Simple Laser-Induced Hexagonal Boron Nitride Nanospheres for Enhanced Tribological Performance
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of the Hexagonal Boron Nitride (h-BN) Spherical Nanoparticles
2.2. Material Characterizations
2.3. Evaluation of the Tribological Property
3. Results and Discussion
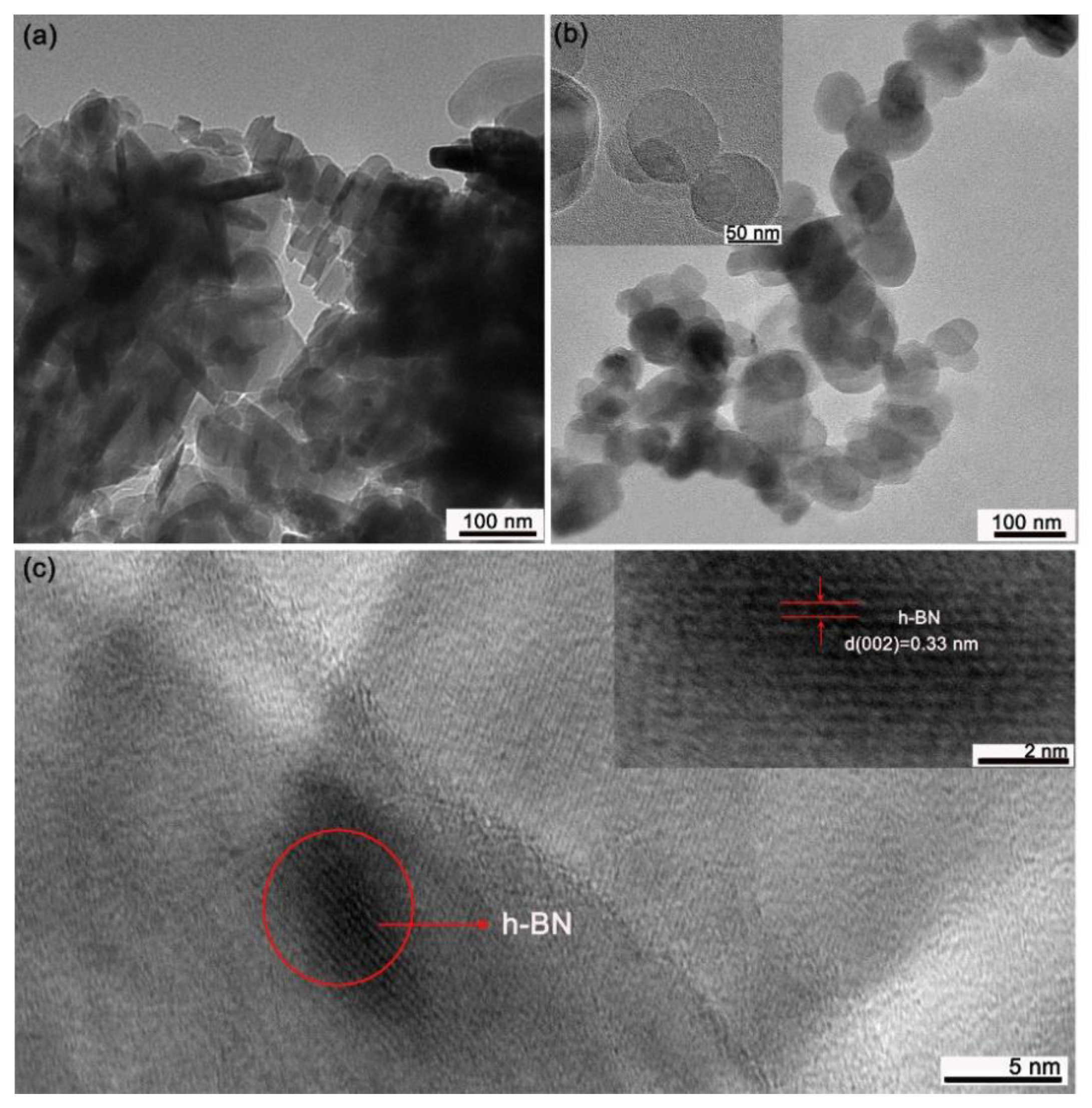
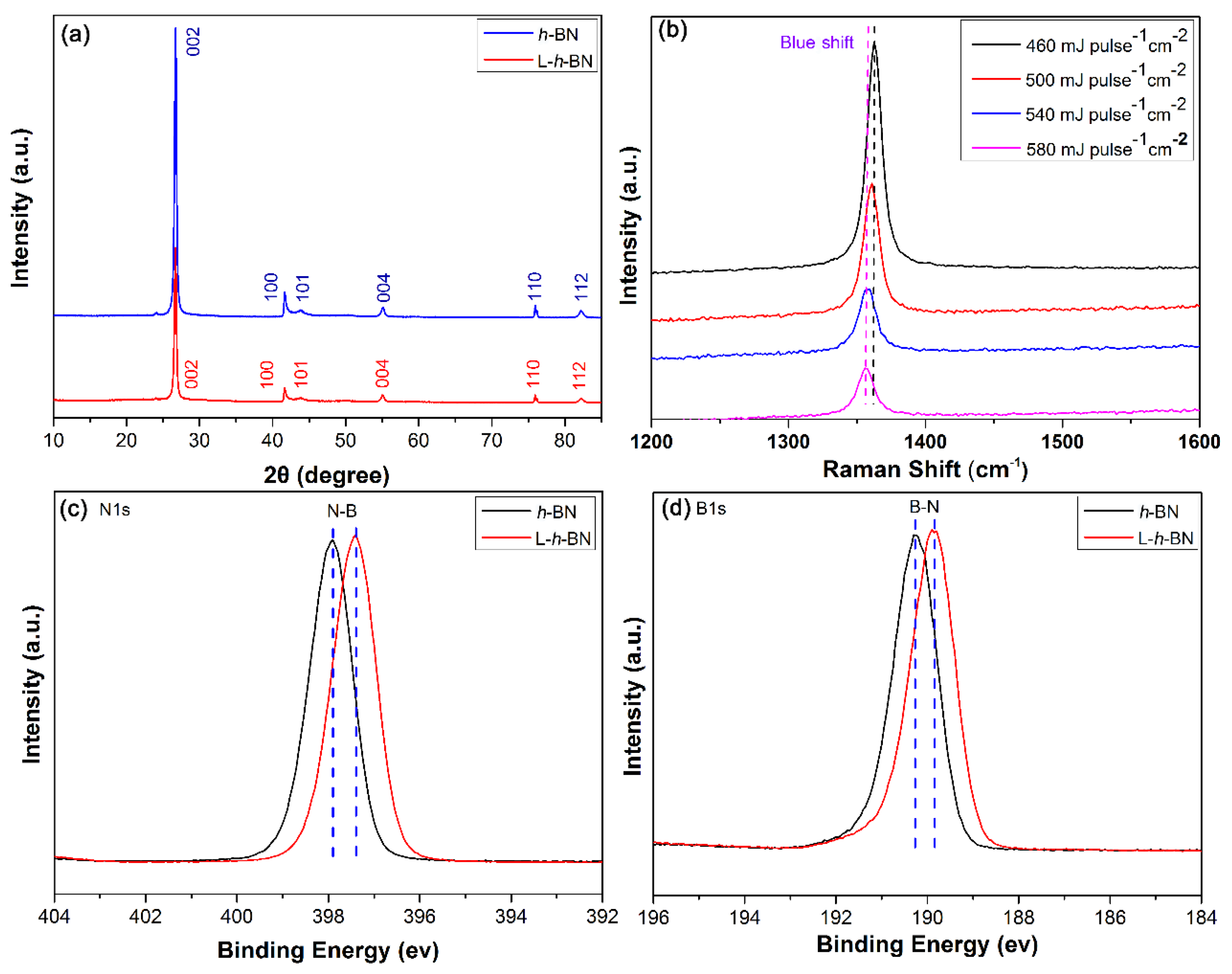
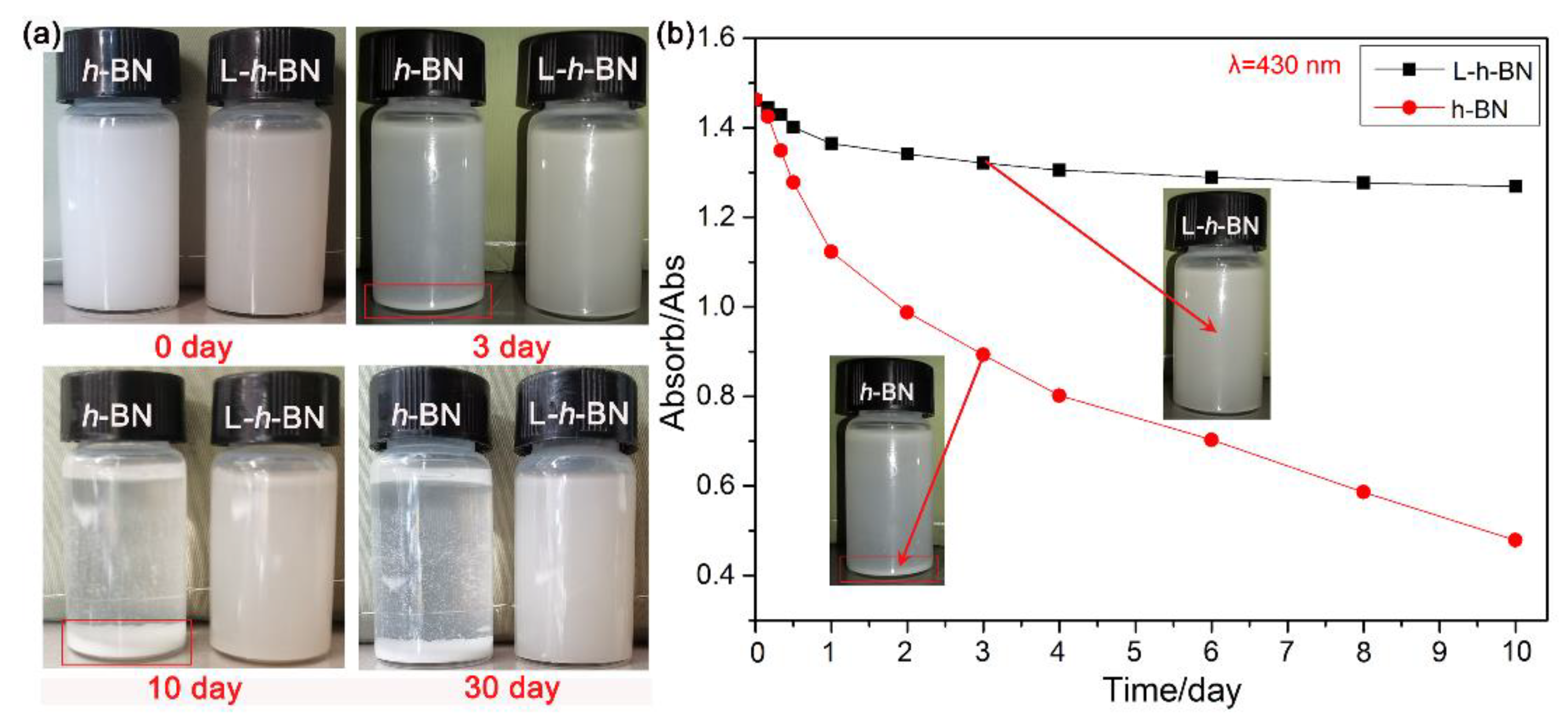
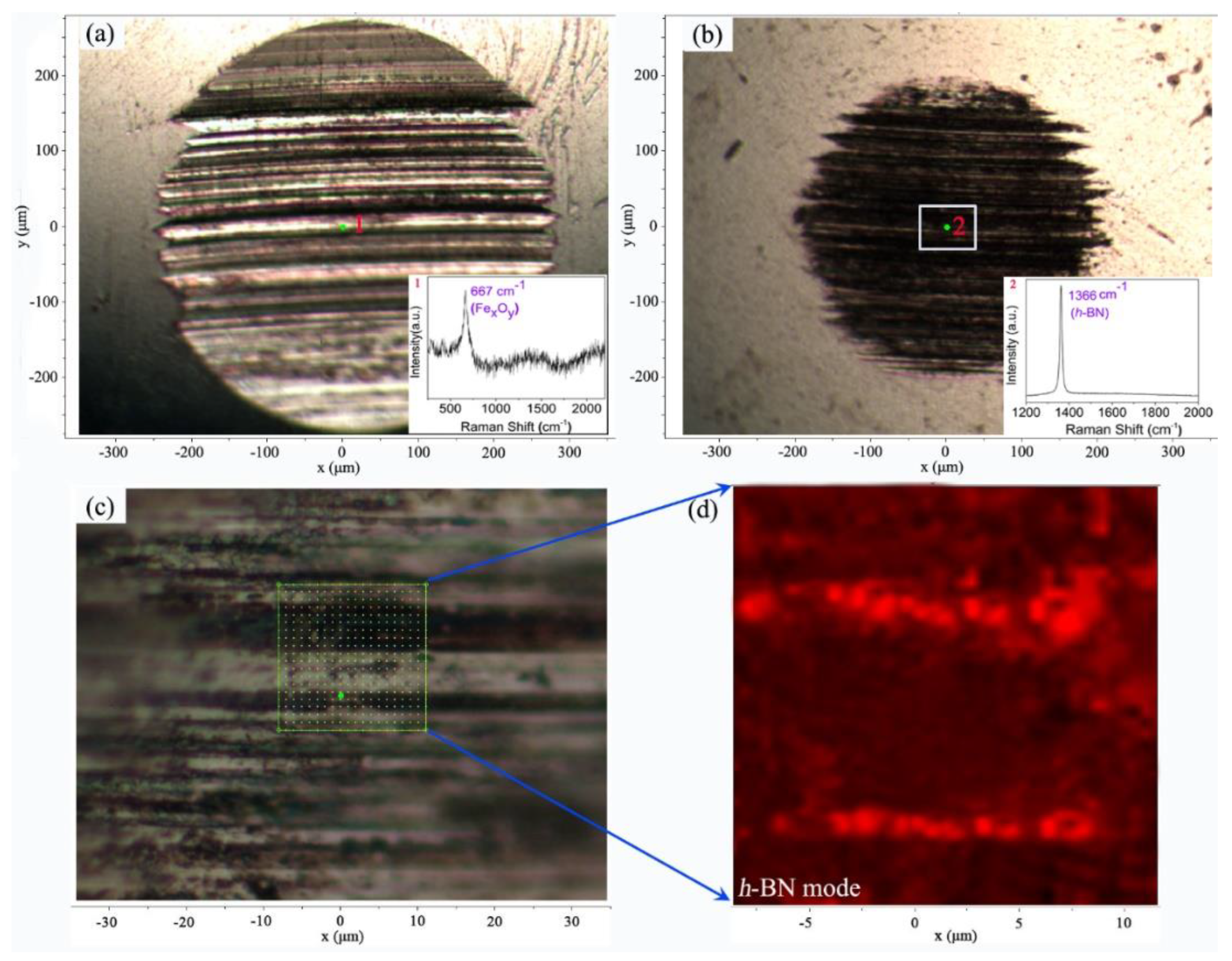
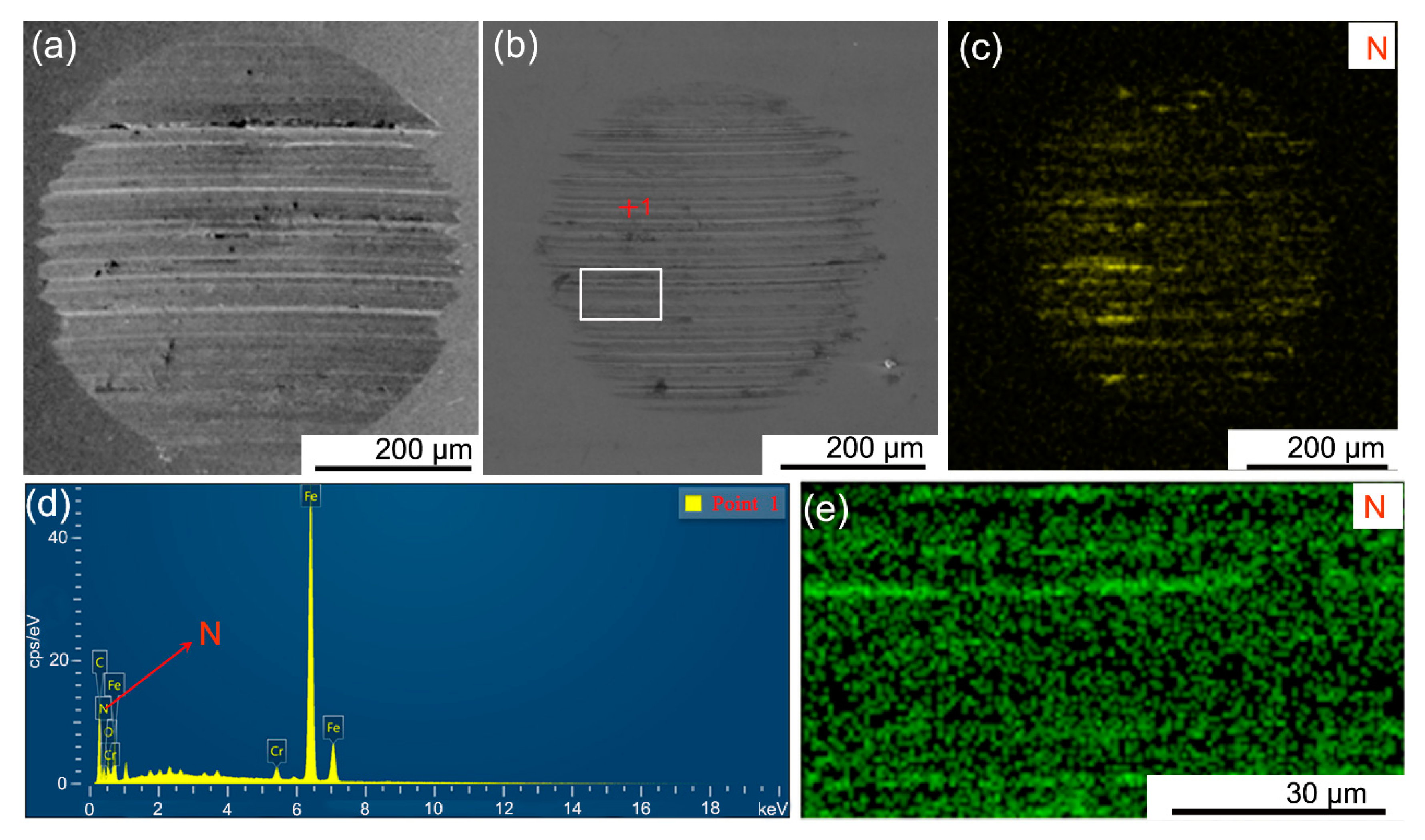
3.1. Preparation and Characterization of the L-h-BN Spheres
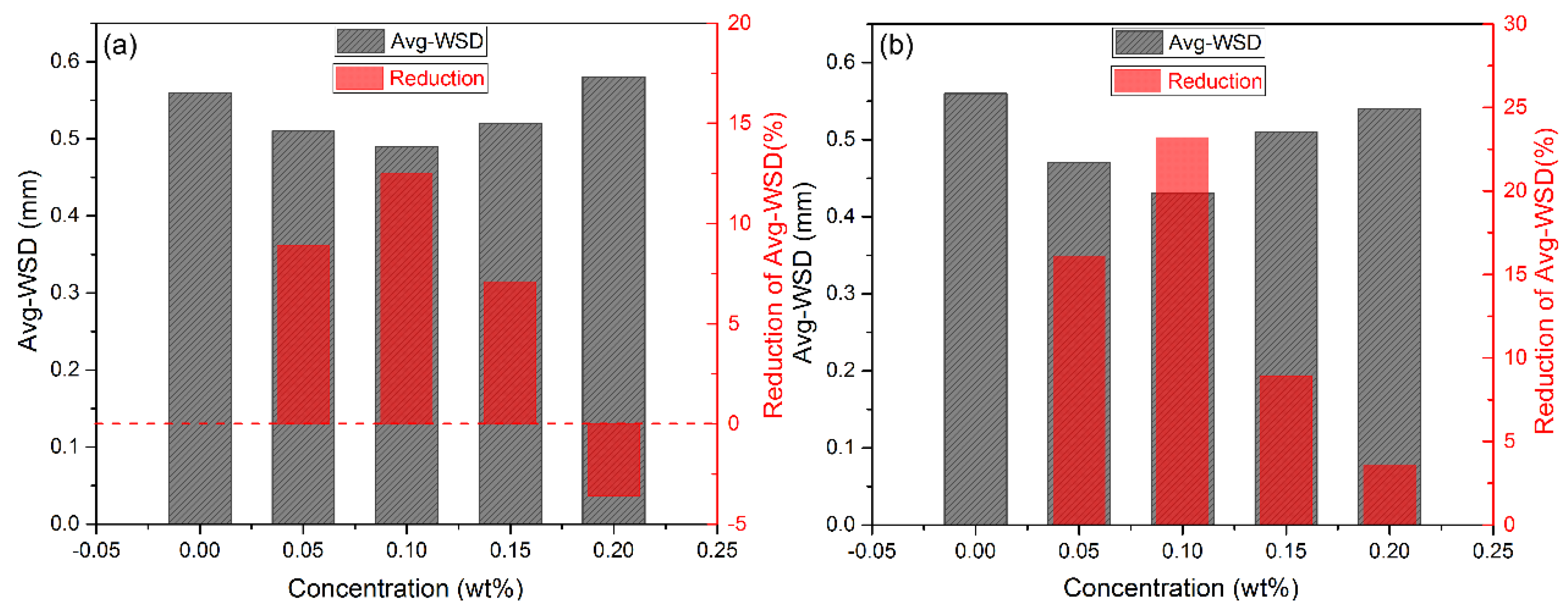
3.2. Friction Experiment
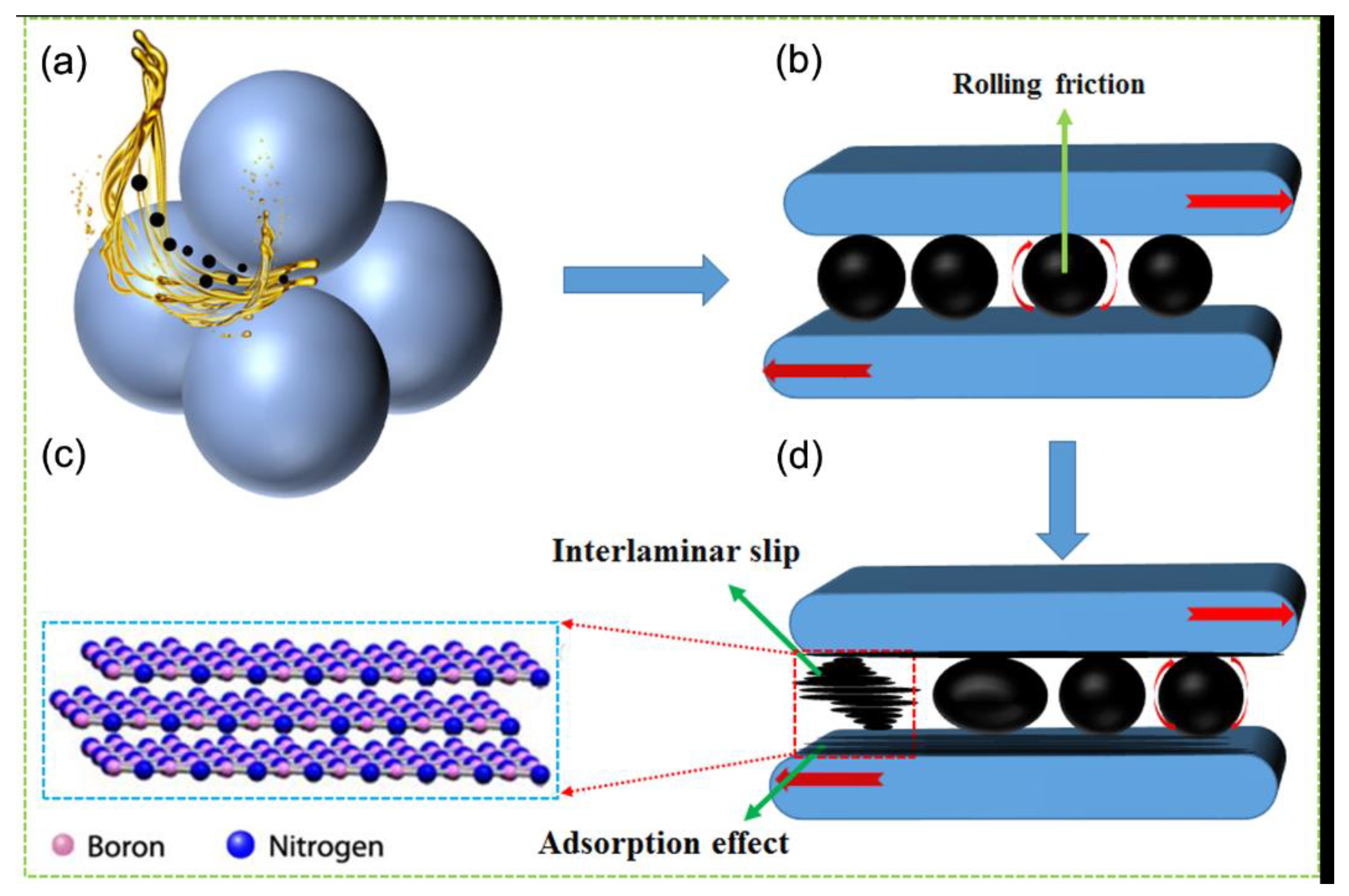
3.3. Mechanism Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mariello, M. Recent Advances on Hybrid Piezo-Triboelectric Bio-Nanogenerators: Materials, Architectures and Circuitry. Nanoenergy Adv. 2022, 2, 64–109. [Google Scholar] [CrossRef]
- Yang, W.; Geng, Z.; Li, Y.; Liu, X.; Tian, X.; Wang, S.; Wu, N.; Wang, Y.; Xu, R.; Yang, F.; et al. Facile synthesis of lipophilic alkylated boron nitride nanosheets as lubricating oil additive to greatly enhance the friction and heat-conducting properties. Tribol. Int. 2022, 173, 107655. [Google Scholar] [CrossRef]
- Altavilla, C.; Sarno, M.; Ciambelli, P.; Senatore, A.; Petrone, V. New ‘chimie douce’ approach to the synthesis of hybrid nanosheets of MoS2 on CNT and their anti-friction and anti-wear properties. Nanotechnology 2013, 24, 125601. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, J.; Yu, W.-J.; Yu, Y.; Li, R.-Y.; Gao, Q.; Ren, K.-F.; Ji, J. A Bioinspired Hydrogel-Elastomer Hybrid Surface for Enhanced Mechanical Properties and Lubrication. ACS Appl. Mater. Interfaces 2021, 13, 50461–50469. [Google Scholar] [CrossRef]
