A Review of Electric Potential-Controlled Boundary Lubrication
Abstract
:1. Introduction
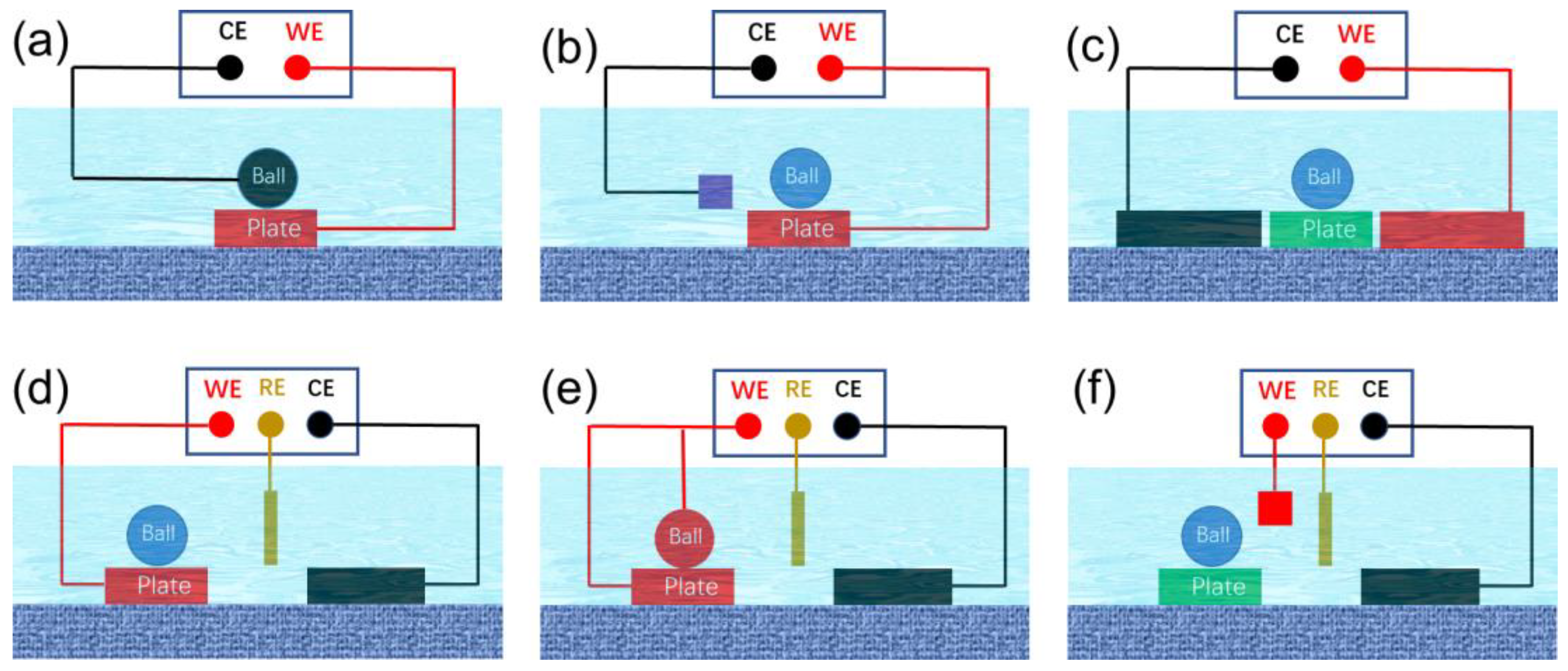
2. Two- and Three-Electrode Systems
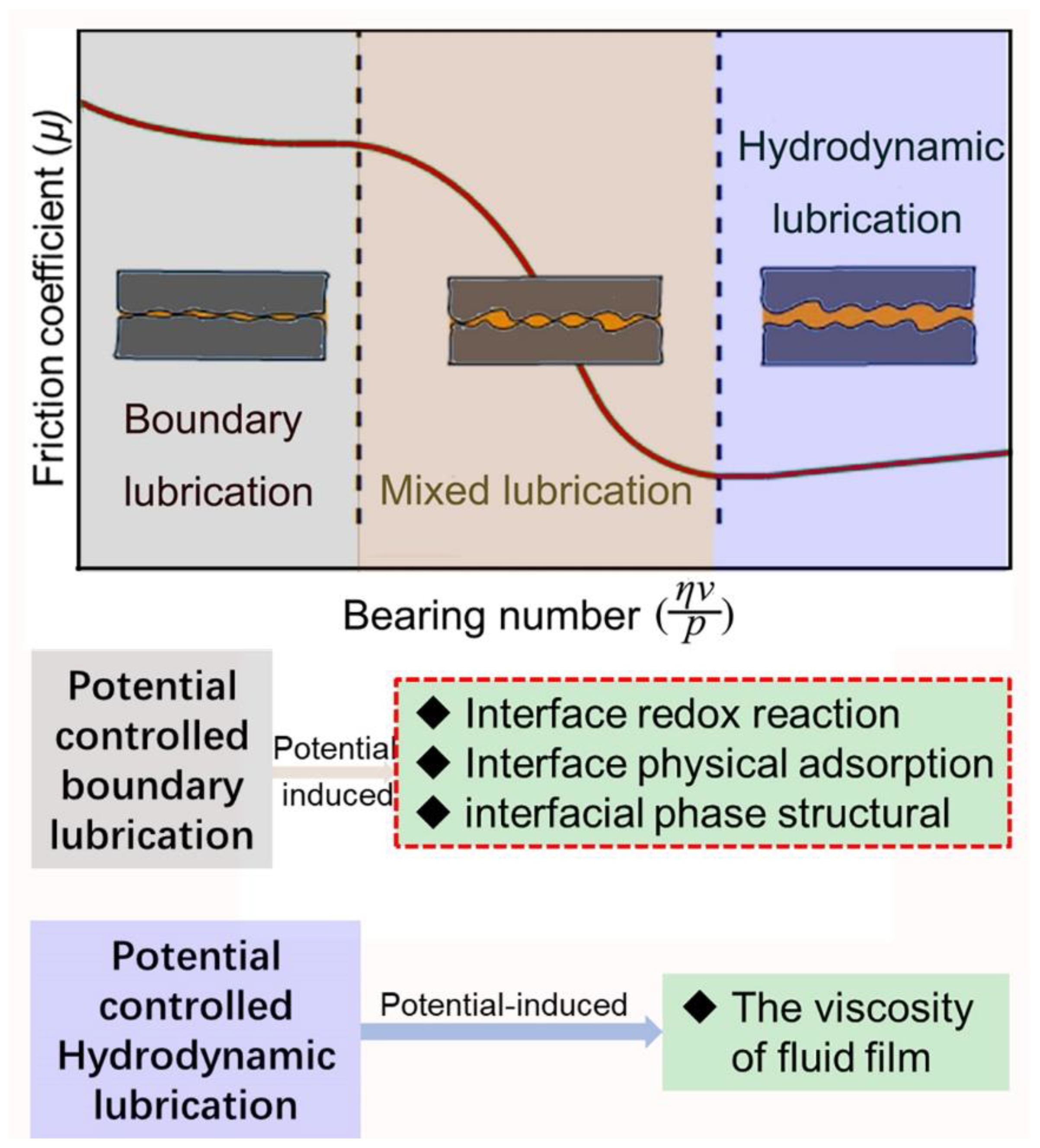
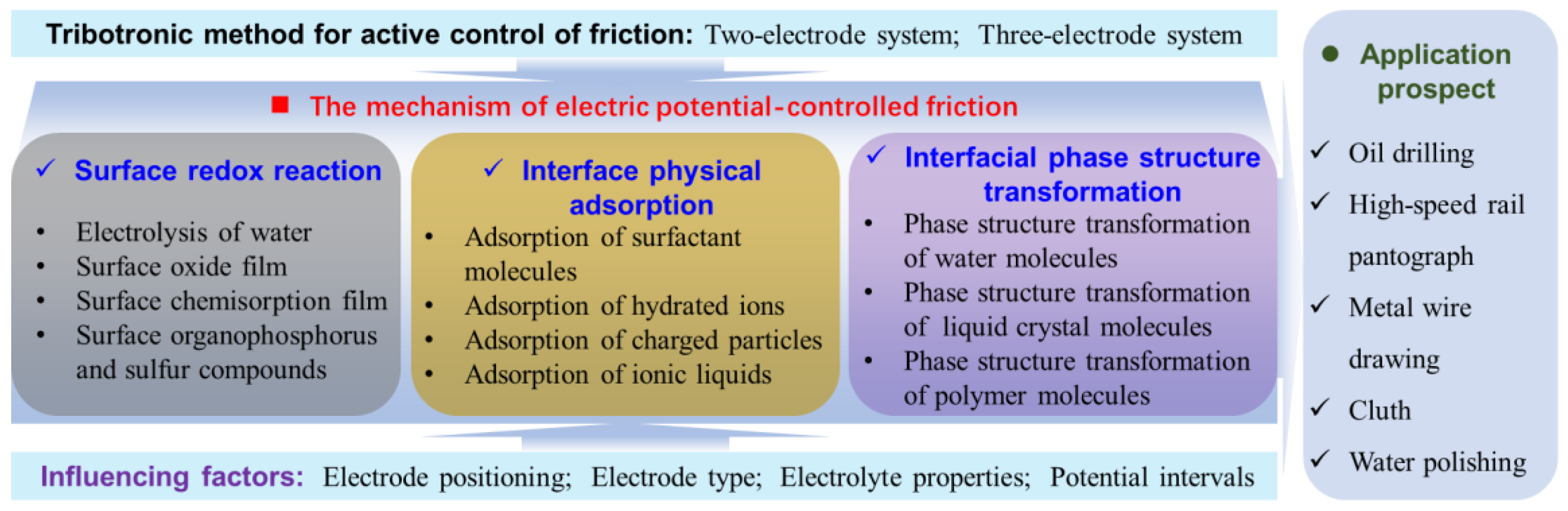
3. The Mechanism of Potential-Controlled Friction
3.1. Interface Redox Reaction
3.1.1. Electrolysis of Water
3.1.2. Surface Oxide Film
3.1.3. Surface Chemisorption Film
3.1.4. Surface Organophosphorus and Sulfur Compounds
3.2. Interface Physical Adsorption
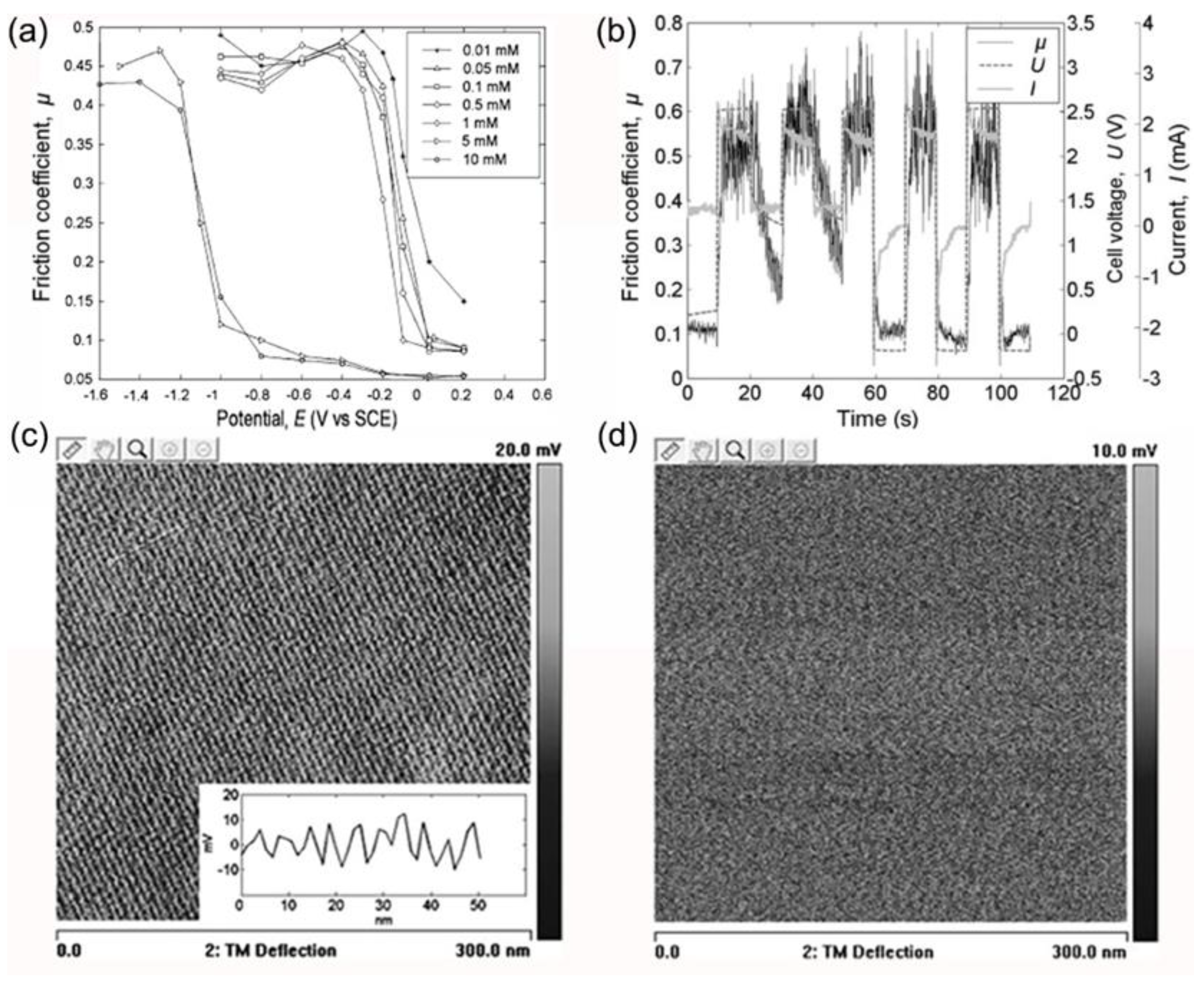
3.2.1. Adsorption of Surfactant Molecules
3.2.2. Adsorption of Surface Hydrated Ions
3.2.3. Adsorption of Charged Particle
3.2.4. Adsorption of Ionic Liquids
3.3. Interfacial Phase Structural Transformation
3.3.1. Phase Structure Transformation of Water Molecules
3.3.2. Phase Structure Transformation of Liquid Crystal Molecules
3.3.3. Phase Structure Transformation of Interfacial Polymer
3.4. Mechanism Summary
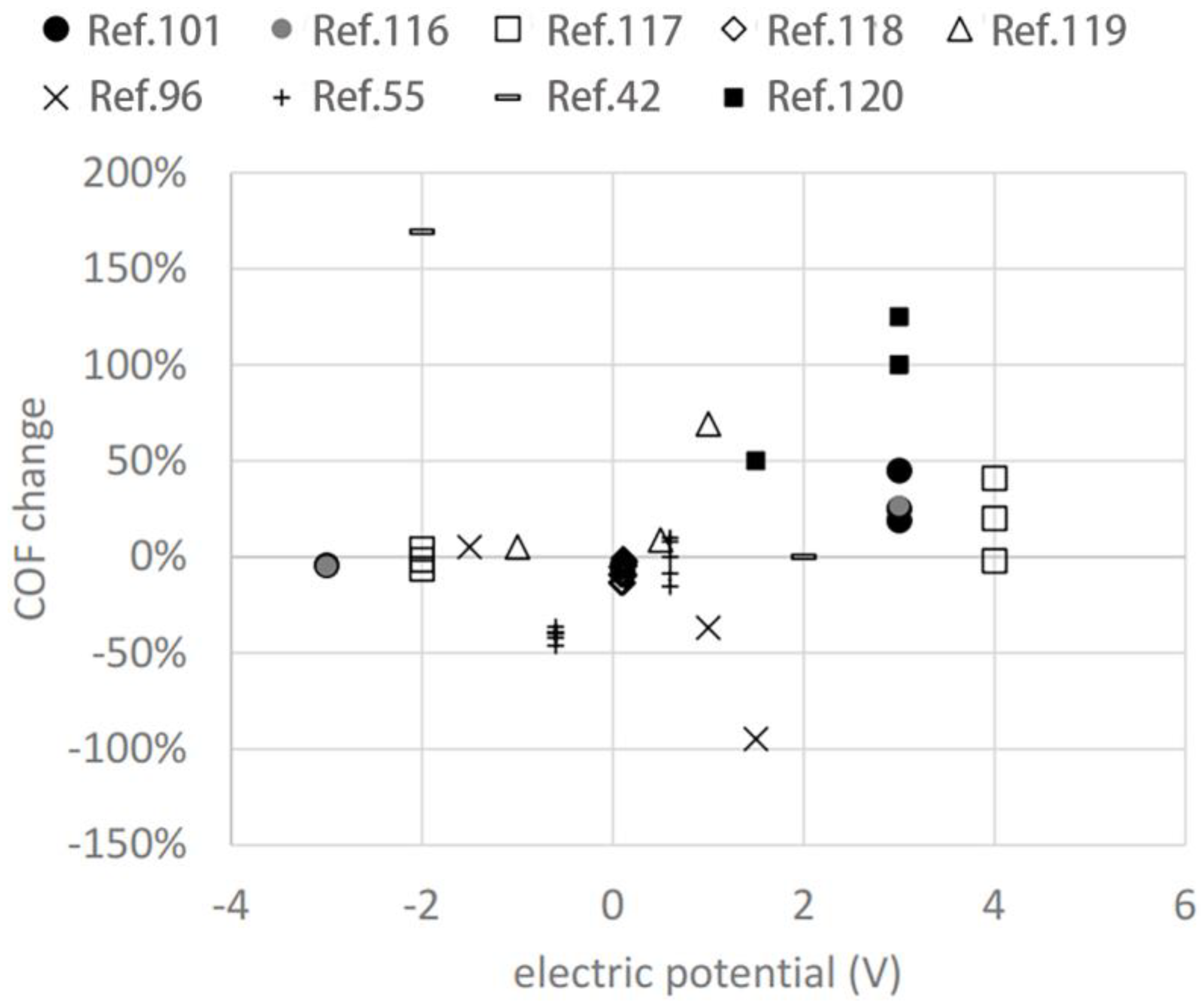
4. Influencing Factors of Electro/Potential-Controlled Friction
5. Application Prospect
6. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Luo, J.; Liu, M.; Ma, L. Origin of friction and the new frictionless technology—Superlubricity: Advancements and future outlook. Nano Energy 2021, 86, 106092. [Google Scholar] [CrossRef]
- Wen, S.; Huang, P. Principles of Tribology; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Persson, B.N.J.; Sivebaek, I.M.; Samoilov, V.N.; Zhao, K.; Volokitin, A.I.; Zhang, Z. On the origin of Amonton’s friction law. J. Phys. Condens. Matter 2008, 20, 395006. [Google Scholar] [CrossRef]
- Ge, X.; Li, J.; Luo, J. Macroscale superlubricity achieved with various liquid molecules: A review. Front. Mech. Eng. 2019, 5, 2. [Google Scholar] [CrossRef]
- Wei, Y.; Li, S.; Huang, H.; Ding, C.; Wang, X. Experimental investigation on synergetic effects of micro grooves and WSe2 in sliding contact. Lubricants 2022, 10, 208. [Google Scholar] [CrossRef]
- Rahman, M.H.; Warneke, H.; Webbert, H.; Rodriguez, J.; Austin, E.; Tokunaga, K.; Rajak, D.K.; Menezes, P.L. Water-based lubricants: Development, properties, and performances. Lubricants 2021, 9, 73. [Google Scholar] [CrossRef]
- Marian, M.; Tremmel, S. Current trends and applications of machine learning in tribology-a review. Lubricants 2021, 9, 86. [Google Scholar] [CrossRef]
- Vazirisereshk, M.R.; Martini, A.; Strubbe, D.A.; Baykara, M.Z. Solid lubrication with MoS2: A review. Lubricants 2019, 7, 57. [Google Scholar] [CrossRef]
- Krim, J. Controlling friction with external electric or magnetic fields: 25 examples. Front. Mech. Eng. 2019, 5, 22. [Google Scholar] [CrossRef]
- Bhel, H.P. Tribology in electrical environments. Lubr. Sci. 2001, 13, 359–369. [Google Scholar] [CrossRef]
- Yang, X.; Meng, Y.; Tian, Y. Potential-controlled boundary lubrication of stainless steels in non-aqueous sodium dodecyl sulfate solutions. Tribol. Lett. 2014, 53, 17–26. [Google Scholar] [CrossRef]
- He, S.; Meng, Y.; Tian, Y. Correlation between adsorption/desorption of surfactant and change in friction of stainless steel in aqueous solutions under different electrode potentials. Tribol. Lett. 2011, 41, 485–494. [Google Scholar] [CrossRef]
- Li, R.; Zhang, X.R.; Zeng, C.; Yang, P.A.; Shou, M.J.; Zhu, D.; Lee, C.H. Magneto-controlled friction behaviors of magnetorheological elastomers with cosine-shaped surface structure. Tribol. Int. 2023, 186, 108658. [Google Scholar] [CrossRef]
- Kumar, S.; Sehgal, R.; Wani, M.F.; Sharma, M.D.; Ziyamukhamedova, U.; Dar, T.A. Next-generation ecofriendly mr fluid: Hybrid GO/ Fe2O3 encapsulated carbonyl iron microparticles with improved magnetorheological, tribological, and corrosion resistance properties. Carbon 2023, 214, 118331. [Google Scholar] [CrossRef]
