Investigating the Anti-Wear Behavior of Polyalphaolefin Oil with Methyl Silicon Resin Using Advanced Analytical Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Friction and Wear Test
3. Results and Discussion
3.1. Characterization of Methyl Silicone Resin
3.2. Tribological Properties
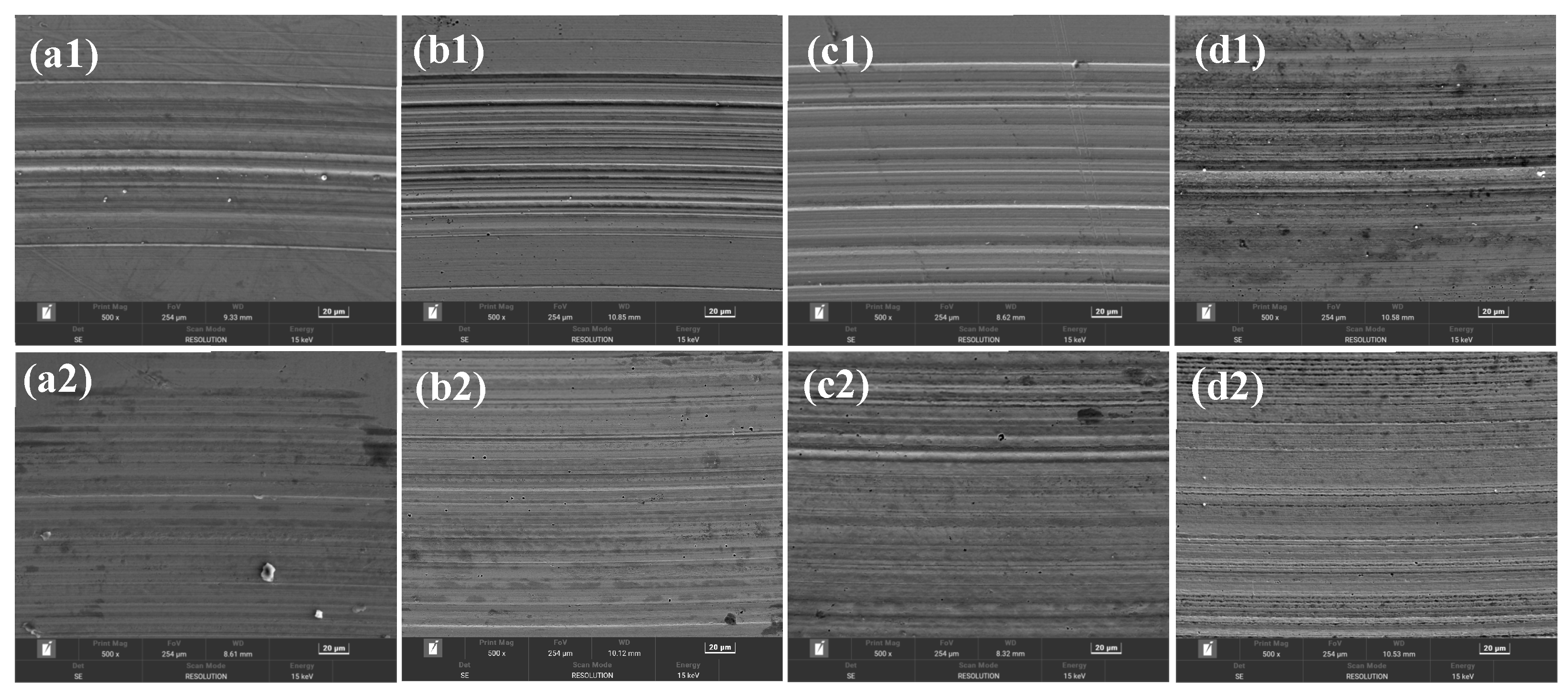
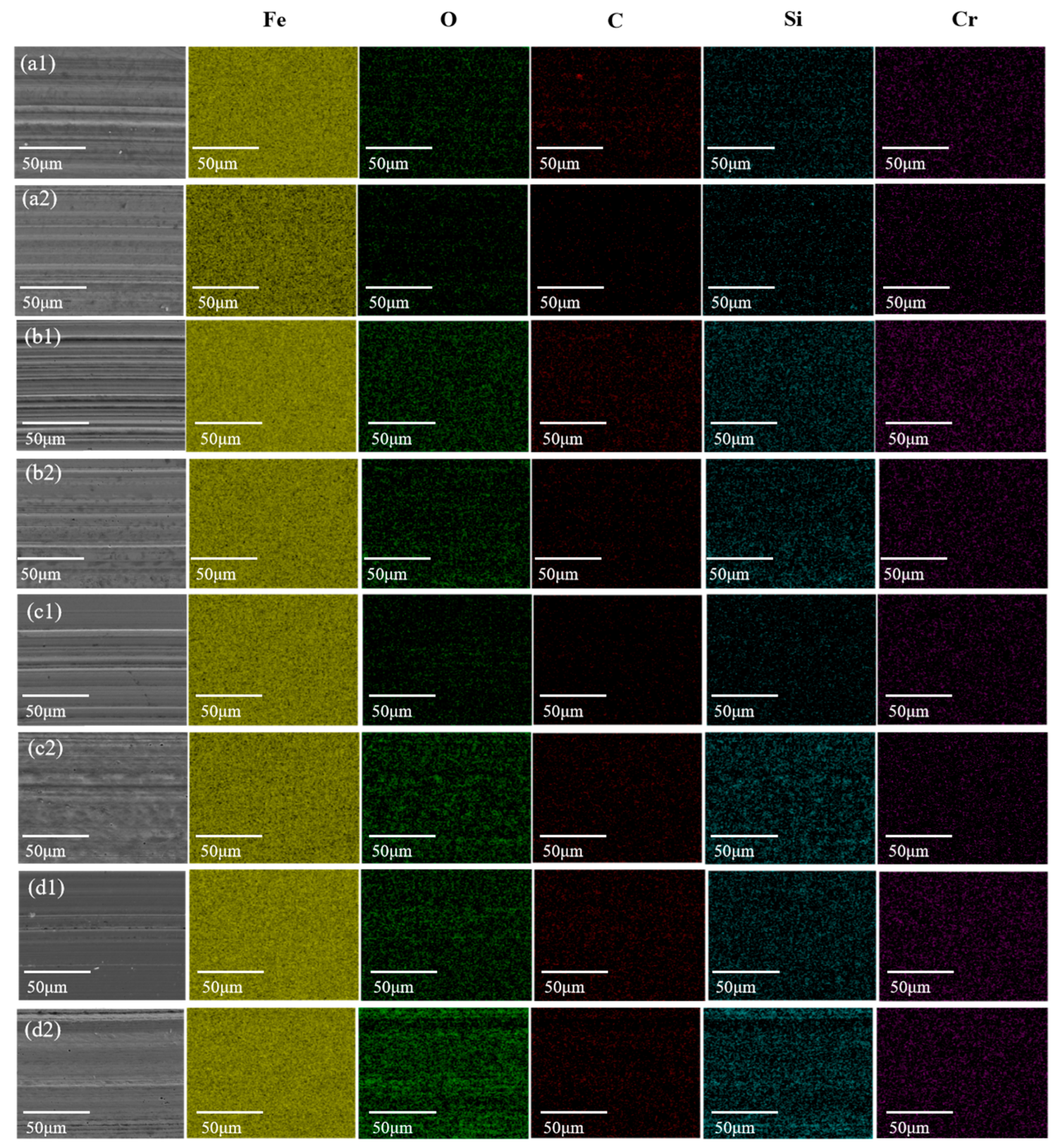
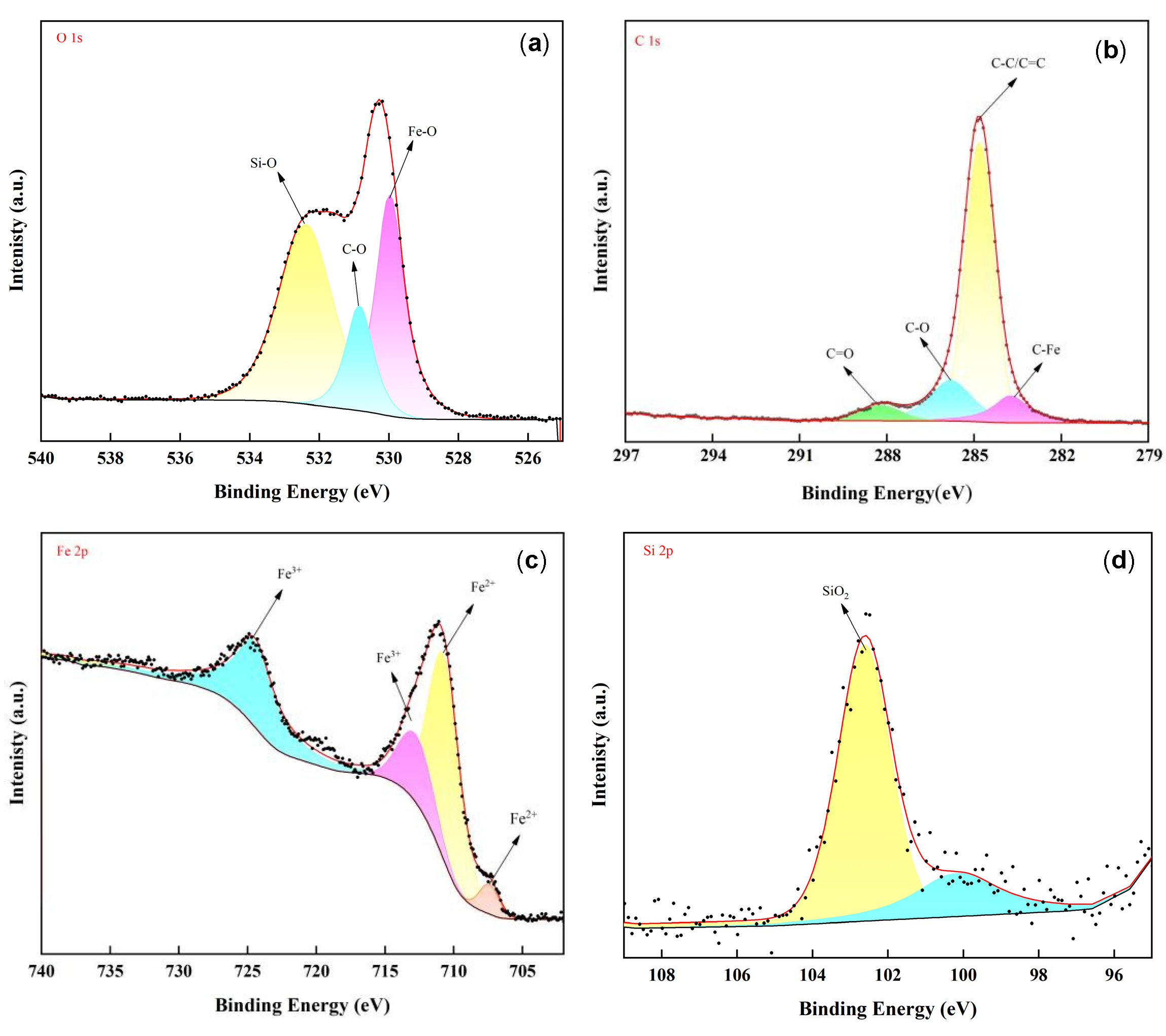
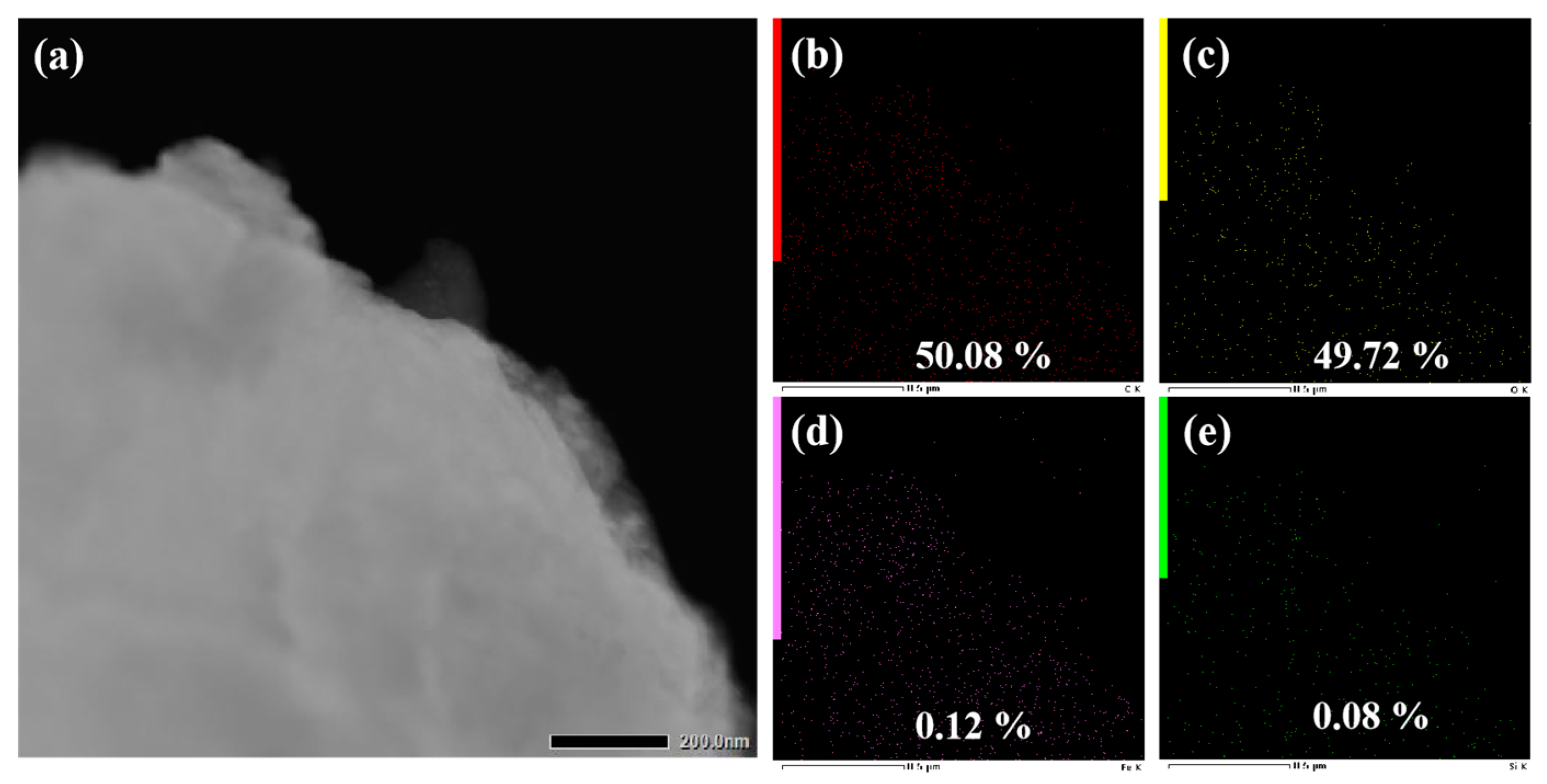
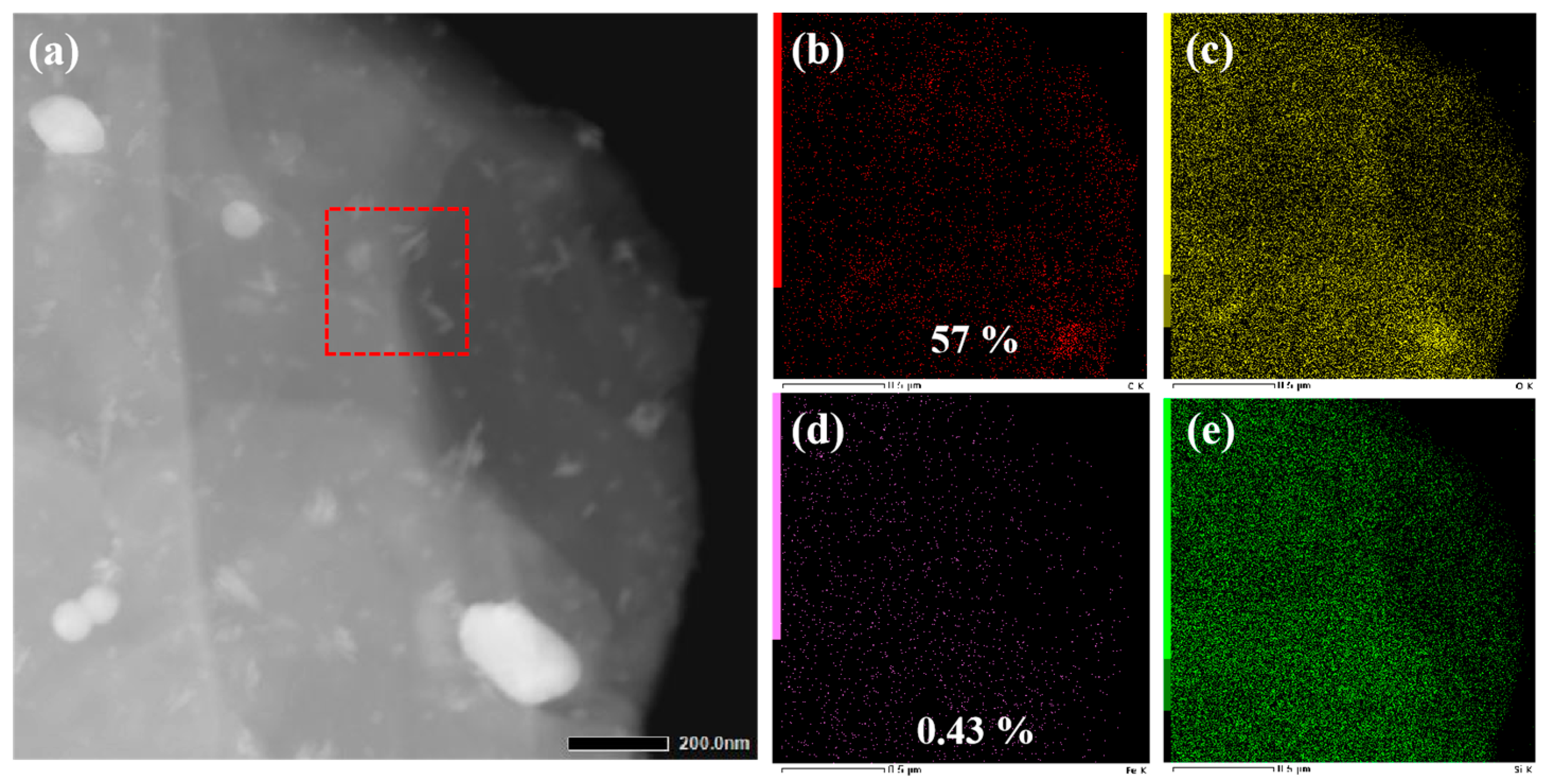
3.3. Analysis of Tribological Mechanisms
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xia, Y.; Wang, Y.; Hu, C.; Feng, X. Conductivity and tribological properties of IL-PANI/WS2composite material in lithium complex grease. Friction 2022, 11, 977–991. [Google Scholar] [CrossRef]
