Sliding Contact Fatigue Damage of Metallic Implants in a Simulated Body Fluid Environment
Abstract
:1. Introduction
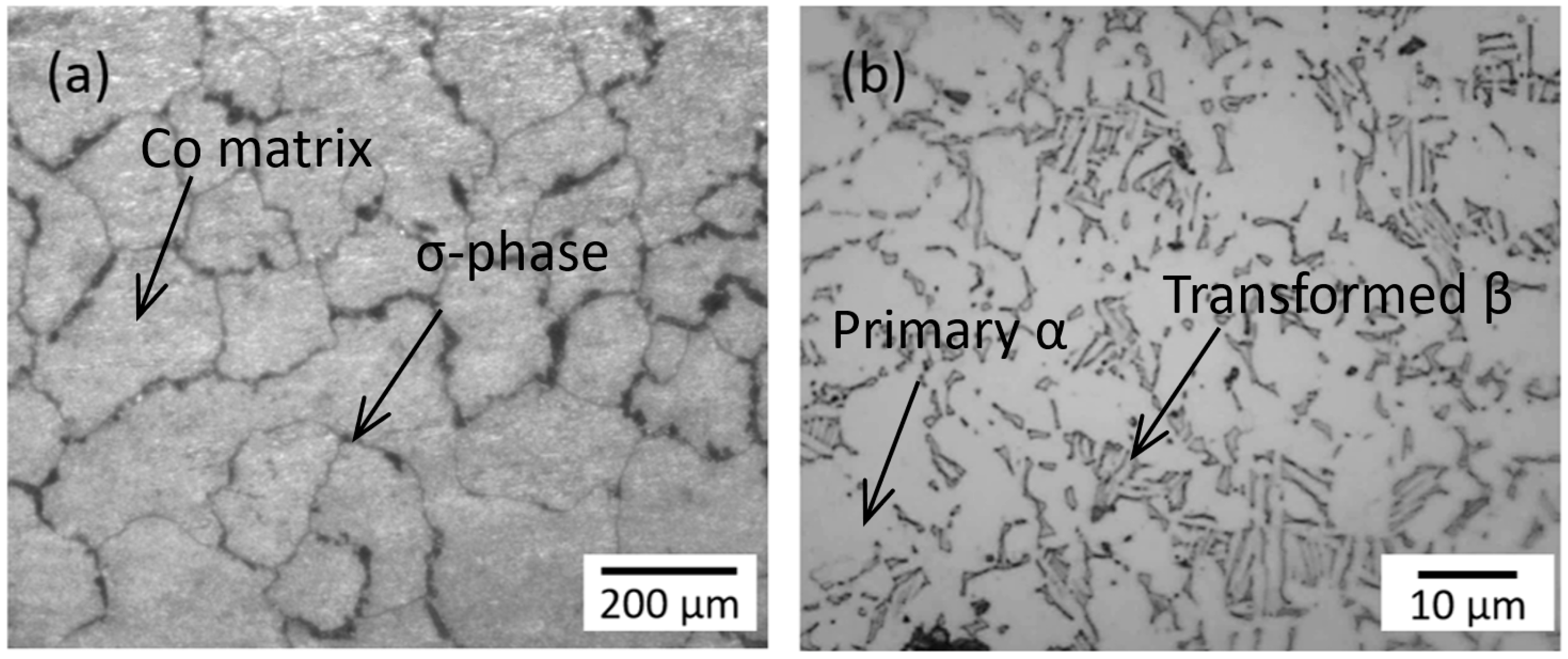
