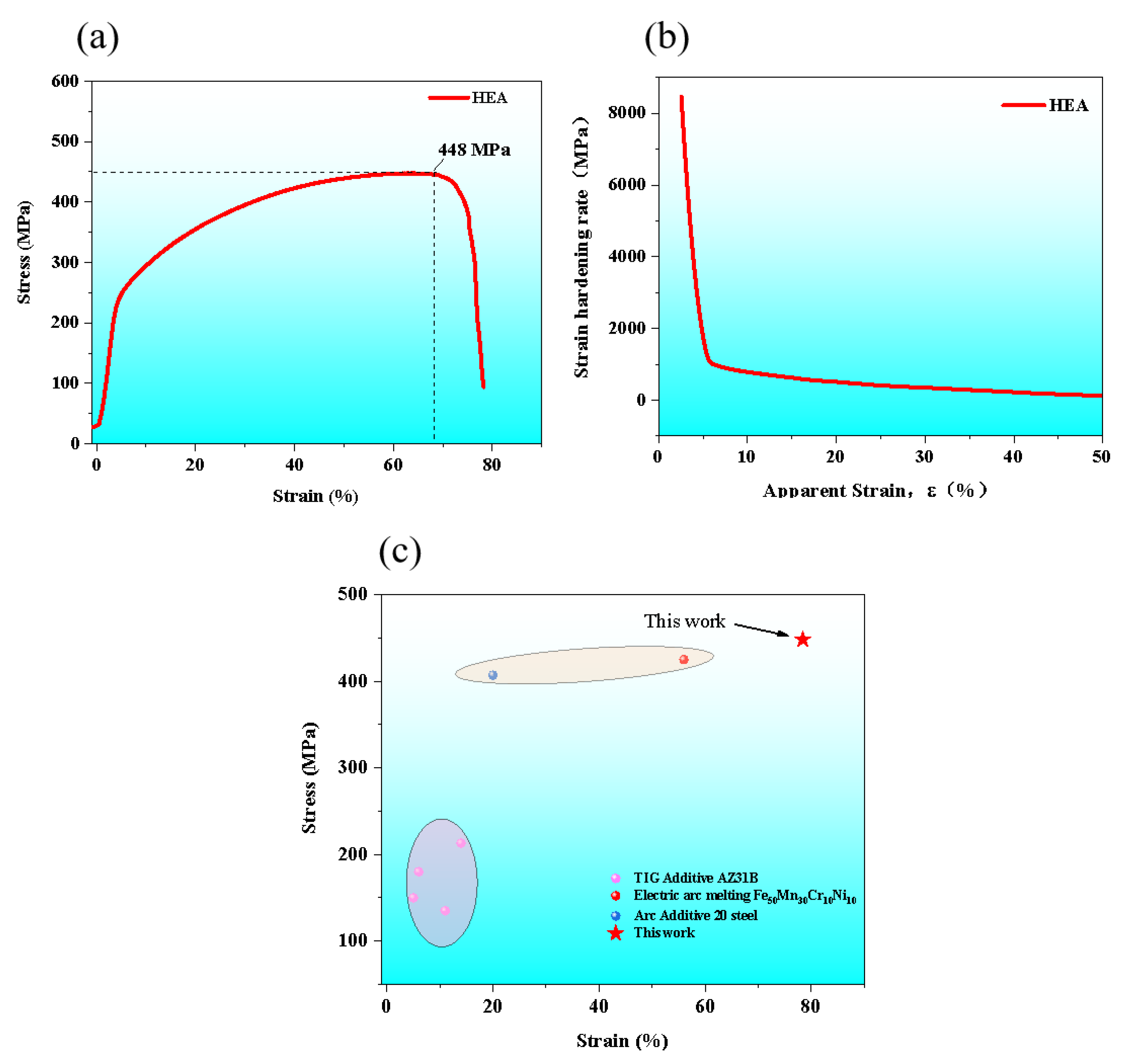
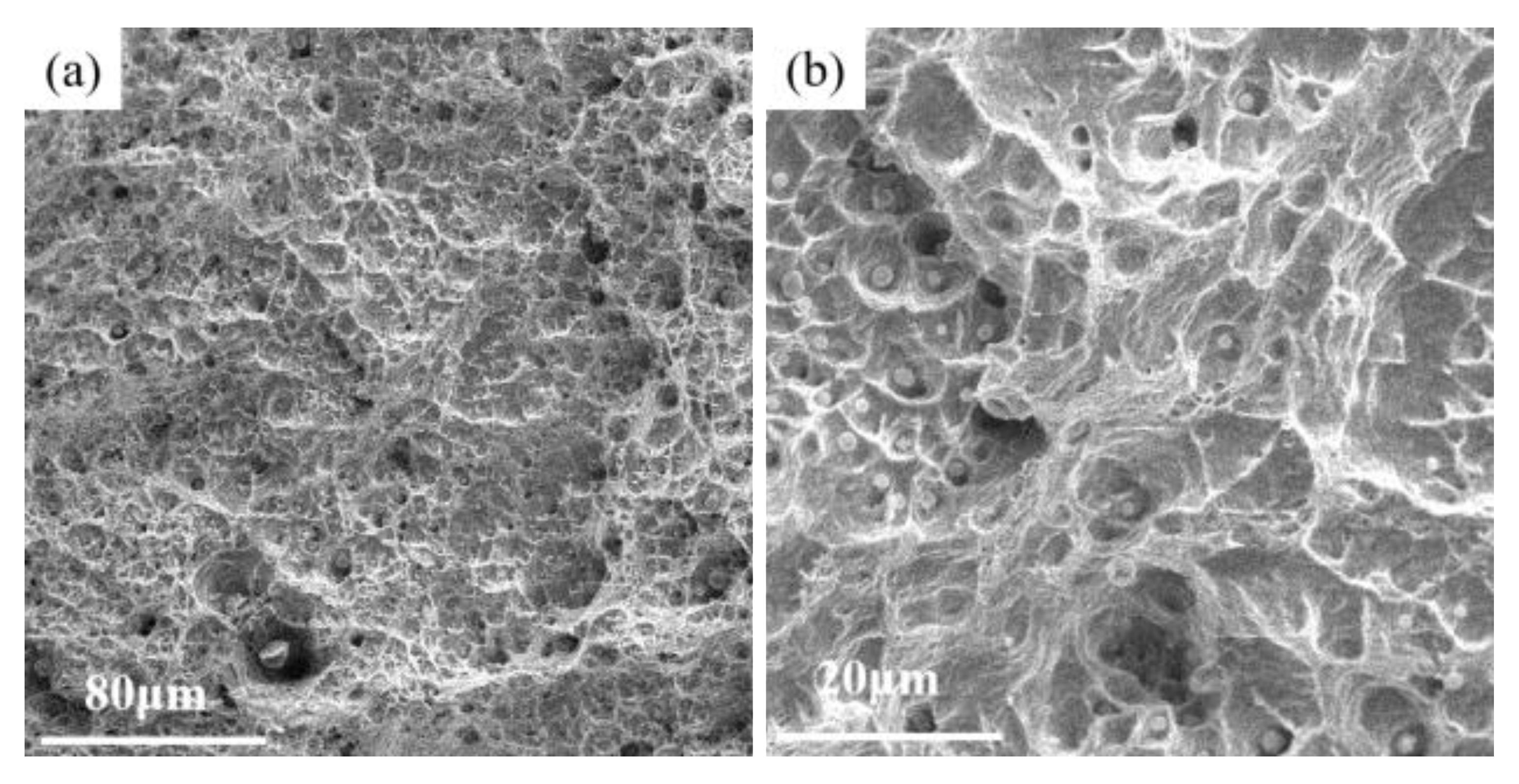
3.2. Mechanical Properties
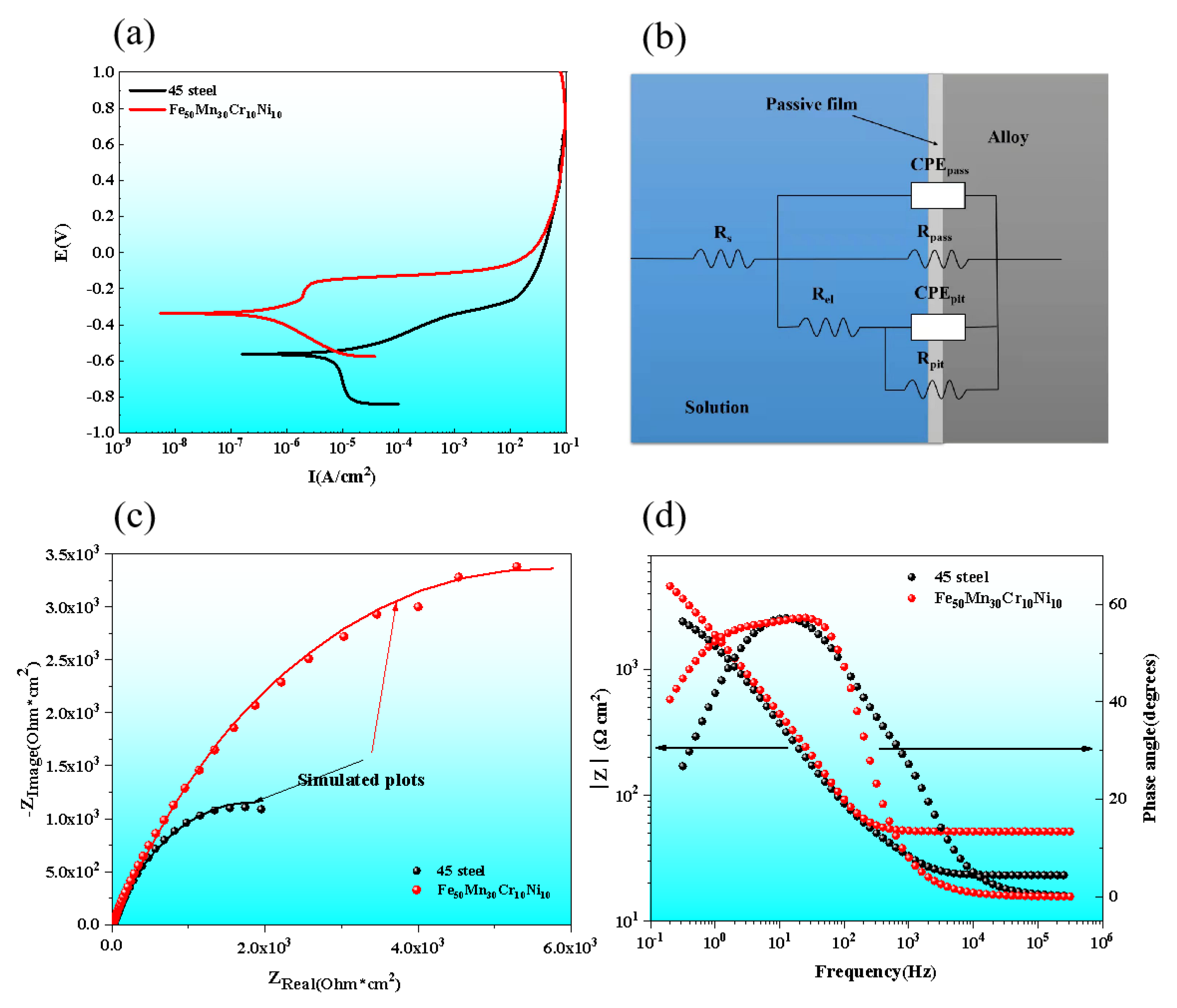
3.3. Electrochemical Corrosion Performance
Polarization and Electrochemical Impedance Spectroscopy Results
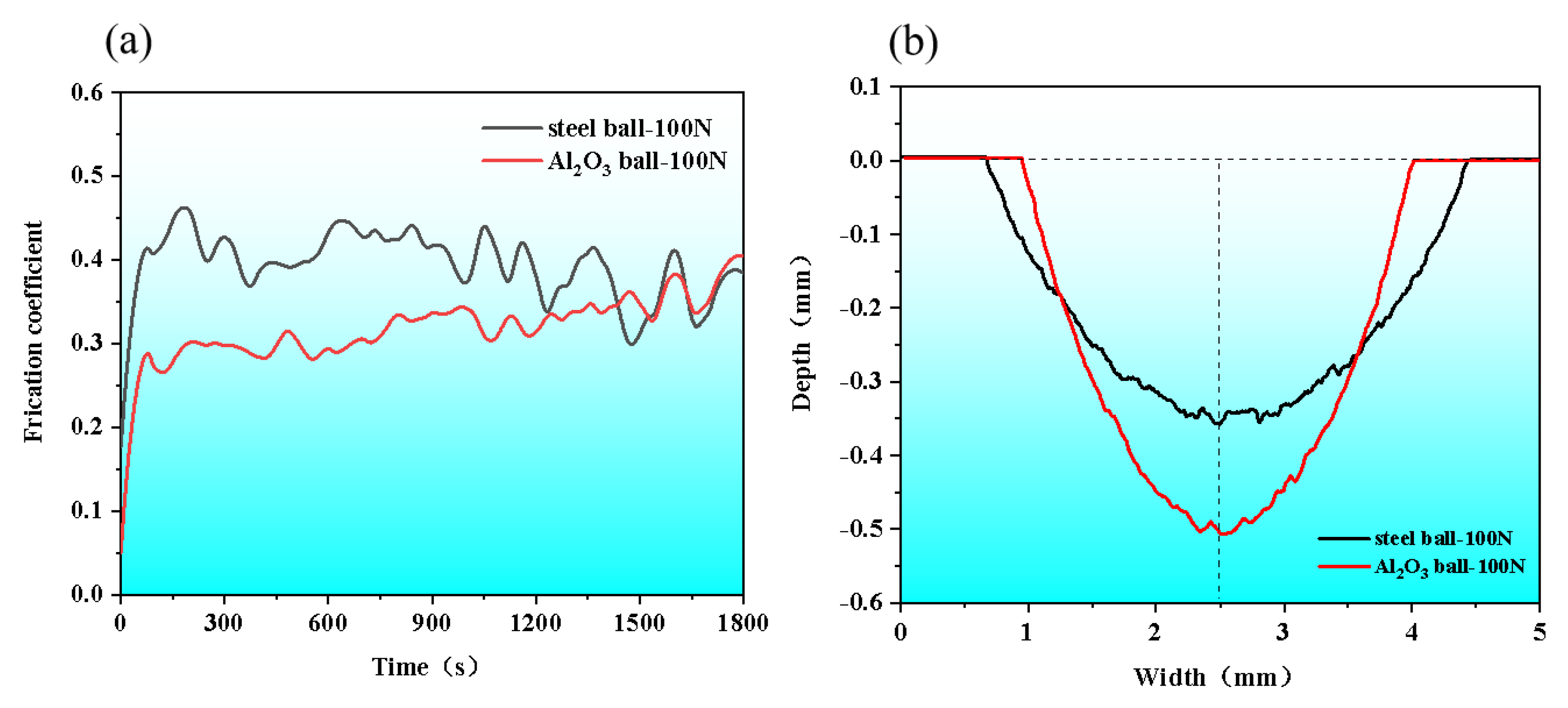
3.4. Wear Performance
4. Conclusions
- (1)
- The FCC phase and σ phase were detected in the AM HEA and the microstructure of the AM HEA is dendritic, consisting of dendrites and inter-dendrites. The grain size decreases from the bottom to the top;
- (2)
- The AM HEA was tested for tensile mechanical properties at room temperature, and its ultimate strength was about 448 MPa with a high plasticity up to 80%;
- (3)
- The corrosion current density of AM HEA was lower, and the corrosion potential was higher. The corrosion current density of AM HEA was 4.81 × 10−6 A/cm2 compared with 1.07 × 10−5 A/cm2 of 45 steel which was much lower. The corrosion potential of AM HEA was −0.3808 V, which was higher than the corrosion potential of 45 steel (−0.5629 V). Compared with 45 steel, the corrosion rate and corrosion tendency of AM HEA was smaller, showing better corrosion resistance;
- (4)
- Two coefficient of friction (COF) curves of the AM HEA were fluctuated at 0.35. The wear marks of the steel ball were lighter and wider with the depth of 0.354 ± 0.01 mm and the width of 3.762 ± 0.01 mm, while those of the Al2O3 ball were deeper and narrower with the depth of 0.506 ± 0.01 mm and the width of 3.074 ± 0.01 mm.