- Rahman, M.H.; Warneke, H.; Webbert, H.; Rodriguez, J.; Austin, E.; Tokunaga, K.; Rajak, D.K.; Menezes, P.L. Water-Based Lubricants: Development, Properties, and Performances. Lubricants 2021, 9, 73. [Google Scholar] [CrossRef]
- Yang, M.; Fan, S.; Huang, H.; Zhang, Y.; Huang, Z.; Hu, H.; Liang, J. In-situ synthesis of calcium borate/cellulose acetate-laurate nanocomposite as efficient extreme pressure and anti-wear lubricant additives. Int. J. Biol. Macromol. 2020, 156, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Kuang, W.; Miao, Q.; Ding, W.; Li, H. A short review on the influence of mechanical machining on tribological and wear behavior of components. Int. J. Adv. Manuf. Technol. 2022, 120, 1401–1413. [Google Scholar] [CrossRef]
- Kuang, S.; Sun, X.; Xiong, L.; Wu, Y.; Li, L.; Guo, L.; He, Z.; Zhang, R. Tribochemistry of mercaptoimidazoline as an additive in emulsion between self-mated GCr15 ball interfaces and its friction-reduction mechanism. Surf. Topogr. Metrol. Prop. 2024, 12, 015002. [Google Scholar] [CrossRef]
