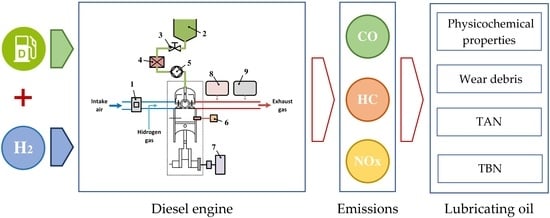
3.1. Characteristics of Engine Exhaust Gases
The temperatures of the exhaust gases for each of the mixtures of diesel with hydrogen are indicated in
Figure 2.
Figure 2 shows that the temperature of the exhaust gases increases with the increase in engine torque, which is a consequence of the greater thermal efficiency of the combustion process at higher loads. In general, it is evident that the presence of hydrogen in the combustion chamber leads to an increase in the temperature of the combustion gases. This behavior is associated with the high calorific value of hydrogen gas, as well as its high vaporization heat. Another factor that can contribute to the increase in temperature is the high autoignition temperature of hydrogen. For the conditions tested, increases of 2.29%, 3.02% and 4.77% in the temperature of the exhaust gases were obtained for the mixtures D100 + 0.75 lpm, D100 + 1.00 lpm, and D100 + 1.25 lpm compared with pure diesel, respectively.
Variations in carbon monoxide (CO) emissions from the engine are shown in
Figure 3.
The formation of CO molecules is associated with insufficient oxygen content during the combustion process, which causes an increase in unburned gases [
34]. With the addition of hydrogen in the diesel engine, a reduction in CO emissions was evident. This may be due to the high flame velocity and the diffusivity of the hydrogen gas [
35,
36]. This allows a more homogeneous mix between diesel fuel and intake air. The reductions in CO emissions were 1.97%, 3.40% and 5.12% for volumetric flows of 0.75 lpm, 1.00 lpm and 1.25 lpm, respectively.
Figure 4 shows the change in hydrocarbon (HC) emissions concerning different engine torque conditions.
The formation of hydrocarbons was a consequence of incomplete combustion and the short distance of extinction of the flame. In general, HC emissions decreased as engine torque increased. As hydrogen gas was added, less HC formation was obtained, which was attributed to the better oxidation during the combustion process as a result of the higher content of hydrogen atoms. The increase in the oxidation of emissions was the result of the higher temperatures in the cylinder chamber, as evidenced in
Figure 2. Another factor that favored reducing HC emissions was the absence of carbon atoms from hydrogen. For the mixtures D100 + 0.75 lpm, D100 + 1.00 lpm and D100 + 1.25 lpm, there were decreases of 3.58%, 6.62% and 10.18%, respectively, compared with pure diesel.
Figure 5 presents the effect of hydrogen gas injection on nitrogen oxide (NOx) emissions.
The main factors that affect the formation of NOx are the duration of the combustion process and the temperature inside the combustion chamber [
37]. In general, the injection of hydrogen gas in the diesel engine favored an increase in NOx emissions. For the fuel conditions evaluated—D100 + 0.75 lpm, D100 + 1.00 lpm and D100 + 1.25 lpm—increases of 2.64%, 4.28% and 6.27% were obtained compared with pure diesel, respectively.
Figure 6 shows the smoke opacity levels for different engine torque conditions.
To analyze the smoke emissions, the opacity of the exhaust gases was measured, which is an indication of the particulate matter content. This is directly associated with the incomplete combustion of fuel. The inclusion of hydrogen in the engine implies a reduction in smoke opacity, which can be related to the oxidation of soot particles due to the higher temperature conditions inside the combustion chamber. For the conditions evaluated, decreases of 2.75%, 4.21% and 6.31% in smoke opacity were obtained with volumetric flow rates of 0.75 lpm, 1.00 lpm and 1.25 lpm of hydrogen gas in the diesel engine, respectively.
3.2. Characteristics of Lubricating Oil
Figure 7 and
Figure 8 show the variations in kinematic viscosity for temperatures of 40 °C and 100 °C.
From the results shown in
Figure 7 and
Figure 8, it is evident that the addition of hydrogen gas in the diesel engine leads to a greater reduction in kinematic viscosity compared with pure diesel. This implies an increase in the contamination of insoluble agents and a loss of anti-wear additives in the lubricating oil. The increase in contaminating agents was evidenced by the increased concentrations of metals, such as Fe, Cu, Al and Cr in the lubricating oil, which are described in
Figure 9. In general, increasing the engine operating time produced a constant decrease in kinematic viscosity.