- Guo, J.; Wu, P.; Zeng, C.; Wu, W.; Zhao, X.; Liu, G.; Zhou, F.; Liu, W. Fluoropolymer grafted Ti3C2Tx MXene as an efficient lubricant additive for fluorine-containing lubricating oil. Tribol. Int. 2022, 170, 107500. [Google Scholar] [CrossRef]
- Sarno, M.; Scarpa, D.; Senatore, A.; Mustafa, W.A.A. rGO/GO Nanosheets in Tribology: From the State of the Art to the Future Prospective. Lubricants 2020, 8, 31. [Google Scholar] [CrossRef]
- Gong, H.; Yu, C.; Zhang, L.; Xie, G.; Guo, D.; Luo, J. Intelligent lubricating materials: A review. Compos. Part B Eng. 2020, 202, 108450. [Google Scholar] [CrossRef]
- Erdemir, A.; Ramirez, G.; Eryilmaz, O.L.; Narayanan, B.; Liao, Y.; Kamath, G.; Sankaranarayanan, S.K.R.S. Carbon-based tribofilms from lubricating oils. Nature 2016, 536, 67–71. [Google Scholar] [CrossRef]
- Spear, J.C.; Ewers, B.W.; Batteas, J.D. 2D-nanomaterials for controlling friction and wear at interfaces. Nano Today 2015, 10, 301–314. [Google Scholar] [CrossRef]
- Zhang, H. Ultrathin Two-Dimensional Nanomaterials. ACS Nano 2015, 9, 9451–9469. [Google Scholar] [CrossRef]
- Bas, H.; Ozen, O.; Bes, I.G.M. Tribological properties of MoS2 and CaF2 particles as grease additives on the performance of block-on-ring surface contact. Tribol. Int. 2022, 168, 107433. [Google Scholar] [CrossRef]
- Kinoshita, H. The use of graphene oxide as a lubricating additive. Carbon 2021, 175, 608–609. [Google Scholar] [CrossRef]