- Wang, B.; Qiu, F.; Barber, G.C.; Zou, Q.; Wang, J.; Guo, S.; Yuan, Y.; Jiang, Q. Role of nano-sizedmaterials as lubricant additives in friction and wear reduction: A review. Wear 2022, 490, 204206. [Google Scholar] [CrossRef]
- Zhu, C.; He, Z.; Xiong, L.; Li, J.; Wu, Y.; Li, L. Study on the influence of the MoS2 addition method on the tribological and corrosion properties of greases. Lubricants 2023, 11, 517. [Google Scholar] [CrossRef]
- Kumara, C.; Armstrong, B.; Lyo, I.; Lee, H.W.; Qu, J. Organic-modified ZnS nanoparticles as a high-performance lubricant additive. RSC Adv. 2023, 13, 7009–7019. [Google Scholar] [CrossRef]
- Kowalczyk, J.; Kulczycki, A.; Madej, M.; Ozimina, D. Effect of ZDDP and Fullerenes Added to PAO8 Lubricant on tribologycal Properties of the Surface Layer of Steel Bare Steel and W-DLC Coating. Q. Tribol. 2022, 299, 19–32. [Google Scholar] [CrossRef]
- Jiang, H.; Hou, X.; Ma, Y.; Su, D.; Qian, Y.; Ali, M.K.A.; Dearn, K.D. The tribological performance evaluation of steel-steel contact surface lubricated by polyalphaolefins containing surfactant-modified hybrid MoS2/h-BN nano-additives. Wear 2022, 504, 204426. [Google Scholar] [CrossRef]
- Wang, S.; Liang, Z.; Wang, B.; Gui, Y.; Ren, G.; Cao, Y.; Hong, Q.; Chen, D. Synthesis of lubricant additive for castor oil: A green and fast approach. J. Mol. Liq. 2024, 411, 125800. [Google Scholar] [CrossRef]
- He, X.; Luo, H.; Mathews, T.J.; Stevenson, L.; Geeza, T.J.; Kumara, C.; Meyer, H.M.; Qu, J. Minimizing Toxicity and Optimizing Lubricity of Ionic Liquids for Eco-Friendly Lubrication. ACS Sustain. Chem. Eng. 2024, 12, 4344–4355. [Google Scholar] [CrossRef]