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yeh, J.-W.; Chen, S.K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Gwalani, B.; Gorsse, S.; Choudhuri, D.; Zheng, Y.; Mishra, R.S.; Banerjee, R. Tensile yield strength of a single bulk Al0. 3CoCrFeNi high entropy alloy can be tuned from 160 MPa to 1800 MPa. Scr. Mater. 2019, 162, 18–23. [Google Scholar] [CrossRef]
- Tong, Y.; Zhang, H.; Huang, H.; Yang, L.; Hu, Y.; Liang, X.; Hua, M.; Zhang, J. Strengthening mechanism of CoCrNiMox high entropy alloys by high-throughput nanoindentation mapping technique. Intermetallics 2021, 135, 107209. [Google Scholar] [CrossRef]
- Zhang, H.; Tong, Y.; Cao, S.; Hu, Y.; Ji, X.; Tang, Q.; Yang, L.; Zhang, X.; Hua, M. Outstanding yield strength of CoCrNiTa0. 1 medium entropy alloy under the synergistic regulated with nanoprecipitation and grain refining. J. Alloys Compd. 2022, 919, 165715. [Google Scholar] [CrossRef]
- Debroy, T.; Wei, H.L.; Zuback, J.; Mukherjee, T.; Zhang, W. Additive manufacturing of metallic components—Process, structure and properties. Prog. Mater. Sci. 2018, 92, 112–224. [Google Scholar] [CrossRef]
- Ding, J.; Colegrove, P.; Mehnen, J.; Ganguly, S.; Almeida, P.S.; Wang, F.; Williams, S. Thermo-mechanical analysis of Wire and Arc Additive Layer Manufacturing process on large multi-layer parts. Comput. Mater. Sci. 2011, 50, 3315–3322. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Lu, Y. Laser 3D printing of CoCrFeMnNi high-entropy alloy. Mater. Lett. 2019, 236, 77–80. [Google Scholar] [CrossRef]