- Wang, D.; Li, F.; Chen, X.; Li, H.; Chen, W.; Zhang, P. Effect of Two Graphene Coatings on the Friction and Wear of Sliding Electrical Contact Interface. Lubricants 2022, 10, 305. [Google Scholar] [CrossRef]
- Sgroi, M.; Asti, M.; Gili, F.; Deorsola, F.; Bensaid, S.; Fino, D.; Kraft, G.; Garcia, I.; Dassenoy, F. Engine bench and road testing of an engine oil containing MoS2 particles as nano-additive for friction reduction. Tribol. Int. 2017, 105, 317–325. [Google Scholar] [CrossRef]
- Tian, Q.; Jia, X.H.; Zhang, Y.C.; Zhang, Y.P.; Yang, J.; Wang, S.Z.; Li, Y.; Shao, D.; Feng, L.; Song, H.J. In-situ growth of amorphous carbon on sucrose-assisted exfoliated boron nitride nanosheets: Exceptional water dispersibility and lubrication performance. Tribol. Int. 2022, 173, 107647. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, H.; Fei, J.; Li, C.; Ma, S.; Cai, X.; Li, B. Effect of modified nano boron nitride on tribological performance of resin-based friction material paired with copper dual disk. Tribol. Int. 2022, 168, 107429. [Google Scholar] [CrossRef]
- Nadiège, N.M.; Manuella, V.; Henry, J.; Philippe, T. Moringa oil with graphite and hexagonal boron nitride particles as additives for lubrication. Diam. Relat. Mater. 2022, 124, 108930. [Google Scholar]
- Kumari, S.; Chouhan, A.; Sharma, O.P.; Tawfik, S.A.; Spencer, M.J.S.; Bhargava, S.K.; Walia, S.; Ray, A.; Khatri, O.P. Alka-li-Assisted Hydrothermal Exfoliation and Surfactant-Driven Functionalization of h-BN Nanosheets for Lubrication Enhancement. ACS Appl. Nano Mater. 2021, 4, 9143–9154. [Google Scholar] [CrossRef]
- Urbaniak, W.; Majewski, T.; Powązka, I.; Śmigielski, G.; Petelska, A.D. Study of Nano h-BN Impact on Lubricating Properties of Selected Oil Mixtures. Materials 2022, 15, 2052. [Google Scholar] [CrossRef]