- Wang, M.; He, H.; Fang, X.; Li, H. The development status and future trends of lubricant additives technology: Based on patents analysis. PLoS ONE 2024, 19, e0304888. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, D.; Zhu, H.; Ma, J.; An, Y. Advanced developments in environmentally friendly lubricants for water-based drilling fluid: A review. RSC Adv. 2022, 12, 22853–22868. [Google Scholar] [CrossRef]
- Saxena, A.; Kumar, D.; Tandon, N. Development of lubricious environmentally friendly greases using synergistic natural resources: A potential alternative to mineral oil-based greases. J. Clean. Prod. 2022, 380, 135047. [Google Scholar] [CrossRef]
- Rastogi, R.B.; Maurya, J.L.; Jaiswal, V. Zero SAPs and ash free antiwear additives: Schif bases of salicylaldehyde with 1,2-phenylenediamine, 1,4-phenylenediamine, and 4,4′-diaminodiphenylenemethane and their synergistic interactions with borate ester. Tribol. Int. 2013, 56, 592–606. [Google Scholar] [CrossRef]
- Hu, H.; Wu, Y.; Gong, X. Organosilicon-Based Carbon Dots and Their Versatile Applications. Small 2024, 20, 2305933. [Google Scholar] [CrossRef]
- Kung, M.C.; Riofski, M.V.; Missaghi, M.N.; Kung, H.H. Organosilicon platforms: Bridging homogeneous, heterogeneous, and bioinspired catalysis. Chem. Commun. 2014, 50, 3262–3276. [Google Scholar] [CrossRef]
- Chen, D.; Lin, W. Advanced Lubricant Formulation Strategies for Modern Engines. Lubr. Eng. 2022, 67, 45–53. [Google Scholar]
- Smith, R.L.; Johnson, M.T. Innovations in Lubricant Additives for Fuel Efficiency and Emission Reduction. J. Pet. Technol. 2022, 64, 289–301. [Google Scholar]
- GBT 3142-1982; Lubricants—Determination of Load Carrying Capacity (Four Balls Method). General Administration of Quality Supervision, Inspection and Quarantine: Beijing, China, 1982.