- Ahamed, R.; Choi, S.B.; Ferdaus, M.M. A state of art on magneto-rheological materials and their potential applications. J. Intell. Mater. Syst. Struct. 2018, 29, 2051–2095. [Google Scholar] [CrossRef]
- Babuska, T.F.; Pitenis, A.A.; Jones, M.R.; Nation, B.L.; Sawyer, W.G.; Argibay, N. Temperature-dependent friction and wear behavior of PTFE and MoS2. Tribol. Lett. 2016, 63, 15. [Google Scholar] [CrossRef]
- Wu, Y.; Cai, M.; Pei, X.; Liang, Y.; Zhou, F. Switching friction with thermal-responsive gels. Macromol. Rapid. Commun. 2013, 34, 1785–1790. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Pei, X.; Wang, X.; Liang, Y.; Liu, W.; Zhou, F. Biomimicking lubrication superior to fish skin using responsive hydrogels. NPG Asia Mater. 2014, 6, e136. [Google Scholar] [CrossRef]
- De Wijn, A.S.; Fasolino, A.; Filippov, A.E.; Urbakh, M. Nanoscopic friction under electrochemical control. Phys. Rev. Lett. 2014, 112, 055502. [Google Scholar] [CrossRef]
- Sweeney, J.; Hausen, F.; Hayes, R.; Webber, G.B.; Endres, F.; Rutland, M.W.; Bennewitz, R.; Atkin, R. Control of nanoscale friction on gold in an ionic liquid by a potential-dependent ionic lubricant layer. Phys. Rev. Lett. 2012, 109, 155502. [Google Scholar] [CrossRef]
- Zhang, Y.B. Boundary lubrication—An important lubrication in the following time. J. Mol. Liq. 2006, 128, 56–59. [Google Scholar] [CrossRef]
- Stephan, S.; Schmitt, S.; Hasse, H.; Urbassek, H.M. Molecular dynamics simulation of the stribeck curve: Boundary lubrication, mixed lubrication, and hydrodynamic lubrication on the atomistic level. Friction 2023, 11, 2342–2366. [Google Scholar] [CrossRef]
- Szeri, A.Z. Fluid Film Lubrication: Theory and Design; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Wong, P.L.; Bullough, W.A.; Feng, C.; Lingard, S. Tribological performance of a magneto-rheological suspension. Wear 2001, 247, 33–40. [Google Scholar] [CrossRef]
- Feng, Y.P.; Su, J.S.; Cheng, H.B. Non-newtonian hydrodynamic modeling of electrorheological finishing feature based on wheel-like finishing tool. J. Intell. Mater. Syst. Struct. 2017, 28, 1407–1414. [Google Scholar] [CrossRef]
- Melrose, J.R.; Itoh, S.; Ball, R.C. Simulations of electrorheological fluids with hydrodynamic lubrication. Int. J. Mod. Phys. B 1996, 10, 3211–3217. [Google Scholar] [CrossRef]
- Kim, P.; Lee, J.I.; Seok, J. Analysis of a viscoplastic flow with field-dependent yield stress and wall slip boundary conditions for a magnetorheological (MR) fluid. J. Non-Newton. Fluid Mech. 2014, 204, 72–86. [Google Scholar] [CrossRef]
- Wang, Y.S.; Wang, Q.J.; Lin, C.; Shi, F.H. Development of a set of stribeck curves for conformal contacts of rough surfaces. Tribol. Trans. 2006, 49, 526–535. [Google Scholar] [CrossRef]
- Bowden, F.P.; Young, L. Influence of interfacial potential on friction and surface damage. Res. J. Sci. Appl. 1950, 3, 235–237. [Google Scholar]
- Drummond, C. Electric-field-induced friction reduction and control. Phys. Rev. Lett. 2012, 109, 154302. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.; Li, X.; Li, W.; Tian, Y.; Meng, Y. On-line feedback control of sliding friction of metals lubricated by adsorbed boundary SDS films. Lubricants 2022, 10, 148. [Google Scholar] [CrossRef]
- Tivony, R.; Zhang, Y.; Klein, J. Modulating interfacial energy dissipation via potential-controlled ion trapping. J. Phys. Chem. C 2021, 125, 3616–3622. [Google Scholar] [CrossRef]
- Li, S.; Bai, P.; Li, Y.; Pesika, N.S.; Meng, Y.; Ma, L.; Tian, Y. Quantification/mechanism of interfacial interaction modulated by electric potential in aqueous salt solution. Friction 2021, 9, 513–523. [Google Scholar] [CrossRef]