2. Materials and Experimental Description
3. Results and Discussion
3.1. Open-Circuit Potential Measurement
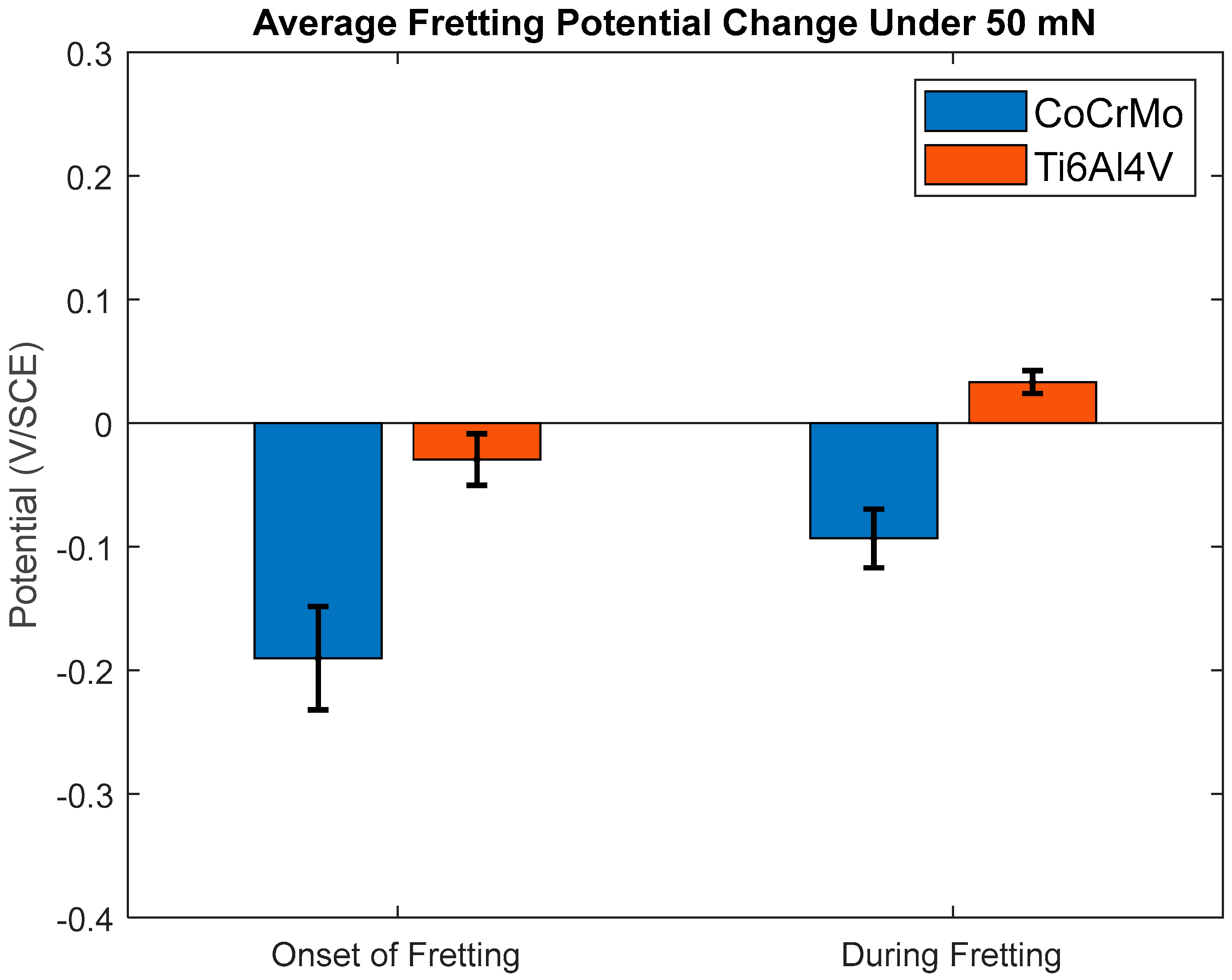