- Ocelík, V.; Janssen, N.; Smith, S.N.; De Hosson, J.T.M. Additive manufacturing of high-entropy alloys by laser processing. Jom 2016, 68, 1810–1818. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Z.; Yao, C.; Feng, K.; Li, Z.; Chu, P.K. Cryogenic deformation mechanism of CrMnFeCoNi high-entropy alloy fabricated by laser additive manufacturing process. Int. J. Lightweight Mater. Manuf. 2018, 1, 33–39. [Google Scholar] [CrossRef]
- Kok, Y.; Tan, X.; Wang, P.; Nai, M.; Loh, N.; Liu, E.; Tor, S. Anisotropy and heterogeneity of microstructure and mechanical properties in metal additive manufacturing: A critical review. Mater. Des. 2018, 139, 565–586. [Google Scholar] [CrossRef]
- Mirsayar, M. A generalized criterion for fatigue crack growth in additively manufactured materials–Build orientation and geometry effects. Int. J. Fatigue 2021, 145, 106099. [Google Scholar] [CrossRef]
- Popovich, V.A.; Borisov, E.V.; Popovich, A.A.; Sufiiarov, V.S.; Masaylo, D.V.; Alzina, L. Functionally graded Inconel 718 processed by additive manufacturing: Crystallographic texture, anisotropy of microstructure and mechanical properties. Mater. Des. 2017, 114, 441–449. [Google Scholar] [CrossRef]
- Bai, L.; Wang, Y.Z.; Yang, X.G.; Wang, R.B. Effect of Cr elements on the microstructure and properties of FeMnNiCr high-entropy alloy. Therm. Process. Process 2020, 49, 5. [Google Scholar] [CrossRef]