- Li, S.; Bai, P.; Li, Y.; Chen, C.; Meng, Y.; Tian, Y. Electric potential-controlled interfacial interaction between gold and hydrophilic/hydrophobic surfaces in aqueous solutions. J. Phys. Chem. C 2018, 122, 22549–22555. [Google Scholar] [CrossRef]
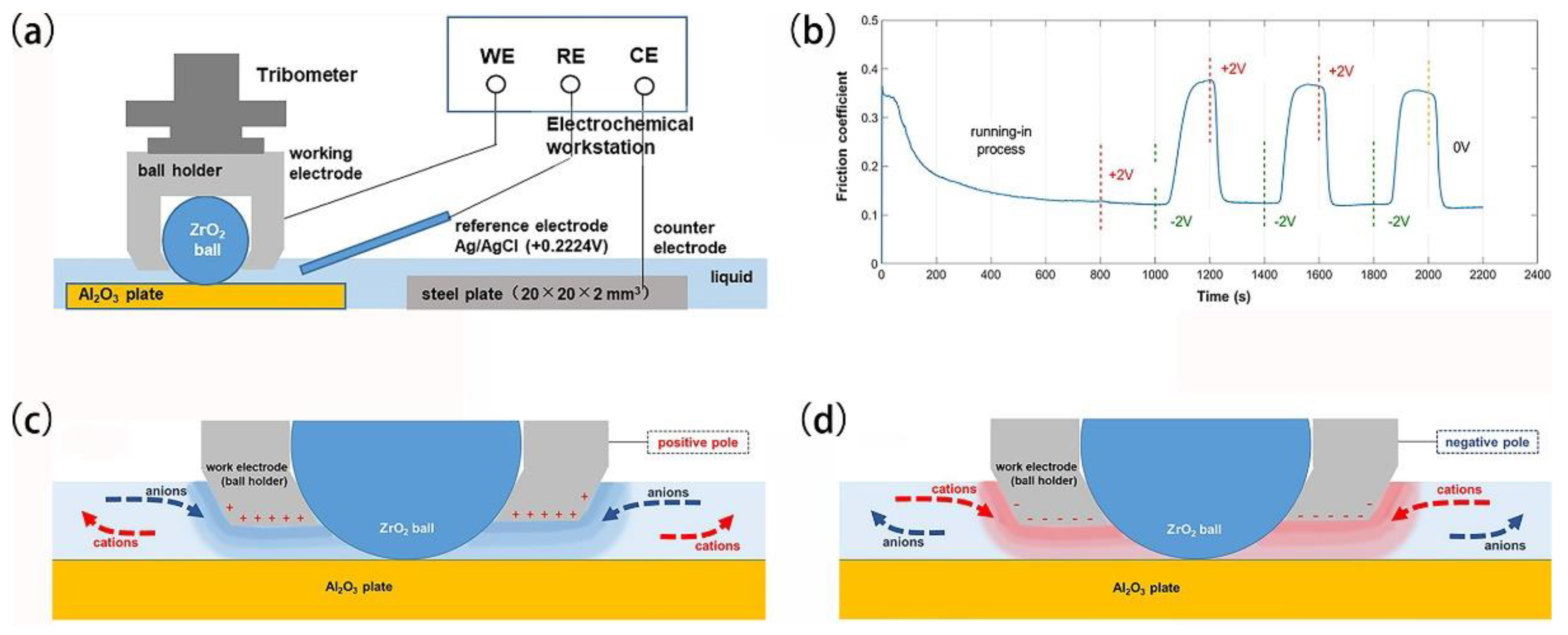
- Cao, H.; Meng, Y. Electrochemical effect on boundary lubrication of zddp additive blended in propylene carbonate / diethyl succinate. Tribol. Int. 2018, 126, 229–239. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Kelsall, G.H.; Spikes, H.A. The influence of electrochemical potentials on the friction and wear of iron and iron-oxides in aqueous systems. Tribol. Trans. 1994, 37, 811–819. [Google Scholar] [CrossRef]
- Xie, G.; Guo, D.; Luo, J. Lubrication under charged conditions. Tribol. Int. 2015, 84, 22–35. [Google Scholar] [CrossRef]
- Spikes, H.A. Triboelectrochemistry: Influence of applied electrical potentials on friction and wear of lubricated contacts. Tribol. Lett. 2020, 68, 90. [Google Scholar] [CrossRef]
- Seed, C.M.; Acharya, B.; Krim, J. Qcm study of tribotronic control in ionic liquids and nanoparticle suspensions. Tribol. Lett. 2021, 69. [Google Scholar] [CrossRef]
- Tysoe, W.T.; Spencer, N.D. Rapid testing of tribotronic materials. Tribol. Lubr. Technol. 2021, 77, 76–77. [Google Scholar]
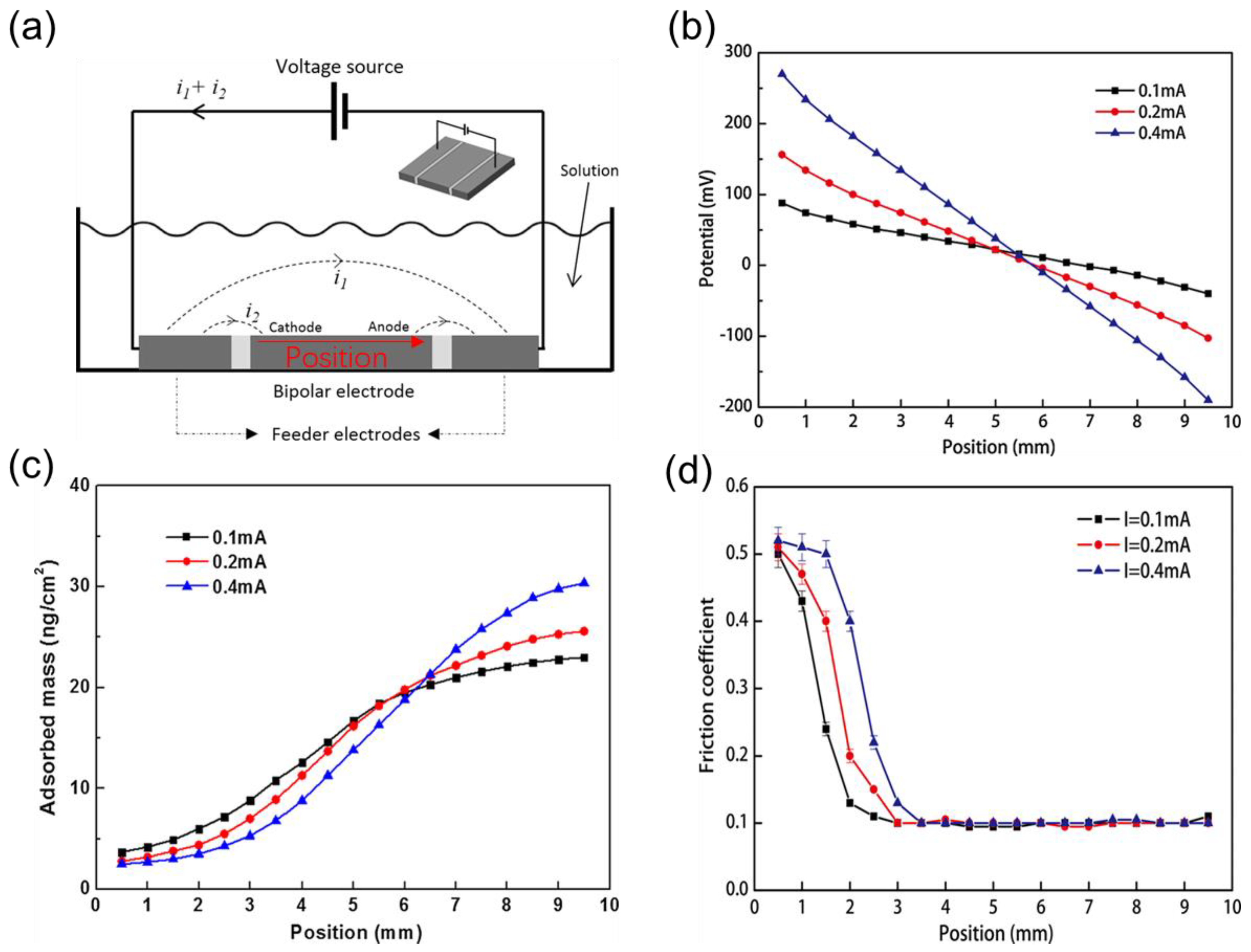
- Zhang, J.; Meng, Y.; Yu, X. Control of friction distribution on stainless steel surface in sodium dodecyl sulfate aqueous solution by bipolar electrochemistry. Tribol. Lett. 2015, 59, 43. [Google Scholar] [CrossRef]
- Liu, C.; Fang, J.; Wen, X.; Tian, Y.; Meng, Y. Active control of boundary lubrication of ceramic tribo-pairs in sodium dodecyl sulfate aqueous solutions. Tribol. Lett. 2021, 69, 144. [Google Scholar] [CrossRef]
- Zhu, Y.; Ogano, S.; Kelsall, G.; Spikes, H.A. The study of lubricant additive reactions using non-aqueous electrochemistry. Tribol. Trans. 2000, 43, 175–186. [Google Scholar] [CrossRef]
- Xiaoyin, X. Application of Electrochemical Techniques to Tribology; Imperial College London (University of London): London, UK, 2004. [Google Scholar]
- Edison, O. Improvement in Telegraph Apparatus. U.S. Patent 158,787, 9 January 1875. [Google Scholar]
- American, N.J.S. The electromotograph. A New Discov. Telegr. 1874, 31, 145. [Google Scholar]
- Pearson, B.R.; Brook, P.A.; Waterhouse, R.B. Influence of electrochemical potential on the wear of metals, particularly nickel. Tribol. Int. 1988, 21, 191–197. [Google Scholar] [CrossRef]
- Kautek, W.; Dieluweit, S.; Sahre, M. Combined scanning force microscopy and electrochemical quartz microbalance in-situ investigation of specific adsorption and phase change processes at the silver/halogenide interface. J. Phys. Chem. B 1997, 101, 2709–2715. [Google Scholar] [CrossRef]
- Labuda, A.; Hausen, F.; Gosvami, N.N.; Grütter, P.H.; Lennox, R.B.; Bennewitz, R. Switching atomic friction by electrochemical oxidation. Langmuir 2011, 27, 2561–2566. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.X.; Tian, Y.; Meng, Y.G. A chemical potential equation for modeling triboelectrochemical reactions on solid-liquid interfaces. Front. Chem. 2021, 9, 650880. [Google Scholar] [CrossRef] [PubMed]
- Brandon, N.P.; Bonanos, N.; Fogarty, P.O.; Mahmood, M.N.; Moore, A.J.; Wood, R.J.K. Influence of potential on the friction and wear of mild-steel in a model aqueous lubricant. J. Appl. Electrochem. 1993, 23, 456–462. [Google Scholar] [CrossRef]
- Ismail, M.N.F.; Harvey, T.J.; Wharton, J.A.; Wood, R.J.K.; Humphreys, A. Surface potential effects on friction and abrasion of sliding contacts lubricated by aqueous solutions. Wear 2009, 267, 1978–1986. [Google Scholar] [CrossRef]
