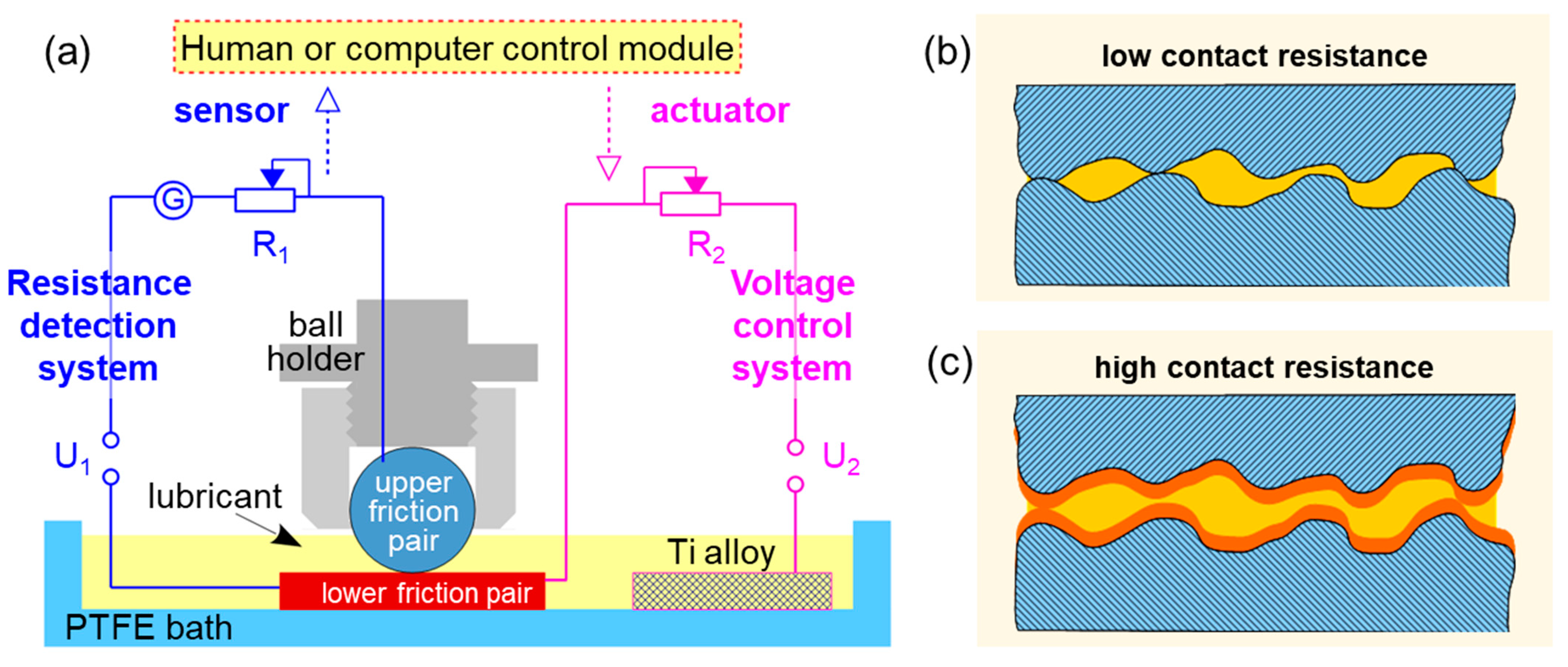
3.1. Effect of External Electric Field on COF and Contact Resistance
By combining the contact resistance with COF, the SDS adsorption film formation and failure on the surface of stainless steel or Cu plate when rubbing against stainless steel or bearing steel ball were analyzed under different applied electrical fields.
Figure 3 shows the COF and contact resistance of 316 L stainless steel plate and 304 stainless steel ball verse time, under dry friction and lubricated by pure water as well as 160 mM SDS aqueous solution. In the first 100 s, the friction pair was under dry friction with a high COF of 1.04 ± 0.07 and a low contact resistance of 3.6 Ω. Then, around 0.25 mL of pure water was injected into the contact area. The COF decreased to 0.37 ± 0.04, and the contact resistance was stable at 3.0 Ω. For macroscale rough surface contacts, the load-carrying capacity of pure water is relatively poor. The addition of some kinds of long-chain organic molecules can improve boundary lubrication of aqueous solutions [
27]. At 200 s, 20 mL of 160 mM SDS aqueous solution was added into the contact area. The COF decreased from 0.37 ± 0.04 to 0.23 ± 0.01. The SDS surfactant shows a better lubrication effect, compared with H
2O molecules. At 300 s, a positive voltage of 3.5 V was applied, with the lower friction pair of 316 L stainless steel as the work electrode and an external Ti alloy plate as the counter electrode as shown in
Figure 1a. The positive voltage represented that the work electrode was contacted with the positive pole of the power supply. The COF decreased to 0.18 ± 0.01, and the contact resistance obviously increased from 3.3 Ω to 12.4 Ω. The mechanism considered is that when the metal plate works as a positive pole, the surface concentration of SDS anions (DS
−) becomes higher due to the field-assisted adsorption. When a negative potential of 3.5 V was applied on the surface of the lower friction pair, the COF increased to 0.45 ± 0.01, and the contact resistance decreased to around 3.4 Ω. The results indicated that a complete boundary lubrication film was formed on the surface of friction pairs in SDS solution under the positive potential of 3.5 V, and destructed owing to the desorption of SDS anions under the negative potential of 3.5 V. In the absence of external electric field, the COF decreased to 0.31 ± 0.01, and the contact resistance was about 2.6 Ω. The changes of the COF under different voltages in SDS solutions are consistent with the regularity reported by previous studies [
11]. Meanwhile, the contact resistance result shows more detailed information on the formation and failure of the boundary film during the friction testing, which will be discussed in
Section 3.3.