- Ma, Z.-S.; Ding, H.-L.; Liu, Z.; Cheng, Z.-L. Preparation and tribological properties of hydrothermally exfoliated ultrathin hexagonal boron nitride nanosheets (BNNSs) in mixed NaOH/KOH solution. J. Alloys Compd. 2019, 784, 807–815. [Google Scholar] [CrossRef]
- Zang, C.; Yang, M.; Liu, E.; Qian, Q.; Zhao, J.; Zhen, J.; Zhang, R.; Jia, Z.; Han, W. Synthesis, characterization and tribological behaviors of hexagonal boron nitride/copper nanocomposites as lubricant additives. Tribol. Int. 2022, 165, 107312. [Google Scholar] [CrossRef]
- Chen, Z.; He, X.; Xiao, C.; Kim, S.H. Effect of Humidity on Friction and Wear—A Critical Review. Lubricants 2018, 6, 74. [Google Scholar] [CrossRef]
- Yu, C.P.; Zhang, Q.C.; Zhang, J.; Geng, R.J.; Tian, W.; Fan, X.D.; Yao, Y.G. One-step in situ ball milling synthesis of polymer-functionalized few-layered boron nitride and its application in high thermally conductive cellulose composites. ACS Appl. Nano Mater. 2018, 1, 4875–4883. [Google Scholar] [CrossRef]
- Soudagar, M.E.M.; Nik-Ghazali, N.-N.; Kalam, M.; Badruddin, I.A.; Banapurmath, N.; Bin Ali, M.A.; Kamangar, S.; Cho, H.M.; Akram, N. An investigation on the influence of aluminium oxide nano-additive and honge oil methyl ester on engine performance, combustion and emission characteristics. Renew. Energy 2020, 146, 2291–2307. [Google Scholar] [CrossRef]
- Song, X.Y.; Qiu, Z.W.; Yang, X.P.; Gong, H.B.; Zheng, S.H.; Cao, B.Q.; Wang, H.Q.; Möhwald, H.; Shchukin, D. Submicron-Lubricant Based on Crystallized Fe3O4 Spheres for Enhanced Tribology Performance. Chem. Mater. 2014, 26, 5113–5119. [Google Scholar] [CrossRef]