- Su, Y.Y. Electrochemical study of the interaction between fatty acid and oxidized copper. Tribol. Int. 1997, 30, 423–428. [Google Scholar] [CrossRef]
- Su, Y.Y. Enhanced boundary lubrication by potential control during copper wire drawing. Wear 1997, 210, 165–170. [Google Scholar] [CrossRef]
- Yang, X.; Meng, Y.; Tian, Y. Effect of imidazolium ionic liquid additives on lubrication performance of propylene carbonate under different electrical potentials. Tribol. Lett. 2014, 56, 161–169. [Google Scholar] [CrossRef]
- Gosvami, N.N.; Bares, J.A.; Mangolini, F.; Konicek, A.R.; Yablon, D.G.; Carpick, R.W. Mechanisms of antiwear tribofilm growth revealed in situ by single-asperity sliding contacts. Science 2015, 348, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, W.; Ouyang, C.; Tian, Y.; Meng, Y. Voltage-assisted tribofilm formation of sulfur- and phosphorus-free organic molybdenum additive on bearing steel surfaces in industrial base oils. Tribol. Lett. 2022, 70, 19. [Google Scholar] [CrossRef]
- Liu, C.; Meng, Y.; Tian, Y. Potential-controlled boundary lubrication using mos2 additives in diethyl succinate. Tribol. Lett. 2020, 68, 72. [Google Scholar] [CrossRef]
- Willermet, P.A.; Dailey, D.P.; Carter, R.O.; Schmitz, P.J.; Zhu, W. Mechanism of formation of antiwear films from zinc dialkyldithiophosphates. Tribol. Int. 1995, 28, 177–187. [Google Scholar] [CrossRef]
- Xu, X.; Spikes, H. Study of zinc dialkyldithiophosphate in di-ethylhexyl sebacate using electrochemical techniques. Tribol. Lett. 2007, 25, 141–148. [Google Scholar] [CrossRef]
- Wang, S.S.; Maheswari, S.P.; Tung, S.C. The nature of electrochemical reactions between several zinc organodithiophosphate antiwear additives and cast iron surfaces. Tribol. Trans. 1989, 32, 91–99. [Google Scholar] [CrossRef]
- Tung, S.C.; Wang, S.S. In-situ electro-charging for friction reduction and wear resistant film formation. Tribol. Trans. 1991, 34, 479–488. [Google Scholar] [CrossRef]
- Wang, S.S.; Tung, S.C. A reaction mechanism for producing low-friction iron phosphate coatings. Tribol. Trans. 1991, 34, 45–50. [Google Scholar] [CrossRef]
- Xu, X.; Brandon, N.; Spikes, H. Study of zinc dialkyldithiophosphate using electrochemical techniques. In Tribology Series; Dowson, D., Priest, M., Dalmaz, G., Lubrecht, A.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2002; pp. 175–181. [Google Scholar]
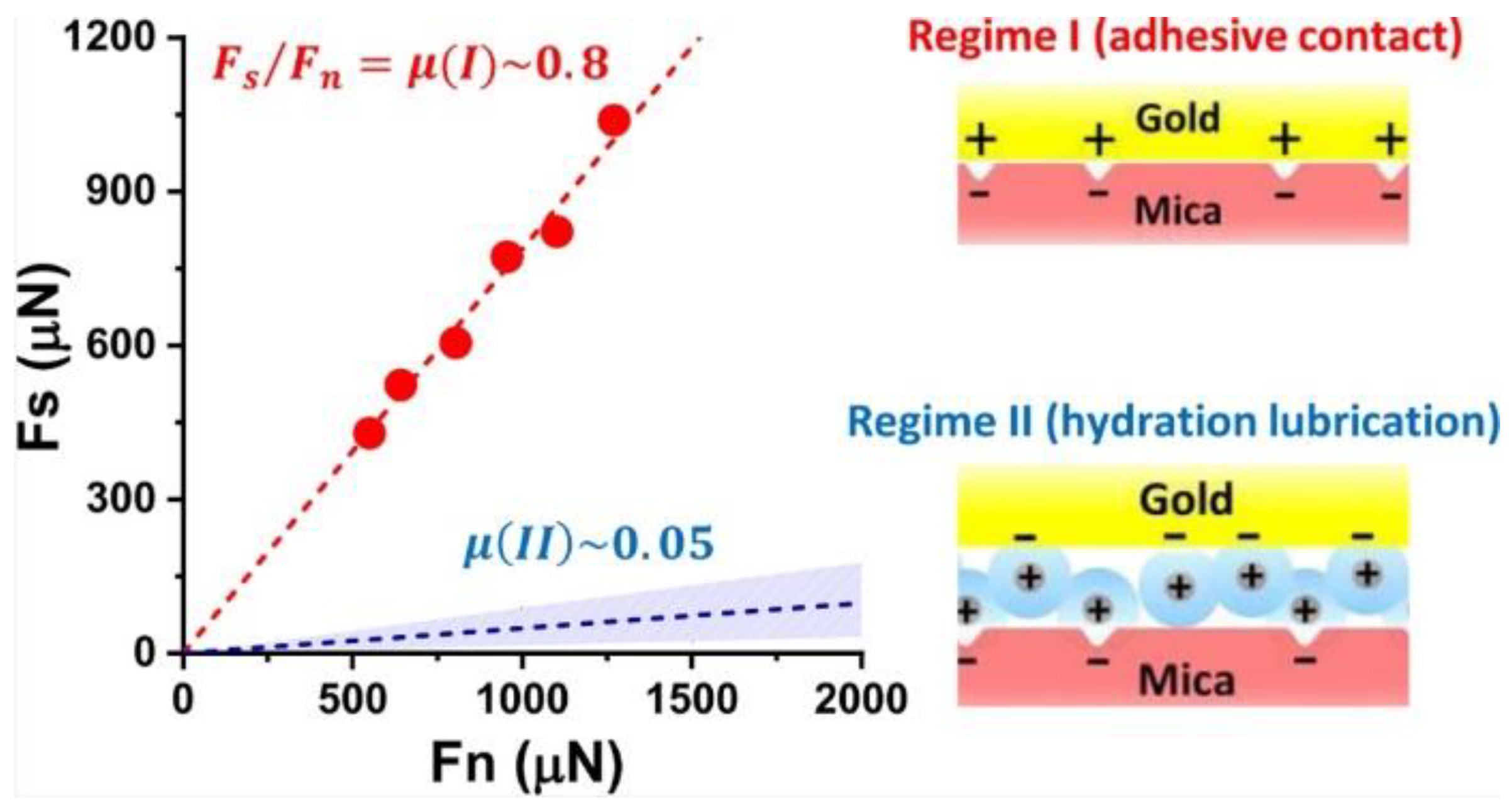
- Klein, J. Hydration lubrication. Friction 2013, 1, 1–23. [Google Scholar] [CrossRef]
- He, S.; Meng, Y.; Tian, Y. Effects of the concentration of sodium lauryl sulfate solution and NaCl additive on the potential-controlled friction. In Advanced Tribology; Luo, J., Meng, Y., Shao, T., Zhao, Q., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 408–412. [Google Scholar]
- Brandon, N.P.; Bonanos, N.; Fogarty, P.O.; Mahmood, M.N. The effect of interfacial potential on friction in a model aqueous lubricant. J. Electrochem. Soc. 1992, 139, 3489–3492. [Google Scholar] [CrossRef]
- He, S.; Meng, Y.; Tian, Y.; Zuo, Y. Response characteristics of the potential-controlled friction of ZrO2/stainless steel tribopairs in sodium dodecyl sulfate aqueous solutions. Tribol. Lett. 2010, 38, 169–178. [Google Scholar] [CrossRef]
- Zhang, J.; Meng, Y.; Tian, Y.; Zhang, X. Effect of concentration and addition of ions on the adsorption of sodium dodecyl sulfate on stainless steel surface in aqueous solutions. Colloids Surf. A 2015, 484, 408–415. [Google Scholar] [CrossRef]
- Gao, T.; Li, J.; Wang, W.; Luo, J. Extremely low friction on gold surface with surfactant molecules induced by surface potential. Friction 2022, 11, 513–523. [Google Scholar] [CrossRef]
