The Effect of Temperature and Asphaltene Content on the Lubricating Properties of Fuel Oils
Abstract
:1. Introduction
- (i)
- A light cycle oil (LFO), which is a distillate fuel oil not containing asphaltenes;
- (ii)
- A medium wax-blend fuel oil (MFO) containing high molecular weight paraffin (wax), low concentration of asphaltenes and solid particles;
- (iii)
- A crude-derived heavy fuel oil (HFO) containing high concentrations of asphaltenes and solid particles.
2. Materials and Methods
2.1. Elemental Analysis
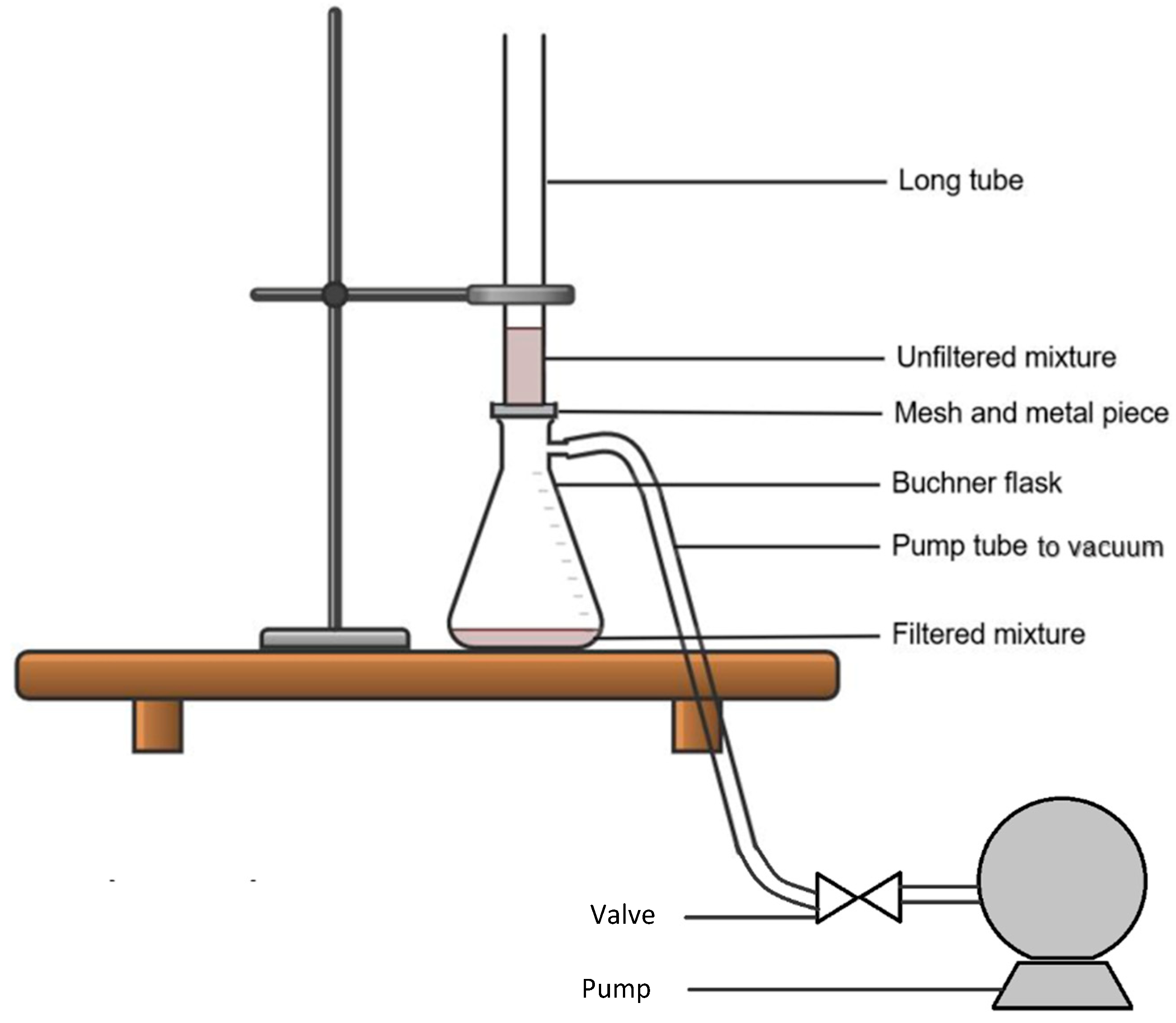
2.2. Precipitation of Asphaltenes
2.3. Lubricity Tests
3. Results
3.1. Viscosity Measurements
3.2. Water Content Measurements
3.3. Elemental Analysis
3.4. Asphaltene Content and Solid Particles Measurements
3.5. Lubricity Tests
3.6. Wear
4. Discussion
- LFO with no asphaltenes (solid particles only) has little impact on the COF and wear from 60 to 100 °C. The trends for COF and for wear are similar for both filtered and unfiltered LFO. The solid particles affect the roughness of the COF, minimal change in the WS1.4 and slight abrasive wear due to the presence of metals and silica in the solid particles. The removal of solid particles results in minimal change in COF except at 25 °C where the unfiltered LFO has a drastic reduction in COF when compared with filtered LFO. The removal of solid particles in filtered fuel oils also removes molecules with good lubricity resulting in increased COF when compared with unfiltered LFO.
