Influence of Different Binders and Severe Environmental Conditions on the Tribological and Electrochemical Behaviour of WC-Based Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Processing
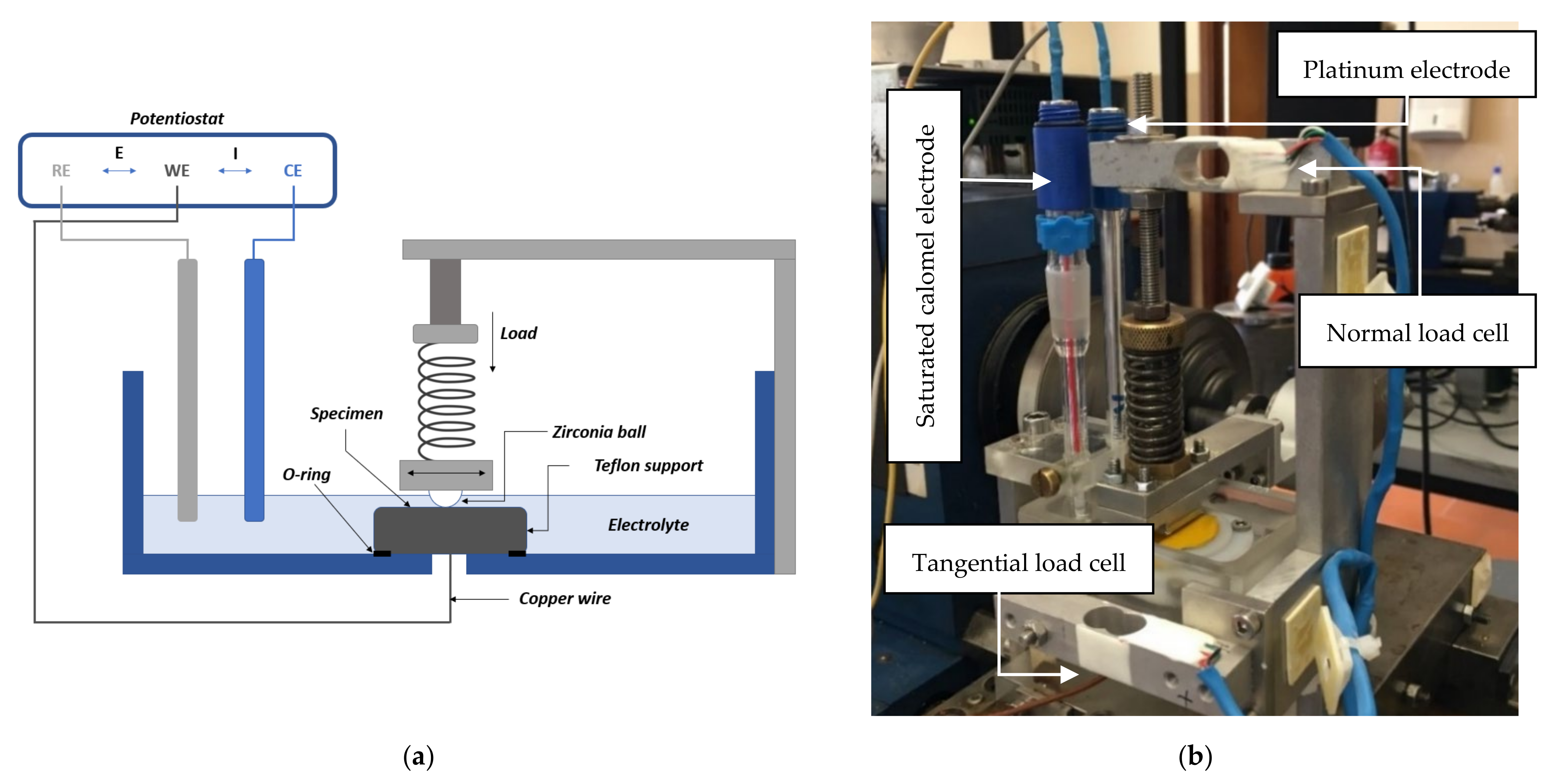
2.2. Triboelectrochemical Test
2.2.1. Electrochemical Test