- Jacob, N. Israelachvili. Intermolecular and Surface Forces, 3rd ed.; Academic Press: Burlington, MA, USA, 2011; pp. 59–65. [Google Scholar]
- Han, T.; Zhang, S.; Zhang, C. Unlocking the secrets behind liquid superlubricity: A state-of-the-art review on phenomena and mechanisms. Friction 2022, 10, 1137–1165. [Google Scholar] [CrossRef]
- Han, T.; Zhao, M.; Sun, C.; Zhao, R.; Xu, W.; Zhang, S.; Zhang, C. Macroscale superlubricity of hydrated anions in the boundary lubrication regime. ACS Appl. Mater. Interfaces 2023, 15, 42094–42103. [Google Scholar] [CrossRef] [PubMed]
- Han, T.Y.; Cao, W.; Xu, Z.; Adibnia, V.; Olgiati, M.; Valtiner, M.; Ma, L.; Zhang, C.; Ma, M.; Luo, J.; et al. Hydration layer structure modulates superlubrication by trivalent La3+ electrolytes. Sci. Adv. 2023, 9, eadf3902. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Li, J.; Yi, S.; Luo, J. Potential-dependent friction on a graphitic surface in ionic solution. J. Phys. Chem. C 2020, 124, 23745–23751. [Google Scholar] [CrossRef]
- Noguchi, H.; Okada, T.; Uosaki, K. Sfg study on potential-dependent structure of water at pt electrode/electrolyte solution interface. Electrochim. Acta 2008, 53, 6841–6844. [Google Scholar] [CrossRef]
- Velasco-Velez, J.J.; Wu, C.H.; Pascal, T.A.; Wan, L.W.F.; Guo, J.H.; Prendergast, D.; Salmeron, M. The structure of interfacial water on gold electrodes studied by x-ray absorption spectroscopy. Science 2014, 346, 831–834. [Google Scholar] [CrossRef]
- Guriyanova, S.; Mairanovsky, V.G.; Bonaccurso, E. Superviscosity and electroviscous effects at an electrode/aqueous electrolyte interface: An atomic force microscope study. J. Colloid. Interface Sci. 2011, 360, 800–804. [Google Scholar] [CrossRef] [PubMed]
- Plausinaitis, D.; Pulmanas, A.; Kubilius, V.; Raudonis, R.; Daujotis, V. Properties of an interfacial solution layer at gold electrode surface in perchlorate and chloride solutions: Piezoelectric resonator and drag force study. Electrochim. Acta 2014, 121, 278–284. [Google Scholar] [CrossRef]
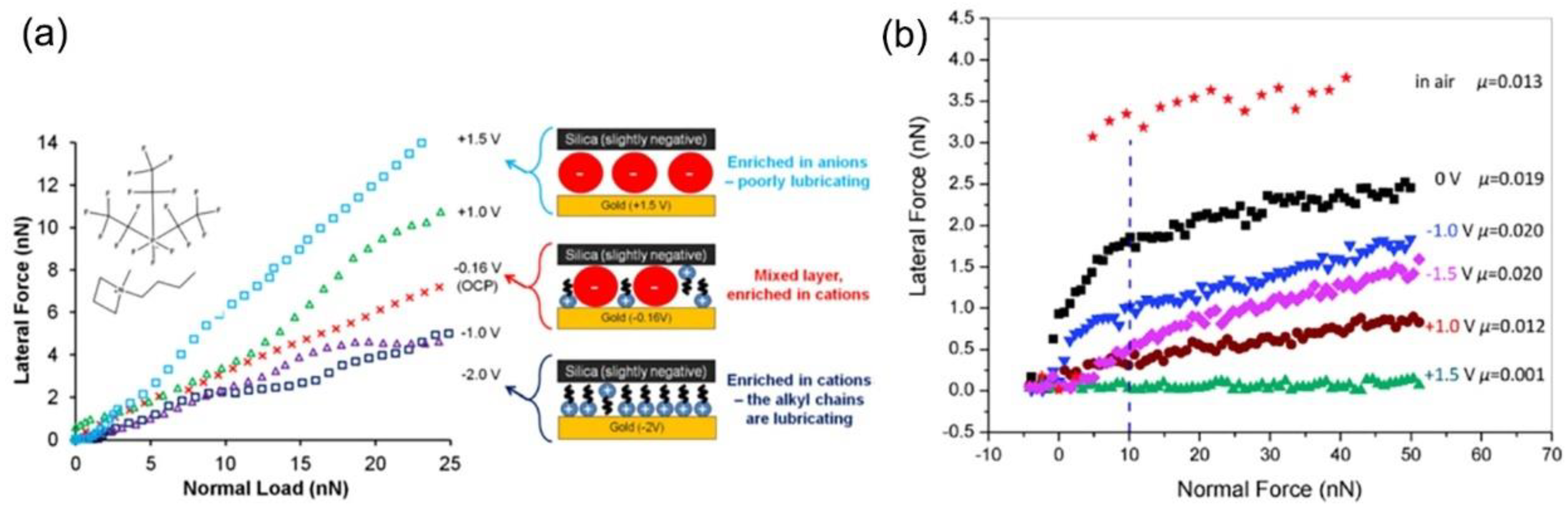
- Pashazanusi, L.; Oguntoye, M.; Oak, S.; Albert, J.N.L.; Pratt, L.R.; Pesika, N.S. Anomalous potential-dependent friction on Au(111) measured by afm. Langmuir 2018, 34, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Pashazanusi, L.; Kristiansen, K.; Li, S.; Tian, Y.; Pesika, N.S. Role of interfacial water and applied potential on friction at Au(111) surfaces. Front. Mech. Eng. 2019, 5, 39. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Bai, P.; Meng, Y.; Tian, Y. Potential-dependent interfacial frictional behavior between charged microspheres and gold in aqueous solutions. J. Phys. Chem. C 2022, 126, 4555–4562. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Chen, X.; Luo, J. Fluorinated graphene: A promising macroscale solid lubricant under various environments. ACS Appl. Mater. Interfaces 2019, 11, 40470–40480. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Friedman, O.; Li, Y.; Li, S.; Tian, Y.; Golan, Y.; Meng, Y. Electric response of cus nanoparticle lubricant additives: The effect of crystalline and amorphous octadecylamine surfactant capping layers. Langmuir 2019, 35, 15825–15833. [Google Scholar] [CrossRef] [PubMed]
- Mou, Z.; Peng, J.; Yan, R.; Yang, Q.; Zhao, B.; Xiao, D. Electrical stimulation enables dynamic regulation of the tribological behaviors of polyelectrolyte-modified carbon dots. Carbon 2023, 203, 11–20. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, L.; Zhang, G.; Wang, D.; Wang, T.; Wang, Q. High lubricity and electrical responsiveness of solvent-free ionic sio2 nanofluids. J. Mater. Chem. A 2018, 6, 2817–2827. [Google Scholar] [CrossRef]