- Qu, J.; Truhan, J.J. An efficient method for accurately determining wear volumes of sliders with non-flat wear scars and compound curvatures. Wear 2006, 261, 848–855. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, R.; He, Z.; Xiong, L. Tribological performance of N-containing heterocyclic triazine derivative as lubricant additive in ethylene glycol. Surf. Interface Anal. 2021, 12, 53. [Google Scholar] [CrossRef]
- Han, J. Additives for Lubricating Oil and Grease: Mechanism, Properties and Applications. Lubricants 2024, 12, 243. [Google Scholar] [CrossRef]
- Chybowski, L.; Szczepanek, M.; Sztangierski, R.; Brożek, P. A Quantitative and Qualitative Analysis of the Lubricity of Used Lubricating Oil Diluted with Diesel Oil. Appl. Sci. 2024, 14, 4567. [Google Scholar] [CrossRef]
- Liu, Y. Effects of POSS on the thermalstability of silicone and on the mechanical properties of quartz fiber/silicone composites. Master’s Thesis, Harbin Institute of Technology, Harbin, China, 2006. [Google Scholar]
- Zhu, Y.; Hu, P.; Li, H.; Lai, X.; Zeng, X. Synthesis and Characterization of Reactive Methylphenyl Silicone Resin. Silicone Mater. 2023, 37, 6–10. (In Chinese) [Google Scholar]
- Yu, B.; Sun, C.; Mou, Z.; Zhou, F.; Liang, Y.; Liu, W. Preparation and Tribological Behavior of the End Hydroxyl Group Phenyl-containing Poly Silicone Film. J. Mater. Sci. Eng. 2005, 23, 412–415. (In Chinese) [Google Scholar]
- Liu, M. Preparation and Properties of UV-Cured Silicone Resin. Master’s Thesis, Xiangtan University, Xiangtan, China, 2018. [Google Scholar]
- Dai, G.; Pei, Y.; Chu, J.; Luo, Y.; Xie, Y.; Xie, D.; Mei, Y. Progress in Modification Methods and Mechanisms of Silicone Resins for High Temperature Resistance. Polym. Mater. Sci. Eng. 2022, 38, 174–182. (In Chinese) [Google Scholar]
- Chen, Q. Tribological Properties of Green and Efficient Alocohol Molecules and Their Lubrication Mechanism. Master’s Thesis, East China Jiaotong University, Nanchang, China, 2022. [Google Scholar]
- Cui, L.; Lu, Z.; Wang, L. Probing the low-friction mechanism of diamond-like carbon by varying of sliding velocity and vacuum pressure. Carbon 2014, 66, 259–266. [Google Scholar] [CrossRef]
- Safdarzadeh, O.; Farahi, A.; Binder, A.; Sezen, H.; Hofmann, J.P. WLI, XPS and SEM/FIB/EDS surface characterization of an electrically fluted bearing raceway. Lubricants 2024, 12, 148. [Google Scholar] [CrossRef]
- Ji, X.; Chen, Y.; Wang, X.; Liu, W. Tribological behaviors of novel tri(hydroxymethyl)propane esters containing boron and nitrogen as lubricant additives in rapeseed oil. Industr. Lubr. Tribol. 2012, 64, 315–320. [Google Scholar] [CrossRef]
- Wu, J.; Yang, G.; Zhang, S.; Zhang, Y.; Sun, L.; Sun, T.; Yu, L.; Zhang, P. Preparation of Nanofluid of Lanthanum Borate Nanosheets and Investigation of Its Tribological Properties and Tribomechanisms in Different Base Oils. Tribol. Lett. 2023, 71, 1. [Google Scholar] [CrossRef]
- Gandla, K.; Kumar, P.K.; Rajasulochana, P.; Charde, M.S.; Rana, R.; Singh, L.P.; Haque, M.A.; Bakshi, V.; Siddiqui, F.A.; Khan, S.L.; et al. Fluorescent-Nanoparticle-Impregnated Nanocomposite Polymeric Gels for Biosensing and Drug Delivery Applications. Gels 2023, 9, 669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, C.; Wang, Q.; Wang, T. High thermal stability and wear resistance of porous thermosetting heterocyclic polyimide impregnated with silicone oil. Tribol. Int. 2019, 140, 105728. [Google Scholar] [CrossRef]