- Qiulin, W.; Yong, L.; Jinbo, Z.; Mingfu, L.; Zhengxing, M. Extreme High Speed Laser Cladding 316L Coating. J. Phys. Conf. Ser. 2021, 1965, 012083. [Google Scholar] [CrossRef]
- Kuhlmann-Wilsdorf, D. Strengthening Through LEDS. Strength Met. Alloys 1989, 1, 221–226. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, S.; Lu, J.; Wei, J.; Wei, D.; He, F. The dual effect of grain size on the strain hardening behaviors of Ni-Co-Cr-Fe high entropy alloys. J. Mater. Sci. Technol. 2022, 131, 177–184. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, H.; Zhang, J.; Xue, H.; Liu, G.; Sun, J. Achieving excellent strength-ductility synergy in twinned NiCoCr medium-entropy alloy via Al/Ta co-doping. J. Mater. Sci. Technol. 2021, 87, 184–195. [Google Scholar] [CrossRef]
- Chen, Y.-M.; Orazem, M.E. Impedance analysis of ASTM A416 tendon steel corrosion in alkaline simulated pore solutions—ScienceDirect. Corros. Sci. 2016, 104, 26–35. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Kissi, M.; Bouklah, M.; Hammouti, B.; Benkaddour, M. Establishment of equivalent circuits from electrochemical impedance spectroscopy study of corrosion inhibition of steel by pyrazine in sulphuric acidic solution. Appl. Surf. Sci. 2006, 252, 4190–4197. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, G.; Liu, W.; Lu, M. The formation mechanism of corrosion scale and electrochemical characteristic of low alloy steel in carbon dioxide-saturated solution. Corros. Sci. 2012, 57, 131–138. [Google Scholar] [CrossRef]