2.2.2. Tribological Test
3. Results and Discussion
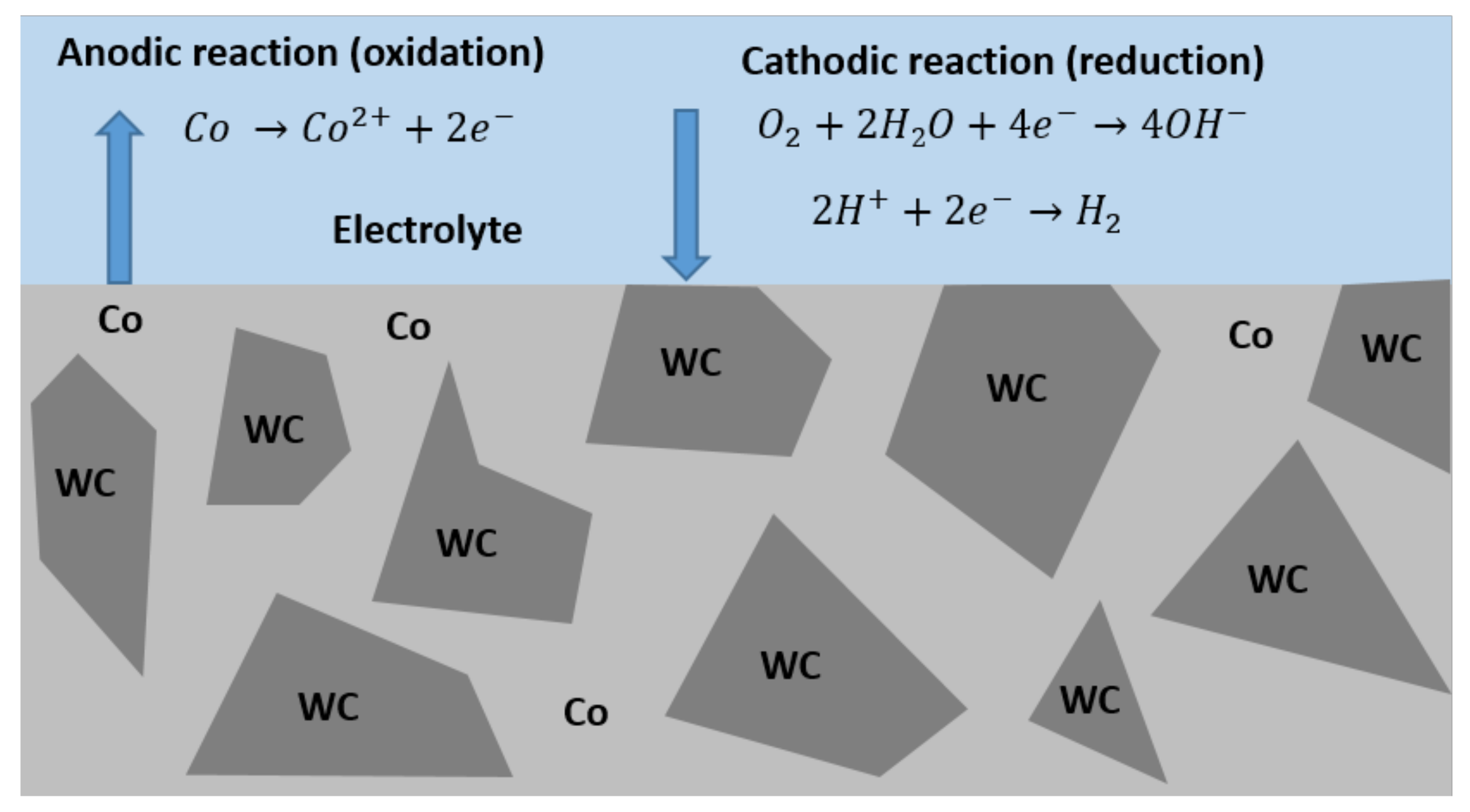
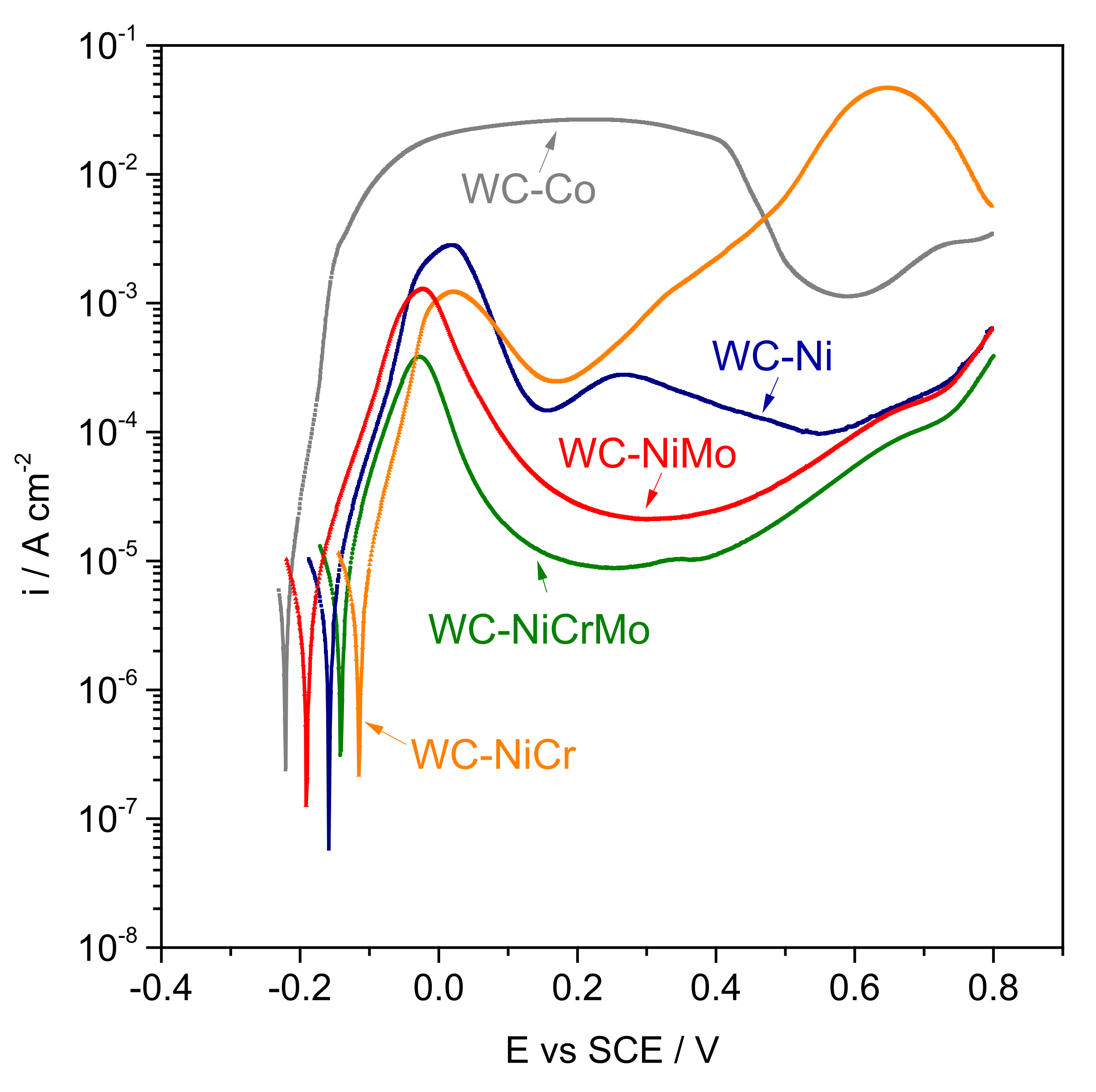
3.1. Electrochemical Behaviour
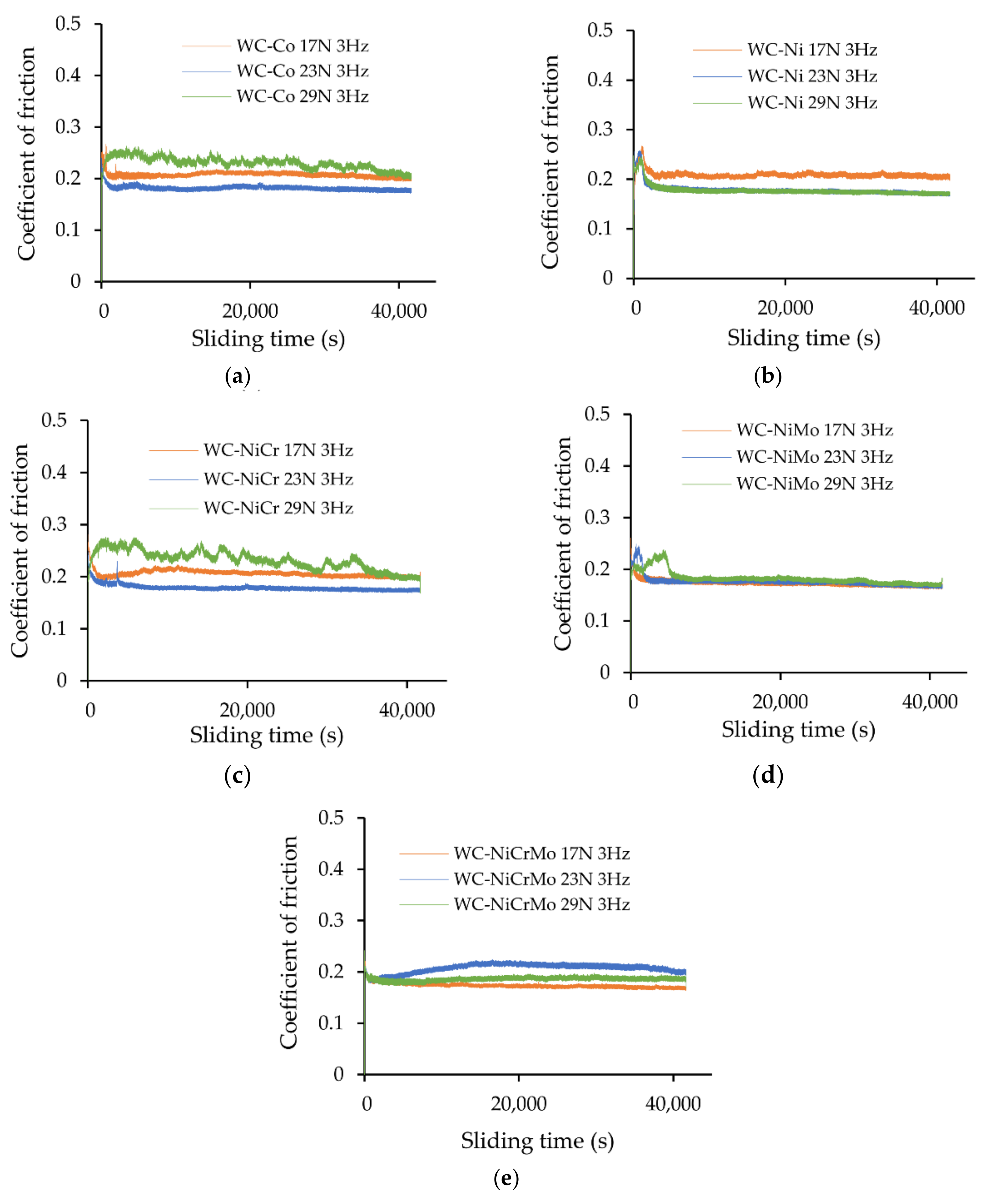
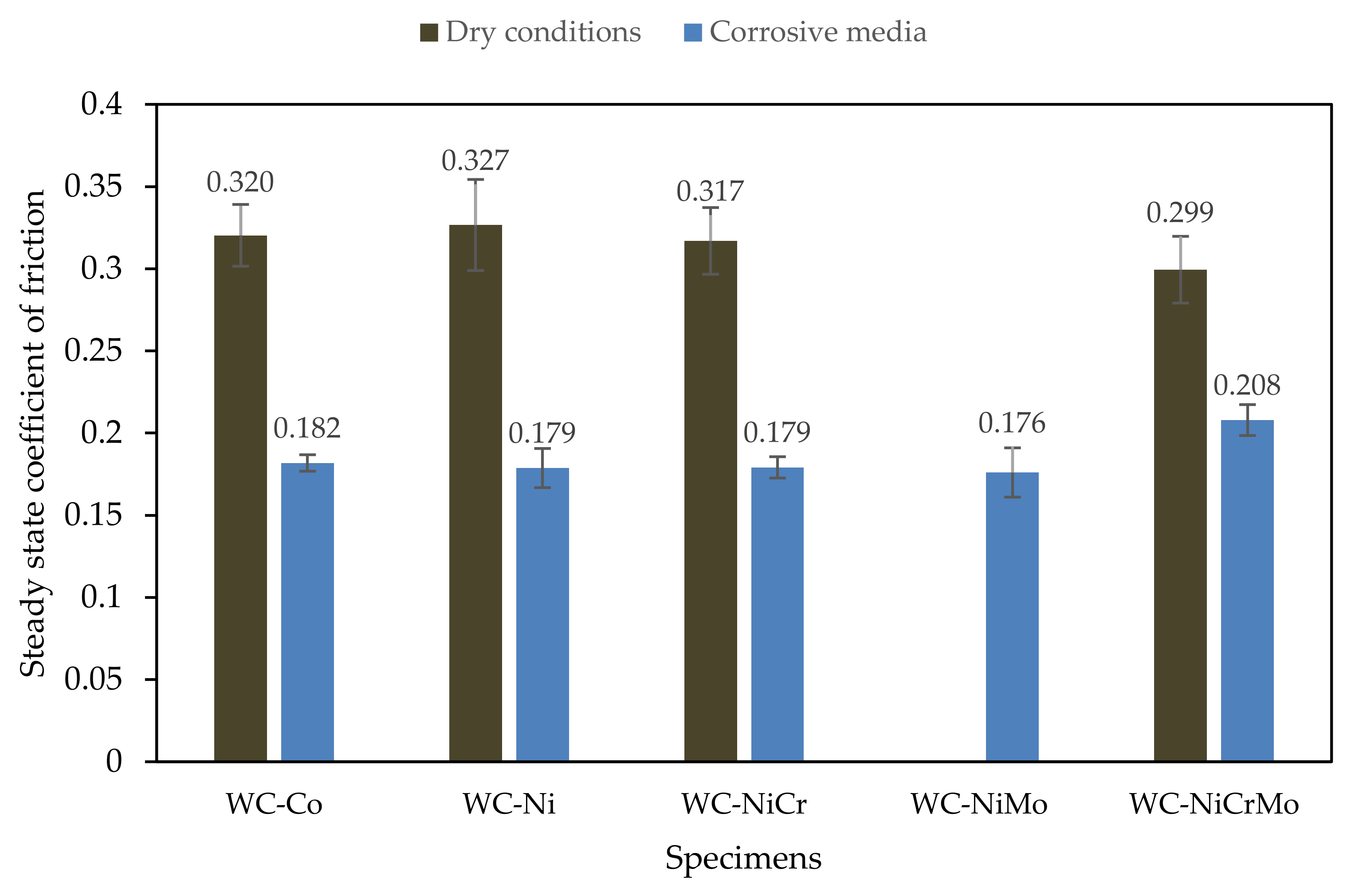
3.2. Friction Behaviour