- Arcifa, A.; Rossi, A.; Espinosa-Marzal, R.M.; Spencer, N.D. Influence of Environmental Humidity on the Wear and Friction of a Silica/Silicon Tribopair Lubricated with a Hydrophilic Ionic Liquid. ACS Appl. Mater. Interfaces 2016, 8, 2961–2973. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Cao, Z.; Yan, X.; Wang, Q. In-situ fabrication of a UHMWPE nanocomposite reinforced by SiO2 nanospheres and its tribological performance. Mater. Chem. Phys. 2019, 236, 121778. [Google Scholar] [CrossRef]
- Sun, W.; Zhou, W.; Liu, J.; Fu, X.; Chen, G.; Yao, S. The Size Effect of SiO2 Particles on Friction Mechanisms of a Composite Friction Material. Tribol. Lett. 2018, 66, 35. [Google Scholar] [CrossRef]
- Seymour, B.T.; Fu, W.; Wright, R.A.; Luo, W.; Qu, J.; Dai, S.; Zhao, B. Improved Lubricating Performance by Combining Oil-Soluble Hairy Silica Nanoparticles and an Ionic Liquid as an Additive for a Synthetic Base Oil. ACS Appl. Mater. Interfaces 2018, 10, 15129–15139. [Google Scholar] [CrossRef]
- Ferrusquía-Jiménez, N.I.; González-Arias, B.; Rosales, A.; Esquivel, K.; Escamilla-Silva, E.M.; Ortega-Torres, A.E.; Guevara-González, R.G. Elicitation of Bacillus cereus-Amazcala (Bc-A) with SiO2 nanoparticles improves its role as a plant growth-promoting bacteria (PGPB) in chili pepper plants. Plants 2022, 11, 3445. [Google Scholar] [CrossRef]
- Gkatziouras, C.; Solakidou, M.; Louloudi, M. Efficient [Fe-imidazole@ SiO2] nanohybrids for catalytic H2 production from formic acid. Nanomaterials 2023, 13, 1670. [Google Scholar] [CrossRef]
- Chen, G.; Ma, Z.; Jiang, S.; Wang, X.; Huang, Y.; Chai, C. Highly lubricating and wear-resistant Ti3C2Tx@SiO2/PI composites based on the action of transfer film at the friction surface. Polym. Compos. 2024, 45, 6332–6343. [Google Scholar] [CrossRef]
- Cortes, V.; Sanchez, K.; Gonzalez, R.; Alcoutlabi, M.; Ortega, J.A. The Performance of SiO2 and TiO2 Nanoparticles as Lubricant Additives in Sunflower Oil. Lubricants 2020, 8, 10. [Google Scholar] [CrossRef]









| Parameters | Density (15 °C, g/cm3) | Dynamic Viscosity (mm2/s) 40 °C | Dynamic Viscosity (mm2/s) 100 °C | Viscosity Index | Pour Point (°C) | Flash Point (°C) | Acid Value (mgKOH/g) |
|---|---|---|---|---|---|---|---|
| 0.8190 | 16.8 | 3.85 | 123 | −58 | 220 | ≤0.03 |
| Lubricant | Element Composition (%) | |||||
|---|---|---|---|---|---|---|
| Fe | C | O | Si | Cr | ||
| 25 °C | PAO | 89.71 | 7.37 | 1.20 | 0.26 | 1.45 |
| PAO+Silicone resin | 92.81 | 3.22 | 1.83 | 0.78 | 1.36 | |
| 50 °C | PAO | 94.85 | 2.81 | 0.69 | 0.19 | 1.46 |
| PAO+Silicone resin | 89.03 | 4.93 | 3.42 | 1.16 | 1.45 | |
| 75 °C | PAO | 93.68 | 3.42 | 1.20 | 0.21 | 1.45 |
| PAO+Silicone resin | 84.08 | 8.85 | 4.10 | 1.59 | 1.38 | |
| 100 °C | PAO | 89.06 | 5.60 | 3.68 | 0.23 | 1.44 |
| PAO+Silicone resin | 88.56 | 4.58 | 4.24 | 1.21 | 1.21 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; He, Z.; Xiong, L.; Qian, L.; Li, L.; Long, Q. Investigating the Anti-Wear Behavior of Polyalphaolefin Oil with Methyl Silicon Resin Using Advanced Analytical Techniques. Lubricants 2024, 12, 416. https://doi.org/10.3390/lubricants12120416
Wang H, He Z, Xiong L, Qian L, Li L, Long Q. Investigating the Anti-Wear Behavior of Polyalphaolefin Oil with Methyl Silicon Resin Using Advanced Analytical Techniques. Lubricants. 2024; 12(12):416. https://doi.org/10.3390/lubricants12120416
Chicago/Turabian StyleWang, Haiyang, Zhongyi He, Liping Xiong, Liang Qian, Lili Li, and Qiyang Long. 2024. "Investigating the Anti-Wear Behavior of Polyalphaolefin Oil with Methyl Silicon Resin Using Advanced Analytical Techniques" Lubricants 12, no. 12: 416. https://doi.org/10.3390/lubricants12120416
APA StyleWang, H., He, Z., Xiong, L., Qian, L., Li, L., & Long, Q. (2024). Investigating the Anti-Wear Behavior of Polyalphaolefin Oil with Methyl Silicon Resin Using Advanced Analytical Techniques. Lubricants, 12(12), 416. https://doi.org/10.3390/lubricants12120416