- Della Rovere, C.; Alano, J.; Silva, R.; Nascente, P.; Otubo, J.; Kuri, S. Characterization of passive films on shape memory stainless steels. Corros. Sci. 2012, 57, 154–161. [Google Scholar] [CrossRef]
- Luo, H.; Li, Z.; Mingers, A.M.; Raabe, D. Corrosion behavior of an equiatomic CoCrFeMnNi high-entropy alloy compared with 304 stainless steel in sulfuric acid solution. Corros. Sci. 2018, 134, 131–139. [Google Scholar] [CrossRef]
- Wei, L.; Liu, Y.; Li, Q.; Cheng, Y.F. Effect of roughness on general corrosion and pitting of (FeCoCrNi)0.89(WC)0.11 high-entropy alloy composite in 3.5 wt.% NaCl solution. Corros. Sci. 2018, 146, 44–57. [Google Scholar] [CrossRef]
- Huang, Y.; Hu, Y.; Zhang, M.; Mao, C.; Tong, Y.; Zhang, J.; Li, K.; Wang, K. On the enhanced wear resistance of laser-clad CoCrCuFeNiTix high-entropy alloy coatings at elevated temperature. Tribol. Int. 2022, 174, 107767. [Google Scholar] [CrossRef]





| AM Current | 140 A |
|---|---|
| Wire feeding rate | 0.4 r/min |
| Welding torch speed | 100 mm/min |
| Swing distance | X5.5 mm, Y5 mm |
| Overlap distance | −4 mm |
| Arcing time | 1 s |
| Interrupting time | 1 s |
| Alloy | Element Content (at.%) | |||
|---|---|---|---|---|
| Fe | Mn | Cr | Ni | |
| Nominal | 50 | 30 | 10 | 10 |
| Overall | 53.02 | 25.62 | 11.31 | 10.05 |