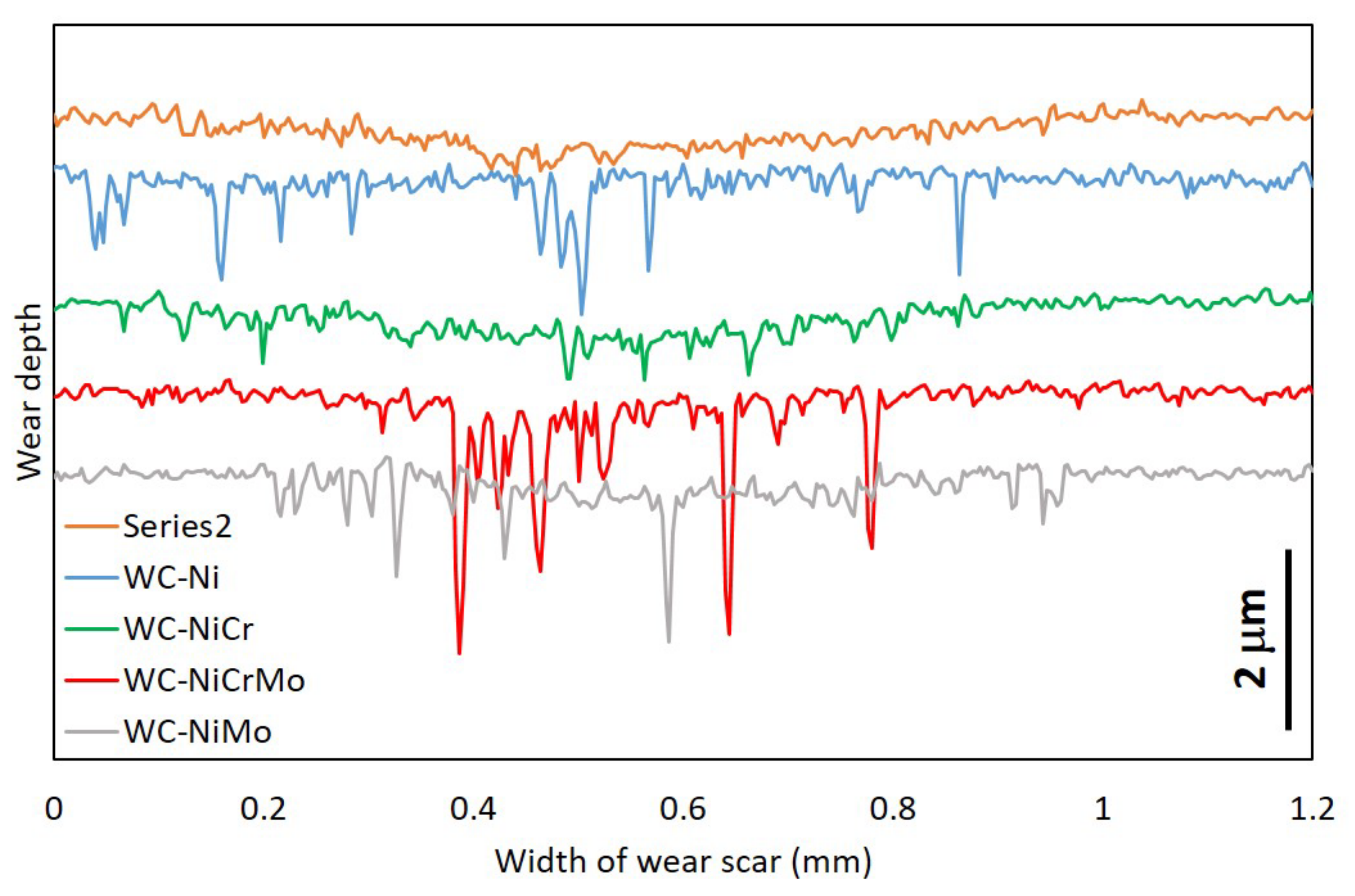
3.3. Wear Behaviour
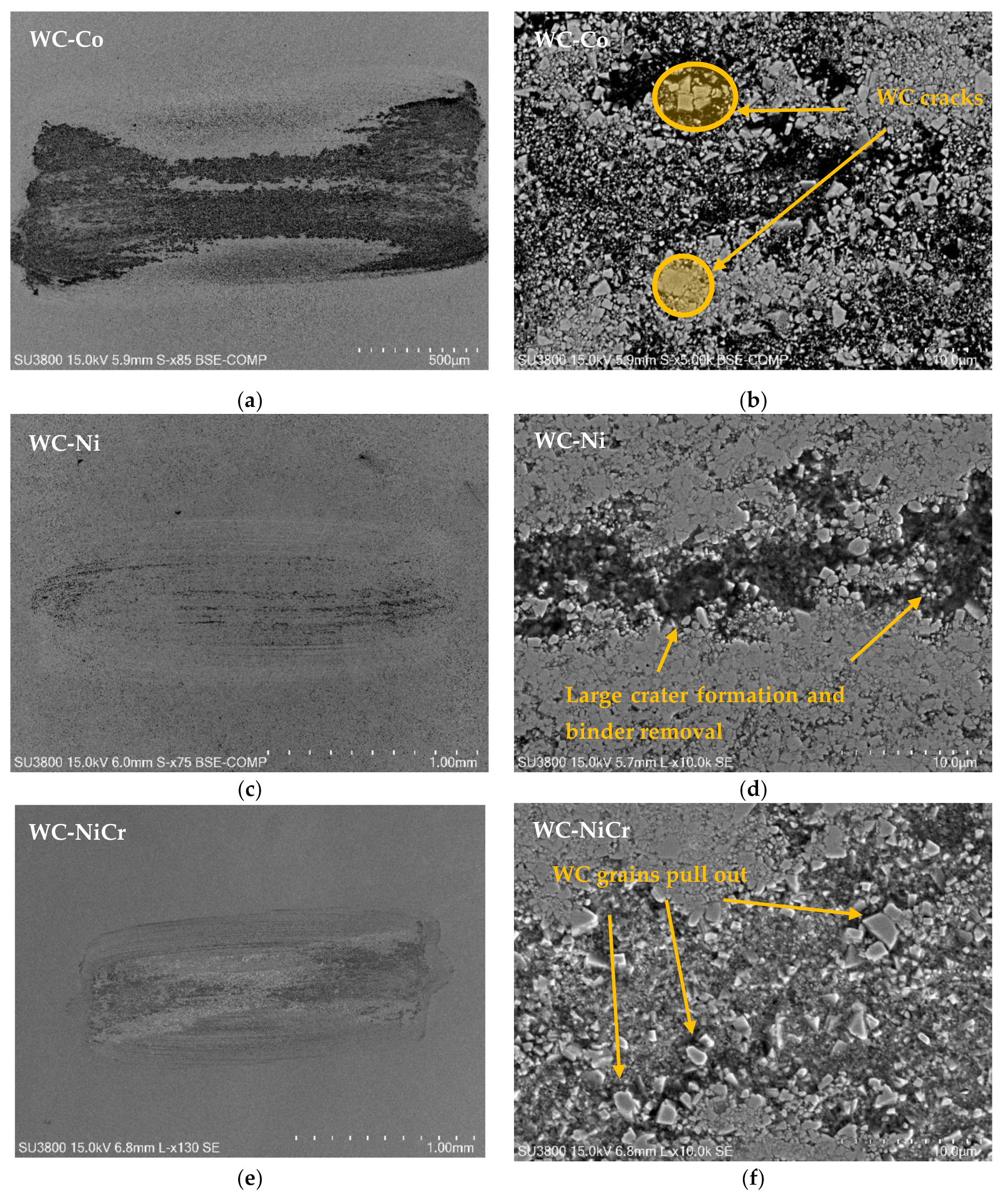
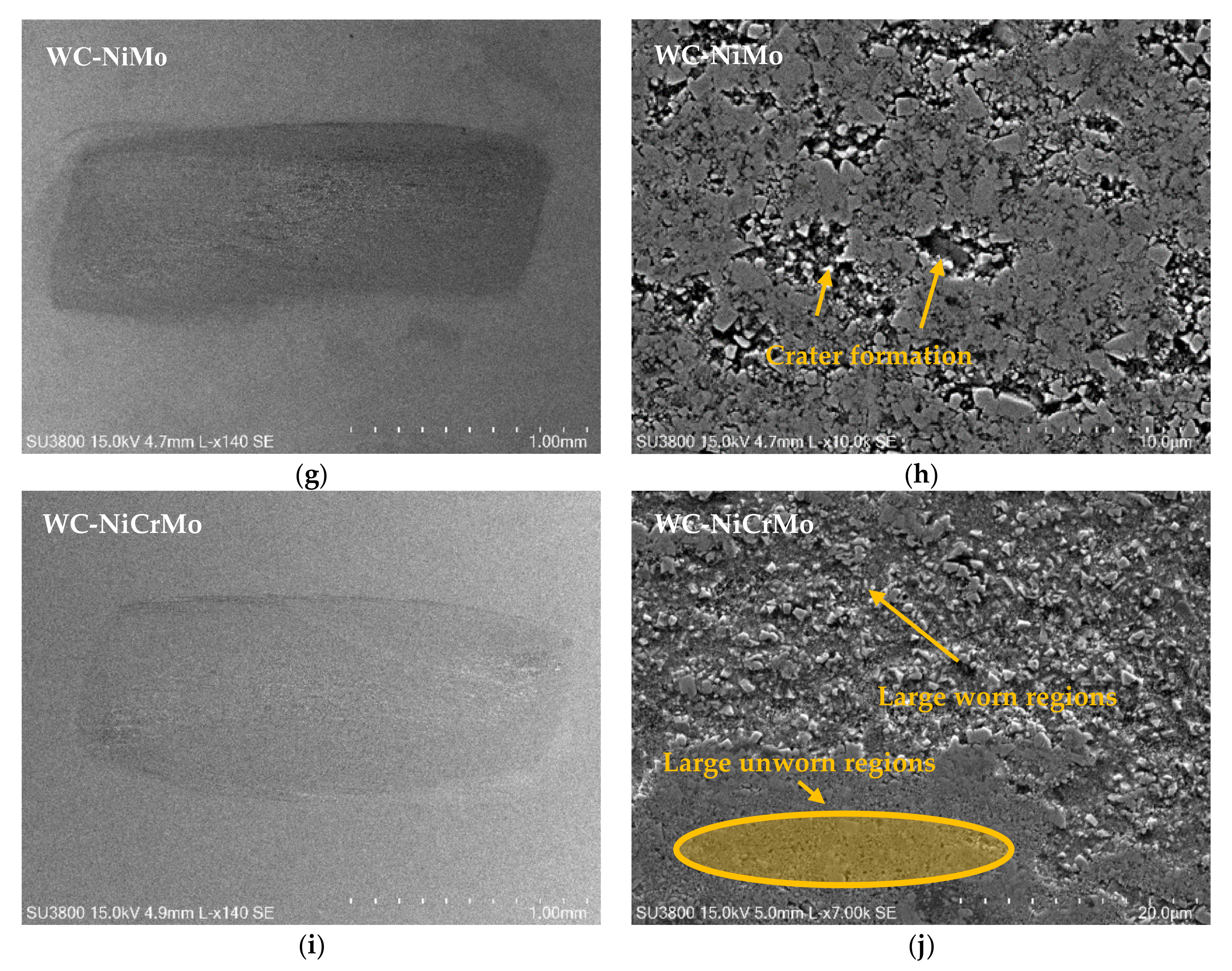
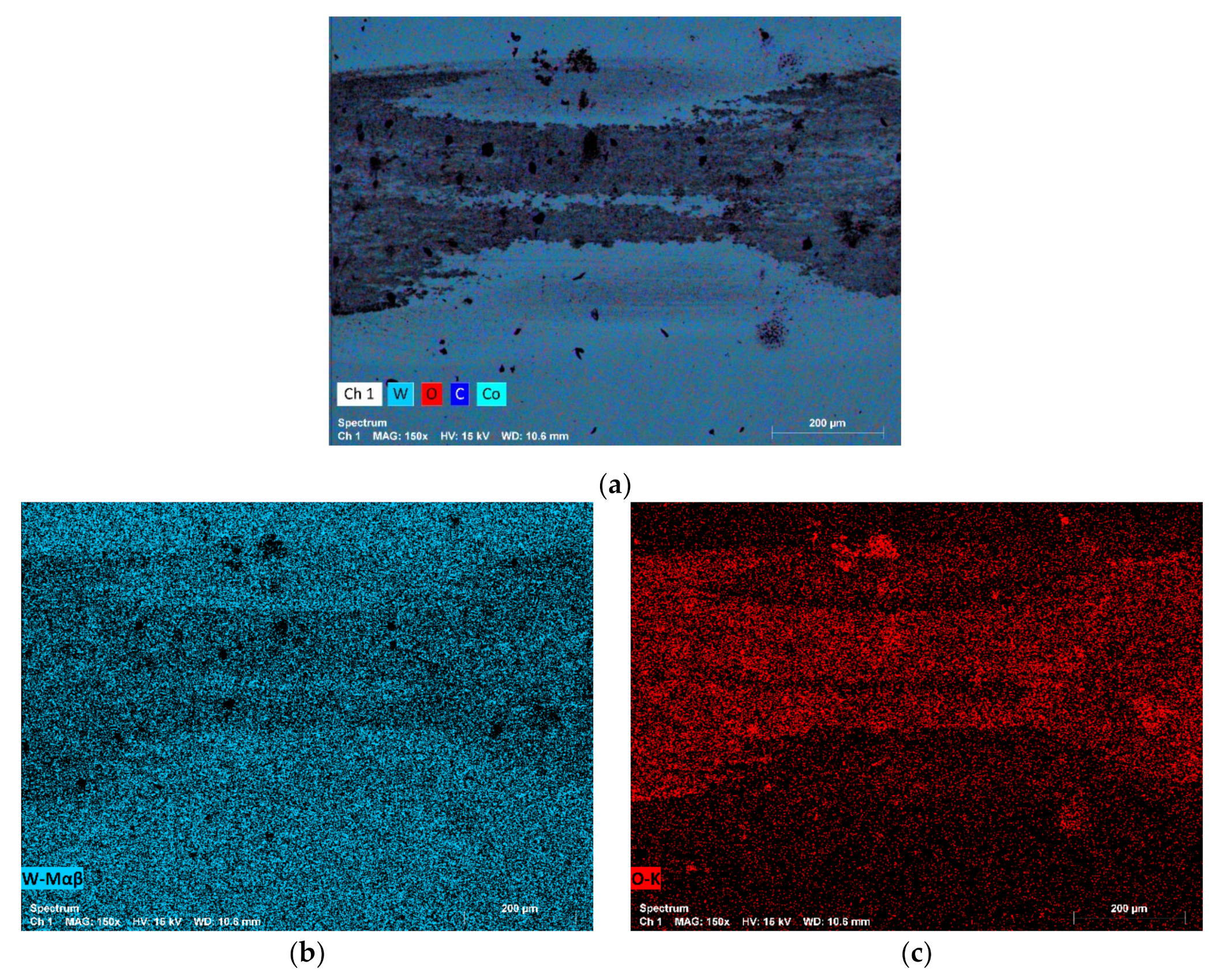
3.4. Wear Mechanisms
4. Conclusions
- Regarding corrosion resistance in 0.5 M NaCl + 0.05 M HCl acidic medium, Ni-based hard metals (WC-Ni, WC-NiCr, WC-NiMo, and WC-NiCrMo) presented similar corrosion behaviour, but the corrosion resistance of WC-NiCr and WC-NiCrMo is slightly higher. WC-Co presented the lowest corrosion resistance.
- For all the specimens investigated in the presence of the corrosive medium (lubricated conditions), the steady state coefficient of friction varied between 0.18 and 0.21. Comparison with previous results under dry conditions showed higher coefficients of friction by a factor of approximately two times (0.30 to 0.33).