- MFO containing high molecular weight paraffin (wax), low concentration of asphaltenes and solid particles produces a stable fuel oil. MFO performs better with asphaltenes present because the wax stabilizes the asphaltenes resulting in the dissolution of asphaltenes at an accelerated rate. The effect of this is good high temperature performance (less friction and wear at high temperatures). For MFO, the removal of asphaltenes results in minimal change in COF and minimal change in WS1.4 from 60 to 100 °C. Due to the presence of waxes in MFO, the wear results for MFO do not follow the typical trend where the increase in temperature usually results in an increase in wear. For MFO, the removal of asphaltenes and solid particles resulted in a decrease in wear at low temperatures and an increase in wear at high temperatures.
- HFO containing high concentrations of asphaltenes and solid particles results in very high COF values and severe abrasive wear at high temperatures. At low and moderate temperatures, unfiltered HFO performs comparable to filtered HFO, whereas at high temperatures COF, abrasive wear and WS1.4 drastically increase when compared with filtered HFO. Overall HFO performs better in the absence of asphaltenes and solid particles due to their abrasive nature—particularly at high temperatures.
5. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kirk, A.D. The cause and effect of particle agglomeration in high pressure diesel injection systems. In Proceedings of the 11th International Tribology Conference of the SAIT, Pretoria, South Africa, 10–12 March 2015. [Google Scholar]
- Zuo, P.; Shijie, Q.; Shen, W. Asphaltenes: Separations, structural analysis and applications. J. Energy Chem. 2019, 34, 186–207. [Google Scholar] [CrossRef]
- Ghanavati, M.; Shojaei, M.; Ramazani, A. Effects of asphaltenes content and temperature on viscosity of Iranian heavy crude oil: Experimental and modelling stud. Energy Fuels 2013, 27, 7217–7232. [Google Scholar] [CrossRef]
- Garaniya, V.; McWilliam, D.; Goldsworthy, L.; Ghiji, M. Extensive chemical characterization of a heavy fuel oil. Fuel 2018, 227, 67–78. [Google Scholar] [CrossRef]
- Ancheyta, J.; Trejo, F.; Rana, M.S. Asphaltenes Chemical Transformation during Hydroprocessing of Heavy Fuel Oils; CRC Press: Boca Raton, FL, USA, 2009; p. 1. [Google Scholar]
- Husin, H.; Aman, Z.; Chyuan, O.H. Correlation between rate of decomposition and temperature of asphaltenes. Mater. Today 2018, 5, 22128–22136. [Google Scholar]
- Mang, T.; Dresel, W. Lubricants and Lubrication, 3rd ed.; Wiley: Weinheim, Germany, 2017; p. 914. [Google Scholar]
- PCS Instruments. HFRR Installation and Test Preparation Manual; Hardware Version 1; PCS Instruments: London, UK, 2005. [Google Scholar]
- Lapuerta, M.; Sánchez-Valdepenas, J.; Bolonio, D.; Sukjit, E. Effect of fatty acid composition of methyl and ethyl esters on the lubricity at different humidities. Fuel 2016, 184, 202–210. [Google Scholar] [CrossRef]
- Nickels, L. Low fuel lubricity in thin distillates. World Pumps 2011, 2, 10. [Google Scholar]
- Bhushan, B. Introduction to Tribology, 2nd ed.; Wiley: West Sussex, UK, 2013; pp. 4–359. [Google Scholar]
- Harker, J.H.; Allen, D.A. Fuel Science; Oliver & Boyde: Edinburgh, Scotland, 1972; p. 120. [Google Scholar]
- Elbaz, A.M.; Khateeb, A.A.; Roberts, W.L. PM from the combustion of heavy fuel oils. Energy 2018, 152, 455–465. [Google Scholar] [CrossRef]
- Litzke, W. A Guide to Fuel Performance; Brookhaven National Laboratory report to the National Oilheat Research Alliance; NORA: Alexandria, VA, USA, 2004. [Google Scholar]
- Gil, L.; Pieniak, D.; Walczak, M.; Ignaciuk, P.; Sawa, J. Impact of acid number of fuels on the wear process of apparatus for fuel injection in diesel engines. Adv. Sci. Technol. 2014, 8, 54–57. [Google Scholar]
- ASTM D6217-18; Standard Test Method for Particulate Contamination in Middle Distillate Fuels by Laboratory Filtration. ASTM International: West Conshohocken, PA, USA, 2018.
