Test Study of the Bridge Cable Corrosion Protection Mechanism Based on Impressed Current Cathodic Protection
Abstract
:1. Introduction
2. Cable ICCP Technique
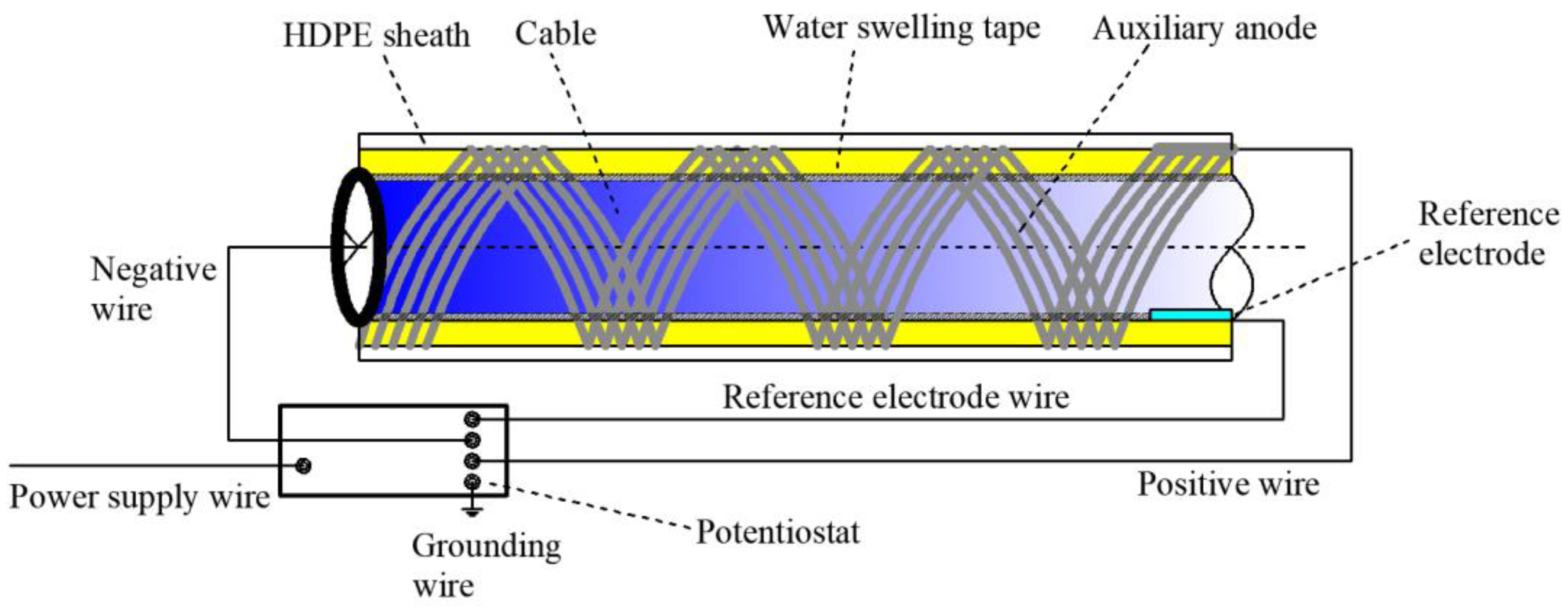
2.1. Working Principle of Cable ICCP System
2.2. ICCP Corrosion Protection Test
2.2.1. Simulated Acid Rain Solvent
2.2.2. Auxiliary Anode and Reference Electrode
2.2.3. Protective Potential
2.2.4. Cable ICCP System
3. Results and Analysis
3.1. Analysis of Surface Configuration of Corroded Cables
3.2. Close Observation and Weight Loss Analysis of Corroded Steel Wire
3.3. Fracture Damage Analysis of Corroded Steel Wire
4. Protection Mechanism of Cable ICCP System
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Borchers, C.; Kirchheim, R. Cold-drawn pearlitic steel wires. Prog. Mater. Sci. 2016, 82, 405–444. [Google Scholar] [CrossRef]
- Guowen, Y.; Shiya, L.; Zengwei, G. Corrosion Fatigue Performance and Damage Mechanism of Bridge Cable Structures; Science Press: Beijing, China, 2022. [Google Scholar]
- Xu, J.; Chen, W. Behavior of wires in parallel wire stayed cable under general corrosion effects. J. Constr. Steel Res. 2013, 85, 40–47. [Google Scholar] [CrossRef]
- McTavish, S.; Raeesi, A.; D’Auteuil, A.; Yamauchi, K.; Sato, H. An investigation of the mechanisms causing large-amplitude wind-induced vibrations in stay cables using unsteady surface pressure measurements. J. Wind Eng. Ind. Aerodyn. 2018, 183, 19–34. [Google Scholar] [CrossRef]
- Ministry of Communications of the People’s Republic of China. Cable of Parallel Steel Wires for Large-Span Cable-Stayed Bridge (JT/T775-2016); China Communications Press: Beijing, China, 2016.