- Xie, M.; Pan, B.; Liu, H.; Li, N.; Chen, Z.; Yan, J.; Fu, Z.; Guo, S.; Wang, H. One-step synthesis of carbon sphere@ 1 T-MoS2 towards superior antiwear and lubricity. Tribol. Int. 2022, 176, 107927. [Google Scholar] [CrossRef]
- Xu, J.X.; Chen, X.C.; Zmacher, P.G.; Rosenkranz, A.; Li, J.J.; Jin, J.; Zhang, C.H.; Luo, J.B. Tribochemical Behaviors of Onion-like Carbon Films as High-Performance Solid Lubricants with Variable Interfacial Nanostructures. ACS. Appl. Mater. Inter. 2019, 11, 25535–25546. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Z.; Yang, M.; Yuan, J.; Li, P.; Guo, F.; Men, X. Mulberry-like carbon spheres decorated with UiO-66-NH2 for enhancing the mechanical and tribological performances of UHMWPE composites. Tribol. Int. 2020, 141, 105916. [Google Scholar] [CrossRef]
- Birleanu, C.; Pustan, M.; Cioaza, M.; Molea, A.; Popa, F.; Contiu, G. Effect of TiO2 nanoparticles on the tribological properties of lubricating oil: An experimental investigation. Sci. Rep. 2022, 12, 5201. [Google Scholar] [CrossRef]
- Hu, N.; Zhang, X.; Wang, X.; Wu, N.; Wang, S. Study on Tribological Properties and Mechanisms of Different Morphology WS2 as Lubricant Additives. Materials 2020, 13, 1522. [Google Scholar] [CrossRef]
- Liu, C.; Fu, K.; Wang, Z.; Cao, C.; Yang, J.; Zhai, Q.; Wang, B.; Zhou, Z.; Ji, J.; Li, M.; et al. Cavitating inside spherical boron nitride nanoparticles dependent on controllably follow-up treated atmospheres. J. Nanopart. Res. 2020, 22, 302. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, H.; Kan, H.; Wang, X.; Long, H.; Zhou, Y. The preparation of high-adsorption, spherical, hexagonal boron nitride by template method. J. Alloys Compd. 2014, 613, 74–79. [Google Scholar] [CrossRef]