- Srivastava, S.P.; Hancsók, J. Fuels and Fuel-Additives; Wiley: Hoboken, NJ, USA, 2014; pp. 336–347. [Google Scholar]
- Fuel Properties. Available online: http://webserver.dmt.upm.es/~isidoro/bk3/c15/Fuel%20properties.pdf (accessed on 20 August 2018).
- Pasadakis, N.; Karonis, D.; Mintza, A. Detailed compositional study of the light cycle oil (LCO) solvent products. Fuel Process. Technol. 2011, 92, 1568–1573. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, X.; Xu, C.; Huang, X.; Wu, Z.; Peng, C.; Duan, A. Selective Hydrocracking of light cycle oil into high-octane gasoline over bi-functional catalysts. J. Energy Chem. 2021, 52, 41–50. [Google Scholar] [CrossRef]
- Toxicological Profile for Fuel Oils. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp75-c3.pdf (accessed on 1 October 2018).
- Chemical and Physical Properties of Refined Petroleum Products. Available online: https://repository.library.noaa.gov/view/noaa/11031/noaa_DS1.pdf (accessed on 20 August 2018).
- SANS 342; Automotive Fuels-Requirements and Test Methods for Diesel. SABS: Pretoria, South Africa, 2016.
- Stachowiak, G.W.; Batchelor, A.W. Engineering Tribology, 4th ed.; Butterworth-Heinemann: Oxford, UK, 2014; pp. 11–38. [Google Scholar]
- Altoé, R.; de Oliveira, M.C.K.; Lopes, H.E.; Cirilo, L.C.M.; Lucas, E.F.; Gonzalez, G. Solution behaviour of asphaltic residues and deasphalted oil prepared by extraction of heavy oil. Colloids Surf. A Physicochem. Eng. Asp. 2014, 445, 59–66. [Google Scholar] [CrossRef]
- Asphaltenes. Available online: https://www.slb.com/-/media/files/oilfield-review/defining-asphaltenes.ashx (accessed on 20 November 2018).
- ASTM D396-18a; Standard Specifications for Fuel Oils. ASTM International: West Conshohocken, PA, USA, 2018.
- Tekie, H.A.; McCrindle, R.I.; Marais, P.J.J.G.; Ambushe, A.A. Evaluation of six sample preparation methods for determination of trace metals in lubricating oils using inductively coupled plasma-optical emission spectrometry. S. Afr. J. Chem. 2015, 68, 76–84. [Google Scholar] [CrossRef]
- Viesca, J.L.; Hernández Battez, A.; González, R.; Reddyhoff, T.; Torres Pérez, A.; Spikes, H.A. Assessing boundary film formation of lubricant additivised with 1-Hexyl-3-methylimidazolium tetrafluoroborate using ECR as qualitative indicator. Wear 2010, 269, 112–117. [Google Scholar] [CrossRef]
- Mettler-Toledo. Introduction to Karl Fischer Titration; KF Guide 1; Mettler-Toledo AG: Schwerzenbach, Switzerland, 2012. [Google Scholar]
- Lei, Y.; Han, S.; Zhang, J. Effect of the dispersion degree of asphaltene on wax deposition in crude oil under static conditions. Fuel Process. Technol. 2016, 146, 20–28. [Google Scholar] [CrossRef]
- Oláh, Z.S.; Szirmai, G.; Resofszki, G. Micro and Macro Analyses of Wear Scar Surfaces—A Complementary Rating Method to the Evaluation of HFRR Test Results; International colloquium; TAE: Ostfildern, Germany, 2005. [Google Scholar]
- Hudedagaddi, C.B.; Raghav, A.G.; Tortora, A.M.; Veeregowda, D.H. Water molecules influence in the lubricity of greases and fuel. Wear 2017, 376–377, 831–835. [Google Scholar] [CrossRef]