| 1 | 55.31 | 26.15 | 10.26 | 8.28 |
| 2 | 51.52 | 25.33 | 10.35 | 12.80 |
| 3 | 52.26 | 24.38 | 11.32 | 12.04 |
| Samples | Electrochemical Parameters | |
|---|---|---|
| Ecorr (VSCE) | Icorr (A/cm2) | |
| 45 steel | −0.5629 | 1.07 × 10−5 |
| HEA | −0.3808 | 4.81 × 10−6 |
| Alloy | Rs | CPEpass Parameter | Rpass | |
|---|---|---|---|---|
| (Ω cm2) | Y0 (μF/cm2) | n | (Ω cm2) | |
| 45 steel | 23.11 | 7.69 × 10−4 | 1 | 3.49 × 103 |
| HEA | 24.07 | 6.78 × 10−5 | 1 | 5.36 × 103 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Tong, Y.; Hu, Y.; Liang, X.; Chen, Y.; Wang, K.; Zhang, M.; Xu, J. Microstructure and Performance of Fe50Mn30Cr10Ni10 High-Entropy Alloy Produced by High-Efficiency and Low-Cost Wire Arc Additive Manufacturing. Lubricants 2022, 10, 344. https://doi.org/10.3390/lubricants10120344
Zhang X, Tong Y, Hu Y, Liang X, Chen Y, Wang K, Zhang M, Xu J. Microstructure and Performance of Fe50Mn30Cr10Ni10 High-Entropy Alloy Produced by High-Efficiency and Low-Cost Wire Arc Additive Manufacturing. Lubricants. 2022; 10(12):344. https://doi.org/10.3390/lubricants10120344
Chicago/Turabian StyleZhang, Xibin, Yonggang Tong, Yongle Hu, Xiubing Liang, Yongxiong Chen, Kaiming Wang, Mingjun Zhang, and Jiaguo Xu. 2022. "Microstructure and Performance of Fe50Mn30Cr10Ni10 High-Entropy Alloy Produced by High-Efficiency and Low-Cost Wire Arc Additive Manufacturing" Lubricants 10, no. 12: 344. https://doi.org/10.3390/lubricants10120344