- Seed, C.M.; Acharya, B.; Nunn, N.; Smirnov, A.I.; Krim, J. Tribotronic and electrochemical properties of platinum–nanofluid interfaces formed by aqueous suspensions of 5 and 40 nm tio2 nanoparticles. J. Chem. Phys. 2023, 159, 114705. [Google Scholar] [CrossRef]
- Seed, C.M.; Acharya, B.; Perelygin, V.; Smirnov, A.I.; Krim, J. Tribotronic control and cyclic voltammetry of platinum interfaces with metal oxide nanofluids. Appl. Surf. Sci. 2021, 566, 150675. [Google Scholar] [CrossRef]
- Hayes, R.; Warr, G.G.; Atkin, R. Structure and nanostructure in ionic liquids. Chem. Rev. 2015, 115, 6357–6426. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Liu, X.; Dong, H.; Zhang, X.; Zhang, S. Multiscale studies on ionic liquids. Chem. Rev. 2017, 117, 6636–6695. [Google Scholar] [CrossRef] [PubMed]
- Rakov, D.A.; Chen, F.F.; Ferdousi, S.A.; Li, H.; Pathirana, T.; Simonov, A.N.; Howlett, P.C.; Atkin, R.; Forsyth, M. Engineering high-energy-density sodium battery anodes for improved cycling with superconcentrated ionic-liquid electrolytes. Nat. Mater. 2020, 19, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, M.; Porcarelli, L.; Wang, X.; Goujon, N.; Mecerreyes, D. Innovative electrolytes based on ionic liquids and polymers for next-generation solid-state batteries. Acc. Chem. Res. 2019, 52, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Lhermerout, R.; Diederichs, C.; Perkin, S. Are ionic liquids good boundary lubricants? A molecular perspective. Lubricants 2018, 6, 9. [Google Scholar] [CrossRef]
- Di Lecce, S.; Kornyshev, A.A.; Urbakh, M.; Bresme, F. Electrotunable lubrication with ionic liquids: The effects of cation chain length and substrate polarity. ACS Appl. Mater. Interfaces 2020, 12, 4105–4113. [Google Scholar] [CrossRef]
- Perkin, S.; Albrecht, T.; Klein, J. Layering and shear properties of an ionic liquid, 1-ethyl-3-methylimidazolium ethylsulfate, confined to nano-films between mica surfaces. Phys. Chem. Chem. Phys. 2010, 12, 1243–1247. [Google Scholar] [CrossRef]
- Li, H.; Wood, R.J.; Rutland, M.W.; Atkin, R. An ionic liquid lubricant enables superlubricity to be “switched on” in situ using an electrical potential. Chem. Commun. 2014, 50, 4368. [Google Scholar] [CrossRef]
- Li, H.; Rutland, M.W.; Watanabe, M.; Atkin, R. Boundary layer friction of solvate ionic liquids as a function of potential. Faraday Discuss 2017, 199, 311–322. [Google Scholar] [CrossRef]
- Kong, L.L.; Huang, W.; Wang, X.L. Ionic liquid lubrication at electrified interfaces. J. Phys. D Appl. Phys. 2016, 49. [Google Scholar] [CrossRef]
- Cooper, P.K.; Li, H.; Rutland, M.W.; Webber, G.B.; Atkin, R. Tribotronic control of friction in oil-based lubricants with ionic liquid additives. Phys. Chem. Chem. Phys. 2016, 18, 23657–23662. [Google Scholar] [CrossRef]
- Pilkington, G.A.; Oleshkevych, A.; Pedraz, P.; Watanabe, S.; Radiom, M.; Reddy, A.B.; Vorobiev, A.; Glavatskin, S.; Rutland, M. Electroresponsive structuring and friction of a non-halogenated ionic liquid in a polar solvent: Effect of concentration. Phys. Chem. Chem. Phys. 2020, 22, 19162–19171. [Google Scholar] [CrossRef]
- Gatti, F.; Amann, T.; Kailer, A.; Baltes, N.; Ruhe, J.; Gumbsch, P. Towards programmable friction: Control of lubrication with ionic liquid mixtures by automated electrical regulation. Sci. Rep. 2020, 10, 17634. [Google Scholar] [CrossRef] [PubMed]
- Antognozzi, M.; Humphris, A.D.L.; Miles, M.J. Observation of molecular layering in a confined water film and study of the layers viscoelastic properties. Appl. Phys. Lett. 2001, 78, 300–302. [Google Scholar] [CrossRef]
- Gao, Y.; Xue, B.; Ma, L.; Luo, J. Effect of liquid crystal molecular orientation controlled by an electric field on friction. Tribol. Int. 2017, 115, 477–482. [Google Scholar] [CrossRef]