- Alcazar-Vara, L.A.; Buenrostro-Gonzalez, E. Characterization of the wax precipitation in Mexican crude oils. Fuel Process. Technol. 2011, 92, 2366–2377. [Google Scholar] [CrossRef]
- Rogel, E.; Ovalles, C.; Vien, J.; Moir, M. Asphaltenes characterisation of paraffinic crude oils. Fuel 2016, 178, 71–76. [Google Scholar] [CrossRef]







| Parameter | Value |
|---|---|
| Stroke length, mm | 1 ± 0.02 |
| Frequency, Hz | 50 ± 1 |
| Humidity, % (RH) | 50 ± 5 (Air) |
| Fluid temperature, °C | 115 ± 2, 100 ± 2, 60 ± 2, 25 ± 2 |
| Load, g | 200 ± 1 |
| Test duration, min | 75 ± 0.1 |
| Fluid volume, mL | 2 ± 0.2 |
| Reservoir surface area, mm2 | 600 ± 100 |
| Sample | HFO (mg/kg) | MFO (mg/kg) | LFO (mg/kg) |
|---|---|---|---|
| V | 14.7 | 4.94 | 2.15 |
| Ni | 10.5 | 2.37 | 1.61 |
| Na | 80.8 | 1.78 | 6.80 |
| Fe | 28.6 | 6.71 | 18.1 |
| Mn | 1.69 | 1.18 | 1.07 |
| Mg | 9.21 | 2.96 | 4.65 |
| K | 11.1 | 2.76 | 4.11 |
| S | 883 | 118 | 159 |
| Cr | 4.32 | 1.58 | 3.76 |
| Ca | 124 | 60.6 | 85.2 |
| Al | 216 | 2.37 | 1.79 |
| P | 18.6 | - | - |
| Zn | 9.77 | 2.57 | 3.04 |
| Si | 5.26 | 8.69 | 11.3 |
| Sample | HFO (mg/kg) | MFO (mg/kg) | LFO (mg/kg) |
|---|---|---|---|
| V | 2.82 | 1.26 | 0.219 |
| Ni | 1.60 | 0.709 | 0.310 |
| Na | 8.96 | 8.37 | 8.40 |
| Fe | 6.67 | 4.77 | 3.50 |
| Mn | 0.415 | 0.236 | 0.346 |
| Mg | 2.19 | 2.01 | 2.22 |
| K | 0.415 | 0.296 | 0.492 |
| S | 1.52 | 1.28 | 1.86 |
| Cr | 1.09 | 0.394 | 0.510 |
| Ca | 41.4 | 30.6 | 44.46 |
| Al | 1.42 | 0.768 | 0.601 |
| P | 0.138 | 0.887 | 0.237 |
| Zn | 1.05 | 0.788 | 1.04 |
| Si | 4.70 | 2.52 | 2.04 |
| 25 °C | 60 °C | 100 °C | 115 °C | |
|---|---|---|---|---|
| Unfiltered LFO |  |  |  |  |
| CR | 3 | 2 | 3 | 4 |
| WS1.4 (μm) | 191 | 360 | 364 | 295 |
| Filtered LFO |  |  |  |  |
| CR | 3 | 2 | 2 | 2 |
| WS1.4 (μm) | 145 | 348 | 375 | 307 |
| 25 °C | 60 °C | 100 °C | 115 °C | |
|---|---|---|---|---|
| Unfiltered MFO |  |  |  |  |
| CR | 3 | 3 | 2 | 2 |
| WS1.4 (μm) | 177 | 191 | 185 | 184 |
| Filtered MFO |  |  |  |  |
| CR | 3 | 2 | 4 | 4 |
| WS1.4 (μm) | 85 | 206 | 188 | 175 |
| 25 °C | 60 °C | 100 °C | 115 °C | |
|---|---|---|---|---|
| Unfiltered HFO |  |  |  |  |
| CR | 6 | 3 | 4 | 5 |
| WS1.4 (μm) | 217 | 222 | 343 | 431 |
| Filtered HFO |  |  |  |  |
| CR | - | 3 | 3 | 3 |
| WS1.4 (μm) | No wear scar | 137 | 201 | 168 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thobejane, T.; de Vaal, P.L. The Effect of Temperature and Asphaltene Content on the Lubricating Properties of Fuel Oils. Lubricants 2023, 11, 162. https://doi.org/10.3390/lubricants11040162
Thobejane T, de Vaal PL. The Effect of Temperature and Asphaltene Content on the Lubricating Properties of Fuel Oils. Lubricants. 2023; 11(4):162. https://doi.org/10.3390/lubricants11040162
Chicago/Turabian StyleThobejane, Trinity, and Philip L. de Vaal. 2023. "The Effect of Temperature and Asphaltene Content on the Lubricating Properties of Fuel Oils" Lubricants 11, no. 4: 162. https://doi.org/10.3390/lubricants11040162
APA StyleThobejane, T., & de Vaal, P. L. (2023). The Effect of Temperature and Asphaltene Content on the Lubricating Properties of Fuel Oils. Lubricants, 11(4), 162. https://doi.org/10.3390/lubricants11040162