- Tang, C.; Bando, Y.; Huang, Y.; Zhi, C.; Golberg, D. Synthetic Routes and Formation Mechanisms of Spherical Boron Nitride Nanoparticles. Adv. Funct. Mater. 2008, 18, 3653–3661. [Google Scholar] [CrossRef]
- Han, W.; Wang, J.; Liu, S.; Ge, C.; Cao, S.; Song, B.; Wang, J.; Zhang, X. Spectral properties of spherical boron nitride prepared using carbon spheres as template. Ceram. Int. 2017, 43, 3569–3575. [Google Scholar] [CrossRef]
- Ren, L.; Zeng, X.; Sun, R.; Xu, J.-B.; Wong, C.-P. Spray-assisted assembled spherical boron nitride as fillers for polymers with enhanced thermally conductivity. Chem. Eng. J. 2019, 370, 166–175. [Google Scholar] [CrossRef]
- Zhang, D.S.; Liu, J.; Liang, C.H. Perspective on how laser-ablated particles grow in liquids. Sci. China Phys. Mech. Astron. 2017, 60, 1–16. [Google Scholar] [CrossRef]
- Adelmann, B.; Hellmann, R. A study of SiC decomposition under laser irradiation. Appl. Phys. A-Mater. 2017, 123, 454–459. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, L.; Hong, M. Enhancement of pulsed laser ablation assisted with continuous wave laser irradiation. Sci. China Phys. Mech. Astron. 2019, 62, 66–74. [Google Scholar] [CrossRef]
- Hu, X.; Gong, H.; Wang, Y.; Chen, Q.; Zhang, J.; Zheng, S.; Yang, S.; Cao, B. Laser-induced reshaping of particles aiming at energy-saving applications. J. Mater. Chem. 2012, 22, 15947. [Google Scholar] [CrossRef]