Figure 4 shows the COF and contact resistance of Cu plate and 304 stainless steel ball verse time, under dry friction, pure PC lubrication, and 5 mM SDS PC solution lubrication. The COF under the dry friction was 0.75 ± 0.05, and the contact resistance was around 1.3 Ω. At 300 s, 0.25 mL pure PC was injected into the contact area. The COF decreased to 0.52 ± 0.03 while the contact resistance remained almost constant. At 600 s, the COF further decreased to 0.17 ± 0.01 when 20 mL SDS PC solution was added as a lubricant. While, the contact resistance was barely changed, around 1.3 Ω. When a positive potential of 20 V was applied on the surface of the lower friction pair, the COF decreased to 0.09, and the contact resistance obviously increased from 1.3 Ω to 210.0 Ω in 170 s. When a negative potential of 20 V was applied, the COF increased to 0.53. The contact resistance decreased to around 1.2 Ω under the negative potential or no applied potential, indicating the desorption of the SDS boundary film on the friction pair in the solution. The effect of the electric field on boundary lubrication was basically consistent, no matter whether H
2O or PC was the base lubricant.
3.2. On-Line Control of COF by Contact Resistance Detection and Applied Electric Field Control
Inspired by the friction results in
Figure 3 and
Figure 4, a tribometer with an ECR module, an external electric field module, and an active control software design was designed to realize the on-line control of sliding friction of metals lubricated by adsorbed boundary SDS films.
Figure 5 shows the contact resistance and applied potentials during the friction test of the 316 L steel plate verse the 304 steel ball in 160 mM SDS aqueous solution. At the initial stage of the friction test, the contact resistance was relatively low because of the incomplete boundary film. After 9 s of an applied voltage of 3.5 V, the contact resistance increased from 2.3 to 8.5 Ω. At this stage, although the change of contact resistance was not significant, the COF decreased significantly. The related tribological results can be found in
Figure 6, involving both the normal boundary lubrication without control and the one under control.
When the contact resistance increased to 12.2 Ω (higher than the pre-set threshold of 10.0 Ω) at 36 s, the power supply was switched off. The COF obtained under the actively controlled boundary lubrication in
Figure 6 did not change significantly. Meanwhile, the contact resistance decreased obviously from 12.2 Ω to 7.1 Ω. The power supply was needed, so as to increase the contact resistance, and kept the COF at a good boundary lubrication state. After 36 s, the contact resistance was relatively high even in the absence of applied potentials, indicating that the boundary film state was relatively stable. This method ensures that the boundary lubrication film is in a good lubrication state while saving electric energy as much as possible. According to
Figure 6, the COF was decreased by 24% (from 0.25 to 0.19), after the on-line control of the contact resistance of steel tribopairs in SDS aqueous solution by an external electric field. Compared to the case in which the external electric field was applied for the whole period of testing, the active controlling can save 7.4% of the electric energy in 80 s for the above friction system.
Figure 7a,b show the optical micrographs of the wear tracks of the 316 L steel plate after the friction tests as described in
Figure 6.
Figure 7c shows a comparison of the linear profiles, measured on the ZYGO profilometer (Middlefield, CT, USA), of these two wear tracks. For the normal boundary lubrication, the depth and length of the wear tracks were 0.42 and 124.37 μm, respectively. For the actively controlled boundary lubrication, the depth of the wear tracks was around 0.05 μm, and the width was around 84.56 μm. The wear rate of the lower friction pair was reduced from 4.56 × 10
−6 mm
3/(N·m) to 1.61 × 10
−6 mm
3/(N·m).