- Amann, T.; Kailer, A. Relationship between ultralow friction of mesogenic-like fluids and their lateral chain length. Tribol. Lett. 2011, 41, 121–129. [Google Scholar] [CrossRef]
- Amann, T.; Dold, C.; Kailer, A. Rheological characterization of ionic liquids and ionic liquid crystals with promising tribological performance. Soft Matter 2012, 8, 9840–9846. [Google Scholar] [CrossRef]
- Olivero, D.; Evangelista, L.R.; Barbero, G. External electric-field effect on nematic anchoring energy. Phys. Rev. E 2002, 65, 031721. [Google Scholar] [CrossRef]
- Nastishin, Y.A.; Polak, R.D.; Shiyanovskii, S.V.; Bodnar, V.H.; Lavrentovich, O.D. Nematic polar anchoring strength measured by electric field techniques. J. Appl. Phys. 1999, 86, 4199–4213. [Google Scholar] [CrossRef]
- Mori, S.; Iwata, H. Relationship between tribological performance of liquid crystals and their molecular structure. Tribol. Int. 1996, 29, 35–39. [Google Scholar] [CrossRef]
- Kimura, Y.; Nakano, K.; Kato, T.; Morishita, S. Control of friction coefficient by applying electric fields across liquid crystal boundary films. Wear 1994, 175, 143–149. [Google Scholar] [CrossRef]
- Morishita, S.; Nakano, K.; Kimura, Y. Electroviscous effect of nematic liquid crystals. Tribol. Int. 1993, 26, 399–403. [Google Scholar] [CrossRef]
- Manzato, C.; Foster, A.S.; Alava, M.J.; Laurson, L. Friction control with nematic lubricants via external fields. Phys. Rev. E 2015, 91, 012504. [Google Scholar] [CrossRef] [PubMed]
- Klein, J. Shear, friction, and lubrication forces between polymer-bearing surfaces. Annu. Rev. Mater. Sci. 1996, 26, 581–612. [Google Scholar] [CrossRef]
- Zeng, H.; Zhang, Y.; Mao, S.; Nakajima, H.; Uchiyama, K. A reversibly electro-controllable polymer brush for electro-switchable friction. J. Mater. Chem. C 2017, 5, 5877–5881. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Jones, S.; Segalman, R.; Warr, G.G.; Atkin, R. Interfacial nanostructure and friction of a polymeric ionic liquid-ionic liquid mixture as a function of potential at Au(1 1 1) electrode interface. J. Colloid. Interface Sci. 2022, 606, 1170–1178. [Google Scholar] [CrossRef]
- Gatti, F.J.; Cai, W.H.; Herzog, R.; Gharavian, A.; Kailer, A.; Baltes, N.; Rabenecker, P.; Mörchel, P.; Balzer, B.N.; Amann, T.; et al. Investigation of programmable friction with ionic liquid mixtures at the nano- and macroscales. Lubricants 2023, 11, 376. [Google Scholar] [CrossRef]
- Gatti, F.; Amann, T.; Kailer, A.; Abicht, J.; Rabenecker, P.; Baltes, N.; Rühe, J. Makroskopische reibwertsteuerung durch elektrochemische potentiale mit ionischen flüssigkeiten: Makroskopische reibwertsteuerung durch elektrochemische potentiale mit ionischen flüssigkeiten. Tribol. Und Schmier. 2019, 66, 51–57. [Google Scholar]
- Kawada, S.; Ogawa, S.; Sasaki, S.; Miyatake, M. Friction control by applying electric potential under lubrication with ionic liquids. Tribol. Online 2019, 14, 71–77. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, G.; Li, G.; Zhao, F.; Zhang, L.; Guo, F.; Zhang, G. Solvent-free ionic silica nanofluids: Smart lubrication materials exhibiting remarkable responsiveness to weak electrical stimuli. Chem. Eng. J. 2020, 383, 123202. [Google Scholar] [CrossRef]
- Michalec, M.; Svoboda, P.; Krupka, I.; Hartl, M.; Vencl, A. Investigation of the tribological performance of ionic liquids in non-conformal ehl contacts under electric field activation. Friction 2020, 8, 982–994. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, J.; Liu, M.; Wang, F.; Zheng, Y.; Cheng, Y.; Liu, Z. Relationship between viscosity and resistance of oil film: A new way to investigate the controllable friction between charged interfaces lubricated by ionic lubricating oil. Adv. Mater. Interfaces 2022, 9, 2200229. [Google Scholar] [CrossRef]