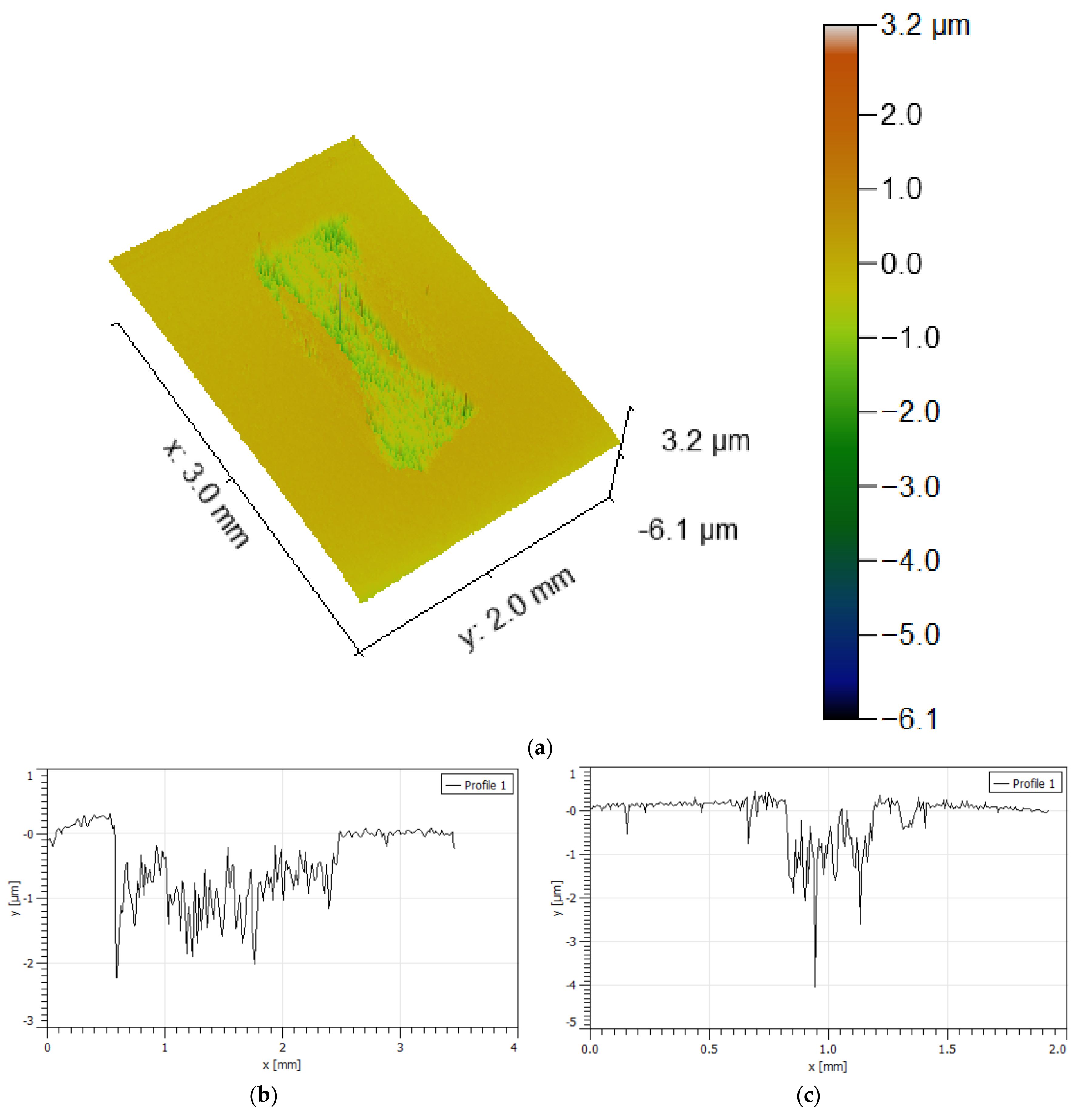
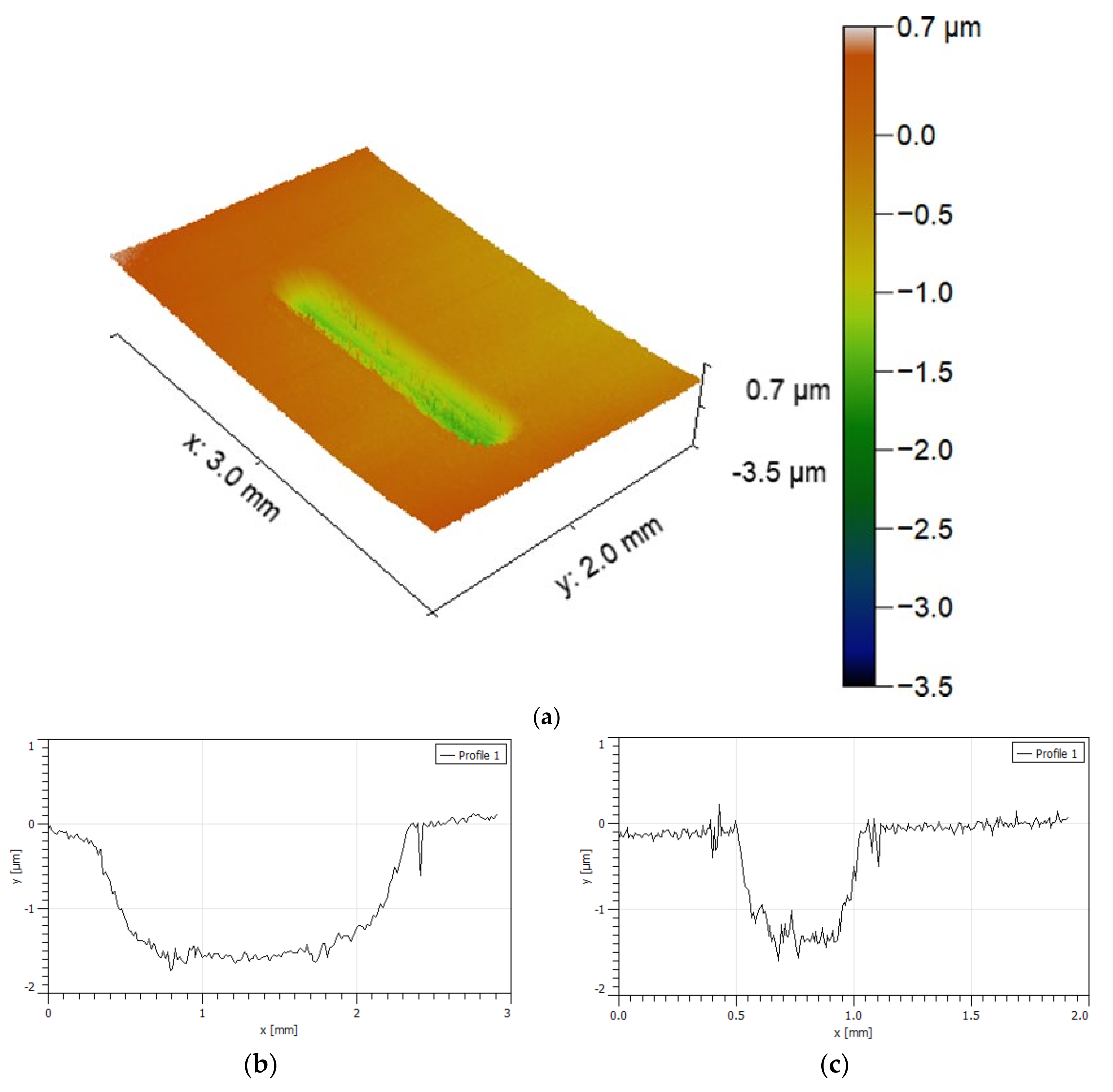
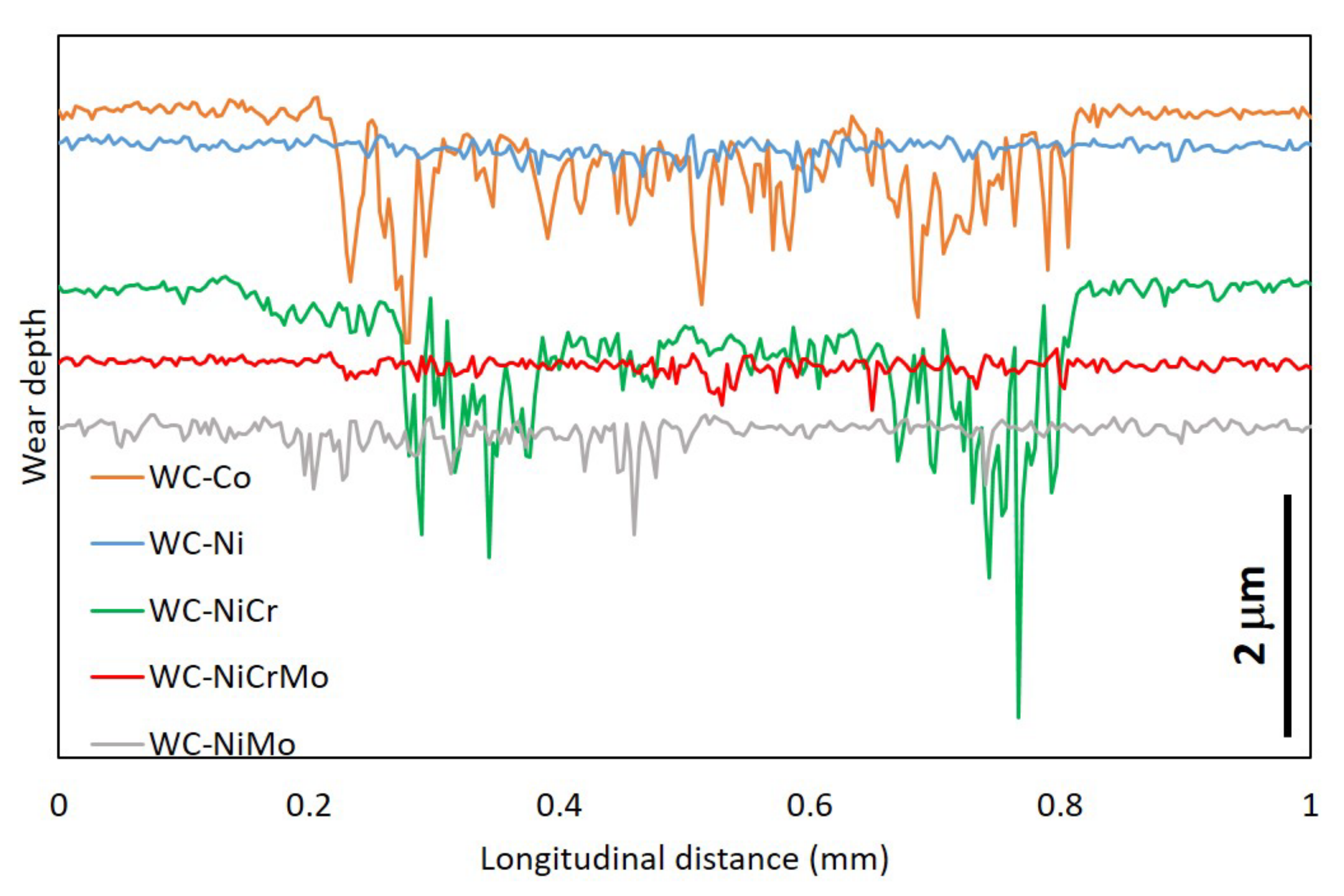
- The wear rate was difficult to estimate since the wear track, for most of the specimens analysed, presented a “dog bone shape”, revealing a hydrodynamic effect, with the visible wear being more significant in zones where the sliding speed is null (extremities of the wear track) and smaller where the sliding speed is higher (in the middle of the wear track). Due to the lubricating effect, they showed much lower wear than in dry sliding tests.
- Wear mechanisms include binder removal, large cratering formation, and carbide grain pull-out, creating vast eroded regions.
- A synergistic effect between wear and corrosion for the contact conditions employed was not verified since the electrolyte contributed mainly to lubricating the contact, reducing friction and wear, and not accelerating wear and corrosion due to load support promoted by hydrodynamic effect and W oxide films formed on the specimen’s surface.
- The specimens that presented higher wear were the WC-Co and WC-NiCr composites showing large portions of worn surfaces, while WC-Ni, WC-NiMo, and WC-NiCrMo showed some confined wear with localized craters.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ASTM. Standard Terminology Relating to Wear and Erosion G40-17; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- López-Ortega, A.; Arana, J.L.; Bayón, R. Tribocorrosion of Passive Materials: A Review on Test Procedures and Standards. Int. J. Corros. 2018, 2018, 7345346. [Google Scholar] [CrossRef]
- Landolt, D.; Mischler, S.; Stemp, M. Electrochemical methods in tribocorrosion: A critical appraisal. Electrochim. Acta 2001, 46, 3913–3929. [Google Scholar] [CrossRef]
- Mischler, S. Triboelectrochemical techniques and interpretation methods in tribocorrosion: A comparative evaluation. Tribol. Int. 2008, 41, 573–583. [Google Scholar] [CrossRef]
- Diomidis, N.; Celis, J.-P.; Ponthiaux, P.; Wenger, F. A methodology for the assessment of the tribocorrosion of passivating metallic materials. Lubr. Sci. 2009, 21, 53–67. [Google Scholar] [CrossRef]
- Trezona, R.; Allsopp, D.; Hutchings, I. Transitions between two-body and three-body abrasive wear: Influence of test conditions in the microscale abrasive wear test. Wear 1999, 225–229, 205–214. [Google Scholar] [CrossRef]
- Landolt, D.; Mischler, S.; Stemp, M.; Barril, S. Third body effects and material fluxes in tribocorrosion systems involving a sliding contact. Wear 2004, 256, 517–524. [Google Scholar] [CrossRef]
- Diomidis, N.; Mischler, S. Third body effects on friction and wear during fretting of steel contacts. Tribol. Int. 2011, 44, 1452–1460. [Google Scholar] [CrossRef]
- Landolt, D.; Mischler, S. Tribocorrosion of Passive Metals and Coatings; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Stachowiak, G.; Salasi, M.; Stachowiak, G. Three-body abrasion corrosion studies of high-Cr Cast irons: Benefits and limi-tations of tribo-electrochemical methods. J. Bio- Tribo-Corros. 2015, 1, 6. [Google Scholar] [CrossRef]
- Landolt, D. Electrochemical and materials aspects of tribocorrosion systems. J. Phys. D: Appl. Phys. 2006, 39, 3121–3127. [Google Scholar] [CrossRef]