Figure 7d reveals that the element compositions on the wear track of 316 L steel plate obtained in the normal boundary lubrication include C, O, Cr, Fe, Ni, Si, and Mo. The elements of Cr, Fe, Ni, Si, and Mo are the components of 316 L stainless steel. C and O are considered to come primarily from contaminants in the air or from lubricants. For the element composition of the wear track obtained in the actively controlled boundary lubrication as shown in
Figure 7e, the compositions and content of the elements are similar to those in
Figure 7d. Furthermore, the surface morphologies and elemental maps of the wear tracks were inspected on SEM and EDX.
Figure 7f,g respectively, show the results of the wear tracks obtained in the normal boundary lubrication and the actively controlled boundary lubrication. The green and blue areas in the figures represent the distributions of O and Fe elements, respectively. In both cases, the distribution of the O element inside and outside the wear tracks is consistent, indicating that no significant oxidation reactions occurred within the wear tracks. These results indicate that the external electric field can control the friction and wear behaviors by changing the adsorption film rather than the formation of the tribofilm or oxidation film on the friction pairs.
Figure 8 shows the COFs verse time for Cu plate/304 steel ball or Cu plate/bearing steel ball with 5 mM SDS PC solution as a lubricant before and after the active control. Here, we set the start time of active regulation as 0 s. As shown in
Figure 8a, the COF obtained in the friction test of Cu plate/304 steel ball without active control (from −50 s to 0 s) was 0.18 ± 0.01. After 0 s, it decreased to 0.11 ± 0.01. In
Figure 8b, the COF decreased from 0.28 ± 0.01 to 0.13 ± 0.01, after the on-line control of the contact resistance between Cu plate and bearing steel ball in SDS PC solution by an external electric field. The average COF was decreased by 39% for Cu plate/304 steel ball and 54% for Cu plate/bearing steel ball.
According to the Hertzian contact model, the different hardness and Young’s modulus of the two kinds of upper friction pairs cause the different Hertz pressures. Compared with the friction pair of Cu plate/304 steel ball, the relatively higher Hertz contact pressure for the friction pair of Cu plate/bearing steel ball causes a higher proportion of boundary film failure, resulting in the higher COF in the normal boundary lubrication condition. When a positive potential is applied, the adsorption capacity increases, and the adsorption strength of the boundary film becomes stronger, so as to achieve a better boundary lubrication effect. According to the adhesion friction theory, the COF is related to the shear strength of the boundary lubrication film and the yield stress of the soft material (pure Cu in this work). Hence, it can be considered that the COFs of the above two cases are similar under good SDS boundary lubrication (0.11 and 0.13). While, due to the different COFs during the normal boundary lubrication, the friction reductions after the actively controlled boundary lubrication are different.
Figure 8c,d show the contact resistance and applied potentials during the actively controlled boundary lubrication processes in
Figure 8a,b, respectively. Similar to
Figure 5, the contact resistances for the two cases were relatively low at the initial stage of the friction test. In
Figure 8c, the contact resistance between the Cu plate and the 304 steel ball increased from 2.7 Ω to 11.1 Ω at around 39 s. Under the action of an electric field, the contact resistance gradually increased to around 190.0 Ω. The relatively high contact resistance indicates the formation of a good boundary lubrication film. In this case, the externally applied potential of 20 V was switched off when the contact resistance was higher than 10.0 Ω. The COF was stabilized at around 0.11, showing the preliminary realization of the smart lubrication and energy saving. For the case of the Cu plate and the bearing steel ball as friction pairs in
Figure 8d, the contact resistance increased from 4.1 Ω to around 6.4 Ω and higher, after 25 s of an applied voltage of 20 V. In this case, the power supply was switched off, when the contact resistance reached 6.0 Ω. The contact resistance obtained under the actively controlled boundary lubrication increased to 177.5 Ω. After 50 s, the contact resistance was relatively high even in the absence of the potentials, indicating that the boundary film state was relatively stable. The active controlling can save about 25.8% and 49.7% of the electric energy for the Cu plate/304 steel ball and the Cu plate/bearing steel ball respectively, in 150 s.
Figure 9a,b show the optical micrographs of the wear tracks of the Cu plate obtained in normal boundary lubrication and actively controlled boundary lubrication of Cu plate vs. 304 steel ball in 5 mM SDS PC solution for 150 s.
Figure 9c shows a comparison of the linear profiles between the two wear tracks described in
Figure 9a,b. Compared with the normal boundary lubrication, the wear resistance is obviously improved in the actively controlled boundary lubrication for the Cu plate/304 steel ball pair in the SDS PC solution. The maximum depth of the wear track is reduced from 0.19 to 0.08 μm.
Figure 9d–f show the optical micrographs and linear profiles of the wear tracks of the Cu plate when rubbing against the bearing steel ball under the same as those in
Figure 9a–c. The maximum depth of the wear track is reduced from 0.12 to 0.04 μm. Combining with the results in
Figure 8 and
Figure 9, we can conclude that the effect of voltages on the wear depth of cross-sections of wear tracks is consistent with that on COF.