- Zhengmao, Z.; Sujuan, W.; Zhenqiu, G. Asymmetric cable replacement technique for cable-stayed bridges in service. World Bridges 2010, 1, 56–58+71. [Google Scholar]
- Editorial Department of China Journal of Highway and Transport. Review on China’s bridge engineering research: 2021. China J. Highw. Transp. 2021, 34, 1–97. [Google Scholar]
- Xuanbo, H.; Zengwei, G.; Hua, X. Vehicle impact effect of suspender in half-through arch bridge considering vehicle-bridge coupled vibration. Noise Vib. Control 2022, 42, 206–213. [Google Scholar]
- Qingkuan, L.; Yunfei, Z.; Yurun, B.; Qi, S.; Xiaobing, L.; Wenyong, M. Parametric optimization of aerodynamic anti-vibration measure for rain-wind induced vibration of cables. J. Vib. Shock 2015, 34, 31–35+54. [Google Scholar]
- Zhongwen, H. Experimental Study on the Fatigue Properties and Time-Dependent Corrosion Model of Coastal Bridge Suspenders; Chongqing Jiaotong University: Chongqing, China, 2020. [Google Scholar]
- Yaliang, C.; Huacong, X.; Yingeng, Y. Accident analysis of lowered/half supported tied arch bridges and enlightments for bridge detection. J. Fujian Univ. Technol. 2013, 11, 213–217. [Google Scholar]
- Jueming, Y.; Jianchi, Z. Corrosion and protection of bridge cable systems. Steel Constr. 2005, 2, 85–89. [Google Scholar]
- Yangguang, Y.; Xiaodong, L.; Guangning, P.; Tao, W.; Dezhi, Z. Temporal and spatial variability of corrosion of high-strength steel wires within a bridge stay cable. Constr. Build. Mater. 2021, 308, 211–213. [Google Scholar]
- Betti, R.; West, A.C.; Vermaas, G.; Cao, Y. Corrosion and embrittlement in high-strength wires of suspension bridge cables. J. Bridge Eng. 2005, 10, 151–162. [Google Scholar] [CrossRef]
- Suzumura, K.; Nakamura, S. Environmental factors affecting corrosion of galvanized steel wires. J. Mater. Civ. Eng. 2004, 16, 1–7. [Google Scholar] [CrossRef]
- Nakamura, S.; Suzumura, K. Hydrogen embrittlement and corrosion fatigue of corroded bridge wires. J. Constr. Steel Res. 2009, 65, 269–277. [Google Scholar] [CrossRef]
- Barton, S.C.; Vermaas, G.W.; Duby, P.F. Accelerated corrosion and embrittlement of high-strength bridge wire. J. Mater. Civ. Eng. 2000, 12, 33–38. [Google Scholar] [CrossRef]
- Guowen, Y.; Chaoyue, L.; Guoqiang, W. Mechanism of corrosion damage of stayed cable under the effect of acid rain and loading coupling. J. Chongqing Jiaotong Univ. (Nat. Sci.) 2016, 35, 6–10. [Google Scholar]
- Nakamura, S.; Furuya, K.; Kitagawa, M.; Suzumura, K. Corrosion Performance of New Suspension Bridge Cable Protection; International Association for Bridge and Structural Engineering: Zurich, Switzerland, 2000. [Google Scholar]
- Rou, L.; Changqing, M.; Tinghua, W. Effect of environmental factors on electrochemical corrosion of galvanized steel wires for bridge cables. Anti-Corros. Methods Mater. 2022, 69, 244–246. [Google Scholar]
- Zengwei, G.; Hanlin, C.; Xiaogang, L.; Jiayu, Y. Simulation of the evolution process of stayed cable electrochemical corrosion based on cellular automata. J. Chongqing Univ. 2019, 42, 19–27. [Google Scholar]
- Lulu, F.; Kaiming, W.; Xiuyu, L.; Wenwei, Q. Research status and development tendency of ultra-high strength steel wire for bridge cables. Mater. China 2020, 39, 395–403. [Google Scholar]
- Zhigang, W.; Xiaoxiong, Z.; Jun, Z.; Jianfeng, C.; Wei, X.; Jianping, H. Performance of galvanized zinc and linc-aluminum alloy for high strength steel cables. Corros. Prot. 2021, 42, 25–29+65. [Google Scholar]
- Heng, Z. Experimental study on water sealing of new type stay cable waterproof cover. Highway 2019, 64, 118–120. [Google Scholar]
- Hailiang, Z. New sealing system technology for bridge cable. Highway 2015, 60, 95–98. [Google Scholar]