- Mathew, M.; Pai, P.S.; Pourzal, R.; Fischer, A.; Wimmer, M.A. Significance of Tribocorrosion in Biomedical Applications: Overview and Current Status. Adv. Tribol. 2009, 2009, 250986. [Google Scholar] [CrossRef] [Green Version]
- Kasar, A.K.; Siddaiah, A.; Ramachandran, R.; Menezes, P.L. Tribocorrosion Performance of Tool Steel for Rock Drilling Process. J. Bio. Tribo-Corros. 2019, 5, 44. [Google Scholar] [CrossRef]
- Katiyar, P.K. A comprehensive review on synergy effect between corrosion and wear of cemented tungsten carbide tool bits: A mechanistic approach. Int. J. Refract. Met. Hard Mater. 2020, 92, 105315. [Google Scholar] [CrossRef]
- Weidow, J.; Andrén, H.-O. Grain and phase boundary segregation in WC–Co with TiC, ZrC, NbC or TaC additions. Int. J. Refract. Hard Met. 2011, 29, 38–43. [Google Scholar] [CrossRef]
- Berg, G.; Friedrich, C.; Broszeit, E.; Berger, C.; Riedel, R. Handbook of Ceramic Hard Materials; Wiley-VCH Weinheim: Weinheim, Germany, 2000. [Google Scholar]
- Beste, U.; Hartzell, T.; Engqvist, H.; Axén, N. Surface damage on cemented carbide rock-drill buttons. Wear 2001, 249, 324–329. [Google Scholar] [CrossRef]
- Beste, U.; Jacobson, S. Friction between a cemented carbide rock drill button and different rock types. Wear 2002, 253, 1219–1221. [Google Scholar] [CrossRef]
- Human, A.; Roebuck, B.; Exner, H. Electrochemical polarisation and corrosion behaviour of cobalt and Co (W, C) alloys in 1 N sulphuric acid. Mater. Sci. Eng. A 1998, 241, 202–210. [Google Scholar] [CrossRef]
- Scholl, H.; Hofman, B.; Rauscher, A. Anodic polarization of cemented carbides of the type [(WC, M): M = Fe, Ni or Co] in sulphuric acid solution. Electrochim. Acta 1992, 37, 447–452. [Google Scholar] [CrossRef]
- Sutthiruangwong, S.; Mori, G.; Kösters, R. Passivity and pseudopassivity of cemented carbides. Int. J. Refract. Met. Hard Mater. 2005, 23, 129–136. [Google Scholar] [CrossRef]
- Rocha, A.F.; Bastos, A.; Cardoso, J.P.V.; Rodrigues, F.; Fernandes, C.M.S.; Soares, E.; Sacramento, J.; Senos, A.; Ferreira, M. Corrosion behaviour of WC hardmetals with nickel-based binders. Corros. Sci. 2018, 147, 384–393. [Google Scholar] [CrossRef]
- Gant, A.; Gee, M.; Gohil, D.; Jones, H.; Orkney, L. Use of FIB/SEM to assess the tribo-corrosion of WC/Co hardmetals in model single point abrasion experiments. Tribol. Int. 2013, 68, 56–66. [Google Scholar] [CrossRef]
- Larsen-Basse, J. Wear of Hard-Metals in Rock Drilling: A Survey Of The Literature. Powder Met. 1973, 16, 1–32. [Google Scholar] [CrossRef]
- Wentzel, E.; Allen, C. The erosion-corrosion resistance of tungsten-carbide hard metals. Int. J. Refract. Met. Hard Mater. 1997, 15, 81–87. [Google Scholar] [CrossRef]
- Engqvist, H.; Beste, U.; Axén, N. The influence of pH on sliding wear of WC-based materials. Int. J. Refract. Met. Hard Mater. 2000, 18, 103–109. [Google Scholar] [CrossRef]
- Gant, A.; Gee, M.; May, A. Microabrasion of WC–Co hardmetals in corrosive media. Wear 2004, 256, 954–962. [Google Scholar] [CrossRef]
- Pereira, P.; Vilhena, L.; Sacramento, J.; Senos, A.; Malheiros, L.; Ramalho, A. Abrasive wear resistance of WC-based com-posites, produced with Co or Ni-rich binders. Wear 2021, 482, 203924. [Google Scholar] [CrossRef]