For the test conditions, a reduction of 25.18% in the kinematic viscosity (for a temperature at 40 °C) of the lubricating oil was observed when the engine was operating with pure diesel. However, the mixtures D100 + 0.75 lpm, D100 + 1.00 lpm and D100 + 1.25 lpm exhibited decreases of 28.29%, 30.05% and 31.83%, respectively. A similar behavior was observed in the kinematic viscosity analysis at 100 °C, in which reductions of 22.90%, 24.82%, 26.58% and 28.89% were obtained with D100, D100 + 0.75 lpm, D100 + 1.00 lpm and D100 + 1.25 lpm fuels, respectively. These behaviors can be attributed to the high temperatures in the combustion chamber caused by the presence of hydrogen gas, causing greater thermal shearing of the oil.
In order to analyze the effect of hydrogen gas on the quality of the lubricating oil, an analysis of wear debris was carried out. For analysis of the metal concentration, an operating period of 180 h was established, which is generally used for this type of study [
38]. The results obtained are shown in
Figure 9.
Figure 9 shows the trends in wear metals Fe, Cu, Al and Cr in the lubricating oil for the different fuel mixtures. In general, it was evident that the injection of hydrogen gas into the diesel engine produced a greater presence of Fe. This behavior can be attributed to wear in the engine’s internal components, such as the cylinder, piston, and bearings, because they are designed to form iron alloys. The increased wear on engine components by hydrogen gas may be a consequence of the greater reduction in oil viscosity, as indicated in
Figure 7 and
Figure 8. This causes a greater friction force between the contact surfaces due to the reduction in the thickness of the lubrication film. For the conditions evaluated, increases in the wear metal Fe of 9.17%, 16.47% and 27.46% were obtained in the mixtures D100 + 0.75 lpm, D100 + 1.00 lpm and D100 + 1.25 lpm, respectively, as compared with pure diesel.
The presence of the wear metal Cu in the lubricating oil also increased with the addition of hydrogen gas in the engine. The results indicate increases of 17.06%, 28.41% and 42.34% in this type of metals for the mixtures D100 + 0.75 lpm, D100 + 1.00 lpm and D100 + 1.25 lpm compared with pure diesel, respectively. In the case of the wear metals Al and Cr, increases of 12.74%, 20.59% and 32.52% were observed for Al, and increases of 10.11%, 24.08% and 48.04% were observed for Cr, with the addition of volumetric flows of 0.75 lpm, 1.00 lpm and 1.25 lpm of hydrogen gas in the diesel engine, respectively. The incremental addition of these metals in the lubricating oil is associated with increased wear on the piston and piston rings.
The increases in wear metals such as Fe, Cu, Al and Cr implied that hydrogen gas accelerates the loss of lubricating oil performance and greater wear on internal engine components.
Variations in the flash point of lubricating oil during engine operation are shown in
Figure 10.
From the results described in
Figure 10, reductions of 10.81%, 12.24%, 14.08% and 15.91% are evidenced in the flash point of the lubricating oil for fuels D100, D100 + 0.75 lpm, D100 + 1.00 lpm and D100 + 1.25 bpm, respectively. In general, the presence of hydrogen gas in the combustion chamber led to a greater reduction in the flash point, which implies greater decomposition of the lubricating oil.
Figure 11 shows the change in the total base number (TBN) of the lubricating oil during engine operation.
The TBN of lubricating oil is an indication of the number of alkaline derivatives. The results in
Figure 11 describe a greater reduction in TBN for blends of diesel with hydrogen gas. In general, the addition of volumetric flows of 0.75 lpm, 1.00 lpm and 1.25 lpm of hydrogen gas caused decreases in the TBN of 6.49%, 9.36% and 11.84%, respectively, as compared with pure diesel. The greater decrease in TBN due to hydrogen led to a lower resistance to corrosion in the lubricating oil.
Figure 12 shows the total acid number (TAN) of the lubricating oil for the different conditions of fuel mixtures.
Figure 12 shows increases in the TAN of the lubricating oil with the addition of hydrogen in the diesel engine. The results indicate increases of 6.94%, 8.23% and 10.29% with the addition of volumetric flows of hydrogen gas of 0.75 lpm, 1.00 lpm and 1.25 lpm compared with pure diesel, respectively. This demonstrated that the presence of hydrogen favors the contamination and oxidation of lubricating oil. This may be a consequence of the greater increase in acidic components due to the increases in nitrogen oxide contents and the combustion temperature.
The additives in lubricating oil improve the lubrication system’s performance and minimize wear on the engine. In the particular case of zinc additives, its purpose is to protect engine components from wear when there is an excessive reduction in the thickness of the lubrication film. The properties of the lubricating oil with the zinc additive are shown in
Table 6.
Figure 13 shows the depletion of the zinc additive for the different fuel mixtures. In general, the results show that after 180 h of operation, the zinc content was reduced by 31.83% when the engine was fueled with pure diesel. However, a higher level of zinc depletion was evidenced with the addition of hydrogen. For volumetric flows of hydrogen gas of 0.75 lpm, 1.00 lpm and 1.25 lpm, decreases of 33.38%, 39.46% and 41.10% were observed, respectively.