- Kumar, A.; Malik, G.; Sharma, S.; Chandra, R.; Mulik, R.S. Precursors controlled morphologies of nanocrystalline h-BN and its growth mechanism. Ceram. Int. 2021, 47, 30985–30992. [Google Scholar] [CrossRef]
- Luo, T.; Wang, P.; Qiu, Z.; Yang, S.; Zeng, H.; Cao, B. Smooth and solid WS2 submicrospheres grown by a new laser fragmentation and reshaping process with enhanced tribological properties. Chem. Commun. 2016, 52, 10147. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Malik, G.; Chandra, R.; Mulik, R.S. Bluish emission of economical phosphor h-BN nanoparticle fabricated via mixing annealing route using non-toxic precursor. J. Solid State Chem. 2020, 288, 121430. [Google Scholar] [CrossRef]
- Annamalai, M.; Gopinadhan, K.; Han, S.A.; Saha, S.; Park, H.J.; Cho, E.B.; Kumar, B.; Patra, A.; Kim, S.-W.; Venkatesan, T. Surface energy and wettability of van der Waals structures. Nanoscale 2016, 8, 5764–5770. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, C.; Du, M.; Wu, Y.; Ren, C.; Ding, K.; Song, M.; Huang, C. Porous Hexagonal Boron Nitride Sheets: Effect of Hydroxyl and Secondary Amino Groups on Photocatalytic Hydrogen Evolution. ACS Appl. Nano Mater. 2018, 1, 4566–4575. [Google Scholar] [CrossRef]
- Chen, X.; Dmuchowski, C.M.; Park, C.; Fay, C.C.; Ke, C. Quantitative Characterization of Structural and Mechanical Properties of Boron Nitride Nanotubes in High Temperature Environments. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Radiman, S.; Daud, A.; Tabet, N.; Al-Douri, Y. XPS and optical studies of different morphologies of ZnO nanostructures prepared by microwave methods. Ceram. Int. 2013, 39, 2283–2292. [Google Scholar] [CrossRef]