- Ulrich, C.; Andersson, O.; Nyholm, L.; Björefors, F. Formation of molecular gradients on bipolar electrodes. Angew. Chem. Int. Ed. 2008, 47, 3034–3036. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, C.; Andersson, O.; Nyholm, L.; Björefors, F. Potential and current density distributions at electrodes intended for bipolar patterning. Anal. Chem. 2009, 81, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.; Ying, C.; Zhao, F. The nature of friction: A critical assessment. Friction 2014, 2, 1–26. [Google Scholar] [CrossRef]
- Kelsall, G.H.; Zhu, Y.; Spikes, H.A. Electrochemical effects on friction between metal oxide surfaces in aqueous solutions. J. Chem. Soc. Faraday Trans. 1993, 89, 267–272. [Google Scholar] [CrossRef]
- Alkan, S.; Gök, M.S. Effect of sliding wear and electrochemical potential on tribocorrosion behaviour of AISI 316 stainless steel in seawater. Eng. Sci. Technol. 2021, 24, 524–532. [Google Scholar] [CrossRef]
- Bonanos, N.; Brandon, N.P.; Mahmood, M.N.; Brown, D. The effect of imposed electrical current on torque release at the metal-mudcake interface. J. Appl. Electrochem. 1996, 26, 977–980. [Google Scholar] [CrossRef]
- Brandon, N.P.; Wood, R.J.K. The influence of interfacial potential on friction and wear in an aqueous drilling mud. Wear 1993, 170, 33–38. [Google Scholar] [CrossRef]
- Sun, Y.; Song, C.; Liu, Z.; Li, J.; Wang, L.; Sun, C.; Zhang, Y. Tribological and conductive behavior of Cu/Cu rolling current-carrying pairs in a water environment. Tribol. Int. 2020, 143, 106055. [Google Scholar] [CrossRef]
- Sun, Y.; Song, C.; Liu, Z.; Li, J.; Sun, Y.; Shangguan, B.; Zhang, Y. Effect of relative humidity on the tribological/conductive properties of cu/cu rolling contact pairs. Wear 2019, 436–437, 203023. [Google Scholar] [CrossRef]
- Hu, B.; Meng, Y.; Wen, S. A preliminary experimental study on voltage-controlled friction clutch. Tribology 2004, 24, 46–50. [Google Scholar]
- Wenjie, Z. Research progress in tribo-electrochemistry and tribo-electrochemical polishing. Tribology 2006, 26, 92–96. [Google Scholar]
- Li, C.H.; Wang, R.J.; Seiler, J.; Bhat, I. Electro-chemical mechanical polishing of silicon carbide. Mater. Sci. Forum. 2004, 33, 481–486. [Google Scholar]
- Zhai, W.; Wang, C. Study on characteristics and mechanism of silicon nitride in electrochemical grinding. Tribology 2004, 24, 360–363. [Google Scholar]
- He, Y.; She, D.; Liu, Z.; Wang, X.; Zhong, L.; Wang, C.; Wang, G.; Mao, S.X. Atomistic observation on diffusion-mediated friction between single-asperity contacts. Nat. Mater. 2022, 21, 173–180. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Liu, C.; He, W.; Zhang, J.; Qiao, X.; Li, J.; Xiang, D.; Qian, G.; Bai, P.; Meng, Y.; et al. A Review of Electric Potential-Controlled Boundary Lubrication. Lubricants 2023, 11, 467. https://doi.org/10.3390/lubricants11110467
Li S, Liu C, He W, Zhang J, Qiao X, Li J, Xiang D, Qian G, Bai P, Meng Y, et al. A Review of Electric Potential-Controlled Boundary Lubrication. Lubricants. 2023; 11(11):467. https://doi.org/10.3390/lubricants11110467
Chicago/Turabian StyleLi, Shaowei, Chenxu Liu, Wang He, Jie Zhang, Xiaoxi Qiao, Jiang Li, Dong Xiang, Gao Qian, Pengpeng Bai, Yonggang Meng, and et al. 2023. "A Review of Electric Potential-Controlled Boundary Lubrication" Lubricants 11, no. 11: 467. https://doi.org/10.3390/lubricants11110467
APA StyleLi, S., Liu, C., He, W., Zhang, J., Qiao, X., Li, J., Xiang, D., Qian, G., Bai, P., Meng, Y., & Tian, Y. (2023). A Review of Electric Potential-Controlled Boundary Lubrication. Lubricants, 11(11), 467. https://doi.org/10.3390/lubricants11110467