- Ball, J.C.; Whitmore, D.W.; Eng, P.; Technologies, V.C. Galvanic Protection for reinforced concrete bridge structures: Case studies and performance assessment. In Proceedings of the 49th Annual Conference of the Australasian Corrosion Association, Harbour, Australia, 15–19 November 2009; pp. 388–396. [Google Scholar]
- Christodoulou, C.; Kilgour, R. The world’s first hybrid corrosion protection systems for prestressed concrete bridges. Corros. Mater. 2014, 39, 36–37. [Google Scholar]
- Rong, L.; Wenyi, Z. Impressed current cathodic protection system for reinforced concrete bridge decks. World Bridges 2008, 2, 69–71+77. [Google Scholar]
- Green, W.; Andrews-Phaedonos, F.; Brewster, G.; Mccormick, D. Lynch’s bridge-a case study in the cathodic protection of reinforced concrete. In Proceedings of the 41st Australasian Corrosion Association Conference 2001, Newcastle, New South Wales, Australia, 18–21 November 2001. [Google Scholar]
- Huyuan, S. Design and research on in-situ ICCP monitoring system for steel pipe piles of shanghai yangtze river bridge. J. Highw. Transp. Res. Dev. 2010, 27, 141–143+154. [Google Scholar]
- Dongxiong, Z. Application of impressed current cathodic protection technology on cable pylon foundation of sea-crossing bridge. Mod. Transp. Technol. 2013, 10, 60–63. [Google Scholar]
- Tao, C. Impressed current cathodic protection techniques for concrete structures and their applications to civil engineering. Bridge Constr. 2006, 3, 12–15+50. [Google Scholar]
- Manning, D.G.; Schell, H.C. Cathodic protection for medieval bridge. Struct. Eng. 2009, 87, 42. [Google Scholar]
- Bennett, J.; Firlotte, C.; Turk, T.; Sexton, M. Testing of very old cathodic protection systems on Ohio bridge decks. In Proceedings of the Corrosion Conference and Expo, Salt Lake City, UT, USA, 11–15 March 2012; pp. 224–234. [Google Scholar]
- Christodoulou, C.; Sharifi, A.; Das, S.; Goodier, C. Cathodic protection on the UK’s midland links motorway viaducts. Proc. Inst. Civ. Eng.-Bridge Eng. 2014, 167, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Cooke, C. Hybrid cathodic protection systems save a New Zealand bridge. Constr. Eng. Aust. 2017, 3, 54–56. [Google Scholar]
- Bahekar, P.V.; Gadve, S.S. Impressed current cathodic protection of rebar in concrete using Carbon FRP laminate. Constr. Build. Mater. 2017, 156, 242–251. [Google Scholar] [CrossRef]
- Wei, L.; Zhu, J.H.; Dong, Z.; Liu, J.; Liu, W.; Su, M.; Xing, F. Anodic and mechanical behavior of carbon fiber reinforced polymer as a dual-functional material in chloride-contaminated concrete. Materials 2020, 13, 222. [Google Scholar] [CrossRef]
- Jin-A, J.; Chung-Kuk, J.; Won-Sub, C. Tidal water effect on the hybrid cathodic protection systems for marine concrete structures. J. Adv. Concr. Technol. 2012, 10, 389–394. [Google Scholar]
- Jianfei, W. Preparation of the Composite Coatings on the Carbon Steel Surface and Their Anticorrosion Properties in Seawater; Ocean University of China: Qingdao, China, 2011. [Google Scholar]
- Yiyan, L.; Jiyue, H.; Li, S.; Wenshui, T. Active and passive protection of steel reinforcement in concrete column using carbon fibre reinforced polymer against corrosion. Electrochim. Acta 2018, 278, 124–136. [Google Scholar]
- Yueping, H.; Ming, X.; Yijun, J.; Chengjun, G.; Yongsheng, D.; Shaozhuang, Z. Examination of localized corrosion and lifetime evaluation for steel cable. Corros. Sci. Prot. Technol. 2006, 18, 132–135. [Google Scholar]
- Guowen, Y.; Shiya, L.; Pengyu, C.; Lifeng, G. A Bridge Cable Impressed Current Cathodic Protection Method, System and Device. CN111593354B, 10 May 2022. [Google Scholar]