- Vilhena, L.M.; Fernandes, C.M.; Soares, E.; Sacramento, J.; Senos, A.M.R.; Ramalho, A. Abrasive wear resistance of WC–Co and WC–AISI 304 composites by ball-cratering method. Wear 2016, 346–347, 99–107. [Google Scholar] [CrossRef]
- Tomlinson, W.J.; Linzell, C.R. Anodic polarization and corrosion of cemented carbides with cobalt and nickel binders. J. Mater. Sci. 1988, 23, 914–918. [Google Scholar] [CrossRef]
- Tomlinson, W.; Molyneux, I. Corrosion, erosion-corrosion, and the flexural strength of WC-Co hardmetals. J. Mater. Sci. 1991, 26, 1605–1608. [Google Scholar] [CrossRef]
- Gee, M. Model scratch corrosion studies for WC/Co hardmetals. Wear 2010, 268, 1170–1177. [Google Scholar] [CrossRef]
- Tarragó, J.M.; Fargas, G.; Jimenez-Pique, E.; Felip, A.; Isern, L.; Coureaux, D.; Roa, J.J.; Al-Dawery, I.; Fair, J.; Llanes, L.; et al. Corrosion damage in WC–Co cemented carbides: Residual strength assessment and 3D FIB-FESEM tomography characterisation. Powder Met. 2014, 57, 324–330. [Google Scholar] [CrossRef]



| Compositions | Nominal Composition (wt. %) | Dens. (g/cm3) | Porosity | HV30 (kgf/mm2) | KIC (MPa.m1/2) | E (GPa) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| WC | Co | Ni | Cr | Mo | ||||||
| WC-Co | 92.0 | 8.0 | - | - | - | 14.72 | A00B00C00 | 1659 | 9.9 | 512 |
| WC-Ni | 92.0 | - | 8.0 | - | - | 14.69 | A00B00C00 | 1508 | 9.4 | 522 |
| WC-NiCr | 91.2 | - | 8.0 | 0.8 | - | 14.58 | A00B00C00 | 1664 | 9.5 | 484 |
| WC-NiMo | 91.2 | - | 8.0 | - | 0.8 | 14.63 | A00B00C00 | 1545 | 9.0 | 491 |
| WC-NiCrMo | 90.6 | - | 8.0 | 0.8 | 0.6 | 14.55 | A00B00C00 | 1616 | 9.0 | 519 |
| Experiment | Load (N) | Sliding Distance (m) | Severity (N.m) | Frequency (Hz) | Sliding Speed (m/s) | Sliding Time (s) |
|---|---|---|---|---|---|---|
| 1 | 17 | 500 | 8500 | 3 | 0.012 | 41,667 |
| 2 | 23 | 500 | 11,500 | 3 | 0.012 | 41,667 |
| 3 | 29 | 500 | 14,500 | 3 | 0.012 | 41,667 |
| Composites | Ecorr (mVSCE) | icorr (A/cm2) |
|---|---|---|
| WC-Co | −221.2 | 4.63 × 10−6 |
| WC-Ni | −158.5 | 5.31 × 10−6 |
| WC-NiCr | −114.9 | 4.56 × 10−6 |
| WC-NiMo | −191.0 | 4.02 × 10−6 |
| WC-NiCrMo | −141.6 | 4.30 × 10−6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, P.; Vilhena, L.; Sacramento, J.; Senos, A.; Malheiros, L.; Ramalho, A. Influence of Different Binders and Severe Environmental Conditions on the Tribological and Electrochemical Behaviour of WC-Based Composites. Lubricants 2022, 10, 145. https://doi.org/10.3390/lubricants10070145
Pereira P, Vilhena L, Sacramento J, Senos A, Malheiros L, Ramalho A. Influence of Different Binders and Severe Environmental Conditions on the Tribological and Electrochemical Behaviour of WC-Based Composites. Lubricants. 2022; 10(7):145. https://doi.org/10.3390/lubricants10070145
Chicago/Turabian StylePereira, Pedro, Luís Vilhena, Joaquim Sacramento, Ana Senos, Luís Malheiros, and Amílcar Ramalho. 2022. "Influence of Different Binders and Severe Environmental Conditions on the Tribological and Electrochemical Behaviour of WC-Based Composites" Lubricants 10, no. 7: 145. https://doi.org/10.3390/lubricants10070145