3.3. Discussions on Mechanism
The mechanism of achieving such an active regulation is summarized in the schematic diagram as shown in
Figure 10. The contact area undergoes no boundary lubrication film, formation of boundary lubrication film, good boundary lubrication, and destruction of boundary lubrication film. No matter whether in water or PC oil, the addition of SDS can decrease the real contact area between the metals, resulting in an obvious decrease in COF. Nonetheless, the increase in contact resistance is not obvious due to some remaining local real contact areas. A relatively complete boundary film can be formed under certain positive potential, causing a significant increase in contact resistance. On the contrary, at the initial stage of boundary film failure, the change of contact resistance is quicker than that of COF. It is obvious that the combination of contact resistance and COF can provide more information on the working states of the boundary film between the metallic tribopairs, as contact resistance can reflect boundary film failure more quickly while COF can evaluate its formation more quickly. Hence, it is feasible to use contact resistance to characterize the state of boundary film and obtain relatively low COF.
To further discuss the effect of adsorption film on the COF and contact resistance during the boundary lubrication, dry friction tests were designed and carried out by excluding other effects such as tribochemical reactions or hydrodynamic lubrication. A clean 316 L stainless steel plate was soaked in a 10 mM SDS aqueous solution at room temperature. After 0.5 h, the steel plate was removed from the solution and dried at 100 °C for another 0.5 h.
Figure 11a shows the schematic diagram of the experimental procedure. The COF and contact resistance of the treated sample and an untreated 304 stainless steel ball were tested on the UMT-3 and the auto ohmmeter.
Figure 11b shows the COF and contact resistance of the SDS treated 316 L steel plate and 304 stainless steel ball verse sliding distance during the dry friction. The friction tests were carried out with a reciprocating velocity of 8 mm/s under a pressure of 442 MPa. In the first 120 mm of the experiment, the COF was around 0.13 and the average contact resistance was around 39.3 kΩ (the maximum value reached 195.8 kΩ).
Figure 11c,d show the wear track of the lower friction pair and wear spot on the upper friction pair after the sliding distance of 80 mm (before the SDS boundary film was destroyed at ~150 mm). The results show that under good boundary lubrication, abrasive particles were invisible around the friction pair. Compared with the contact resistance between 316 L steel plate and 304 steel ball in SDS aqueous solution, the contact resistance between the SDS treated 316 L steel plate and 304 steel ball in the air under its good boundary lubrication condition is much higher, which is considered to be caused by the adsorption film gradually transferred onto the top of the upper friction pair (as shown in
Figure 11d) and the lack of solution conductivity. The contact resistance decreased from 10.8 kΩ to 1.4 kΩ at 105 m and further decreased to around 21 Ω at 128 m.
The increase of the COF before 150 m was not obvious. At around 150 m, the COF increased from 0.20 to 0.82. After the failure of the adsorbed film at 150 m, the average COF was around 0.71 and the average contact resistance was 6 Ω. These results reveal that the SDS adsorption film can decrease the COF and increase contact resistance. Moreover, the contact resistance is more sensitive to the failure of the boundary lubrication film, compared to the COF.
Contact resistance is generally composed of constriction resistance and film resistance [
23,
24]. Here, we discuss three cases as follows.
Peak contacts without covering film: Constriction resistance. The constriction resistance can be calculated from a solution of Laplace’s equation using appropriate boundary conditions [
28]. The electrical constriction resistance of one contact peak between the metallic tribopairs can be given as
where
and
is the electrical resistivity of the upper and lower friction pairs, and
is the constriction radius.
If the researched interface has a total of n contact peaks, the total contact resistance is .
All peak contacts covered with film: Film resistance. According to the definition of surface resistivity (
) of films [
29], the resistance encountered by electrons passing through a conductive film is given as
Its total contact resistance
is the shunt resistance consisting of
n series resistances of the constriction resistance and film resistance, as shown in Equation (3).
Partially peak contacts are covered with film. Assume that the proportion of the number of contact peaks with boundary films is
x of the total number of contact peaks. Then, the reciprocal of the total contact resistance is
therefore,
and
where
A is equal to
.
The total contact resistance is related to
x and
A. When
x is near 1, we can obtain that
According to the experimental result in
Figure 11,
obtained under the good boundary lubrication is much larger than
, and
A is around 1 × 10
3~30 × 10
3. When 0 <
x < 1, it was obtained that the contact resistance was similar to that without boundary lubrication film (
x = 0). Therefore,
. These results indicate that once the boundary lubrication film starts to fail, the contact resistance will quickly drop in the absence of external voltage assistance.