- Xuan, W.; Zhe, D. Analysis on short circuit voltage drop in shutdown aluminum reduction cells. Light Met. 2022, 6, 20–24+44. [Google Scholar]
- Pengyu, C. Research on Impressed Current Cathodic Protection Method of Bridge Cable Structure; Chongqing Jiaotong University: Chongqing, China, 2020. [Google Scholar]
- Fei, H.; Xin, L.; Xusheng, H.; Chaoyang, F.; Kaidong, Q. Analysis and characterization of precious metal particles captured by filters. Precious Met. 2022, 43, 42–46. [Google Scholar]
- Xianliang, C. Experimental Study on Corrosion Charcteristics and Mechanical Properties of Bridge Cable Wires; Southeast University: Dhaka, Bangladesh, 2015. [Google Scholar]
- Lijun, N.; Ailing, D.; Likun, X.; Xiangbo, L. Corrosion Behavior of Galvanized Steel in NaCl Solution. Corros. Sci. Prot. Technol. 2012, 24, 291–295. [Google Scholar]
- Xujie, Y.; Junxi, Z.; Shiming, Z.; Tian, T. Corrosion behavior of galvanized steel for power transmission tower with breakage of zinc coating in polluted environment. J. Chin. Soc. Corros. Prot. 2013, 33, 395–399. [Google Scholar]









| SO42− | NO3− | F− | Cl− | NH4+ | Ca2+ | Mg2+ | Na+ | K+ | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Concentration (mg/L) | Location1 | 7.14 | 3.14 | 0.10 | 0.51 | 1.83 | 1.27 | 0.12 | 0.23 | 0.33 |
| Location2 | 10.24 | 4.61 | 0.12 | 0.47 | 2.54 | 2.80 | 0.20 | 0.17 | 0.46 | |
| Serial Number | Material Name | Specification | Technical Parameter | Quantity |
|---|---|---|---|---|
| 1 | Ammonium sulfate analytically pure | 500 g | AR | 1 bottle |
| 2 | Magnesium sulfate analytically pure | 500 g | AR | 1 bottle |
| 3 | Calcium sulfate analytically pure | 500 g | AR | 1 bottle |
| 4 | Potassium chloride analytically pure | 500 g | AR | 1 bottle |
| 5 | Sodium chloride analytically pure | 500 g | AR | 1 bottle |
| 6 | Diluted sulfuric acid | 500 mL | pH = 1 | 4 bottles |
| 7 | Diluted nitric acid | 500 mL | pH = 1 | 2 bottles |
| Corrossion Time (h) | Group | Mass before Corrosion (g) | Mass after Corrosion (g) | Weight Loss Quantity (g) | Mean Corrosion Rate (g·m−2·h−1) |
|---|---|---|---|---|---|
| 168 | Without voltage protection (rust not removed) | 75.32 | 74.88 | 0.44 | 0.3335 |
| 336 | Without voltage protection (rust not removed) | 75.32 | 74.09 | 1.23 | 0.4661 |
| Without voltage protection | 75.32 | 73.66 | 1.66 | 0.6290 | |
| With protection of −1.070 V | 75.10 | 74.96 | 0.14 | 0.0531 | |
| With protection of −1.130 V | 75.11 | 75.05 | 0.06 | 0.0227 | |
| With protection of −1.200 V | 75.27 | 75.22 | 0.05 | 0.0189 | |
| With protection of −1.300 V | 75.18 | 75.15 | 0.03 | 0.0114 |
| −1.070 V Protection | −1.130 V Protection | −1.200 V Protection | −1.300 V Protection | |
|---|---|---|---|---|
| Tensile strength (MPa) | 1648 | 1693 | 1586 | 1572 |
| Strength loss rate (%) | 6.89 | 4.35 | 10.40 | 11.19 |
| Elongation rate after fracture (%) | 4.19 | 5.06 | 3.23 | 2.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, G.; He, X.; Liu, J.; Guo, Z.; Chen, P. Test Study of the Bridge Cable Corrosion Protection Mechanism Based on Impressed Current Cathodic Protection. Lubricants 2023, 11, 30. https://doi.org/10.3390/lubricants11010030
Yao G, He X, Liu J, Guo Z, Chen P. Test Study of the Bridge Cable Corrosion Protection Mechanism Based on Impressed Current Cathodic Protection. Lubricants. 2023; 11(1):30. https://doi.org/10.3390/lubricants11010030
Chicago/Turabian StyleYao, Guowen, Xuanbo He, Jiawei Liu, Zengwei Guo, and Pengyu Chen. 2023. "Test Study of the Bridge Cable Corrosion Protection Mechanism Based on Impressed Current Cathodic Protection" Lubricants 11, no. 1: 30. https://doi.org/10.3390/lubricants11010030