- Luo, T.; Chen, X.; Wang, L.; Wang, P.; Li, C.; Zeng, H.; Cao, B. Green laser irradiation-stimulated fullerene-like MoS2 nanospheres for tribological applications. Tribol. Int. 2018, 122, 119–124. [Google Scholar] [CrossRef]
- Zhao, B.; Yu, X.; Liu, Y.; Yang, L.; Zhang, Z.; Zhang, B. Frictional characteristics of heterostructure film composed of graphene and H-BN with the consideration of defects. Tribol. Int. 2021, 153, 106607. [Google Scholar] [CrossRef]
- Garcia, M.A.L.; Martinez, A.I.; Castro, M.R.; Falcony, C.; Escobar, L.A. Correlation between structural and magnetic properties of sprayed iron oxide thin films. Phys. B. 2011, 406, 1496–1500. [Google Scholar] [CrossRef]
- Li, W.; Luo, T.; Zhu, C.; Zhang, B.; Cao, B. Graphene/h-BN Nanosheet/Nanosphere Composites Constructed by In Situ Laser Irradiation with Synergistically Improved Tribological Performance. Ind. Eng. Chem. Res. 2023, 62, 435–444. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Luo, T.; Zhu, C.; Xu, D.; Dong, Y.; Cao, B. Simple Laser-Induced Hexagonal Boron Nitride Nanospheres for Enhanced Tribological Performance. Lubricants 2023, 11, 199. https://doi.org/10.3390/lubricants11050199
Li W, Luo T, Zhu C, Xu D, Dong Y, Cao B. Simple Laser-Induced Hexagonal Boron Nitride Nanospheres for Enhanced Tribological Performance. Lubricants. 2023; 11(5):199. https://doi.org/10.3390/lubricants11050199
Chicago/Turabian StyleLi, Wei, Ting Luo, Changxu Zhu, Dalong Xu, Yifan Dong, and Bingqiang Cao. 2023. "Simple Laser-Induced Hexagonal Boron Nitride Nanospheres for Enhanced Tribological Performance" Lubricants 11, no. 5: 199. https://doi.org/10.3390/lubricants11050199