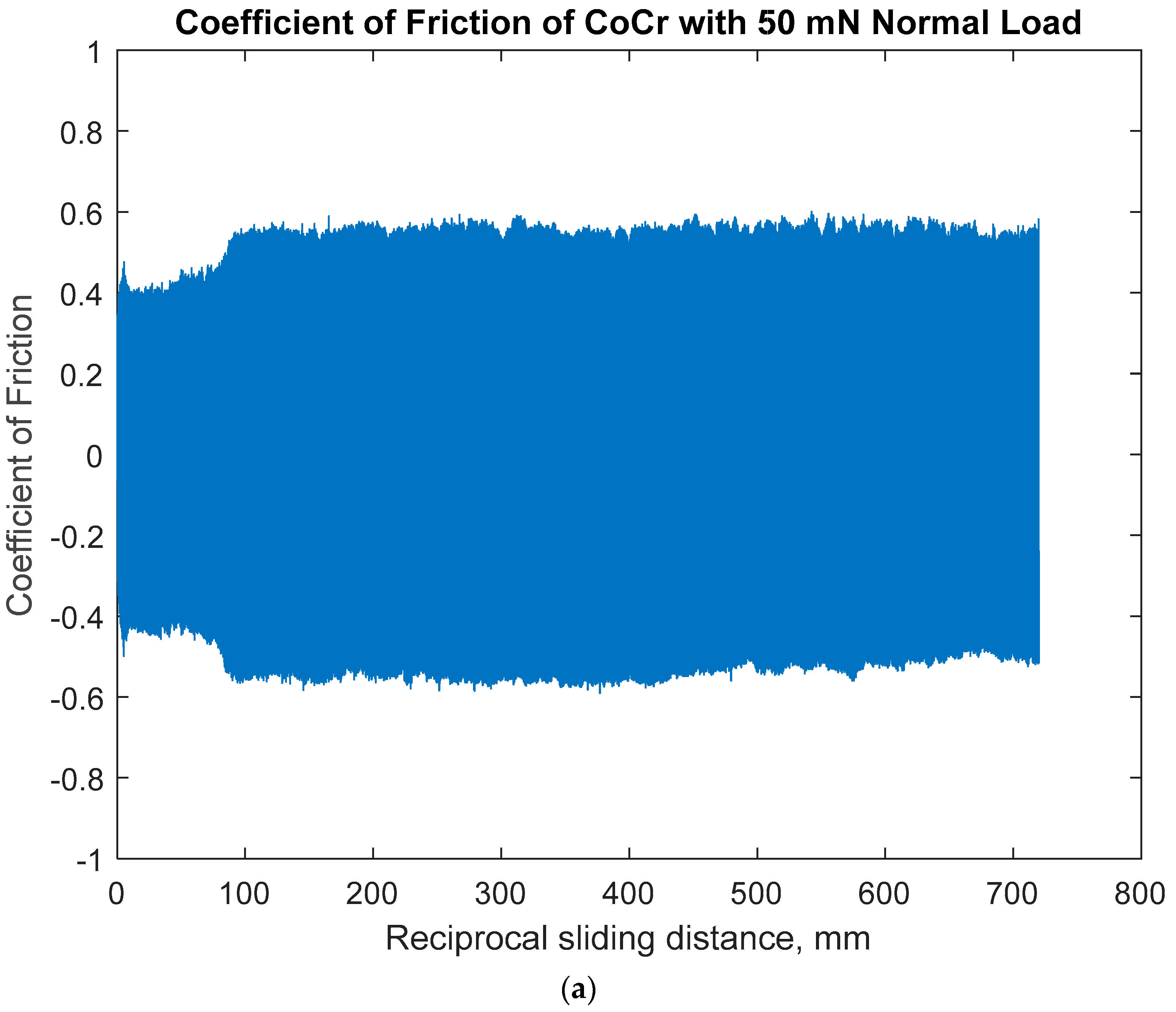
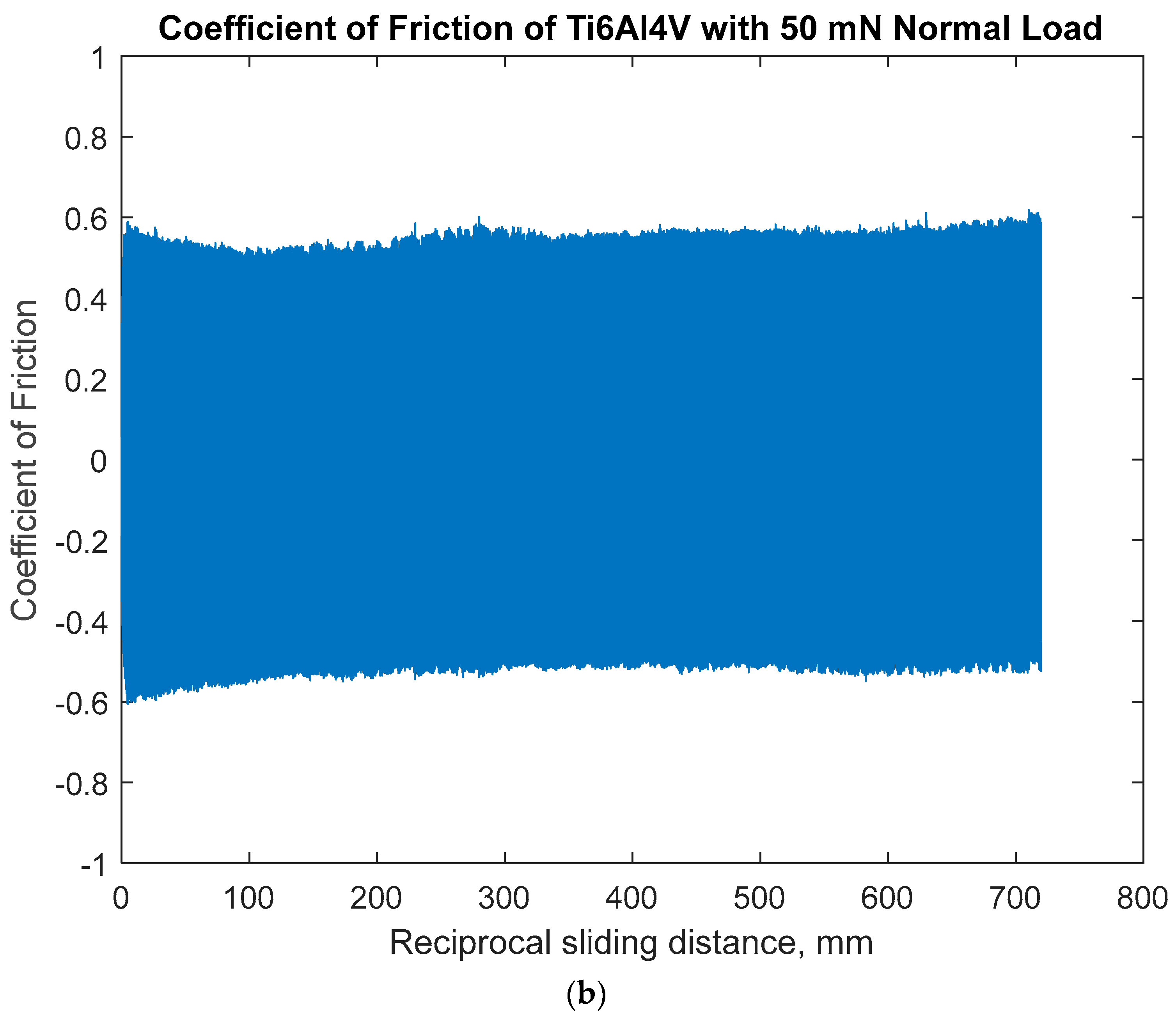
3.1.1. Open-Circuit Potential Under Normal Force of 50 mN
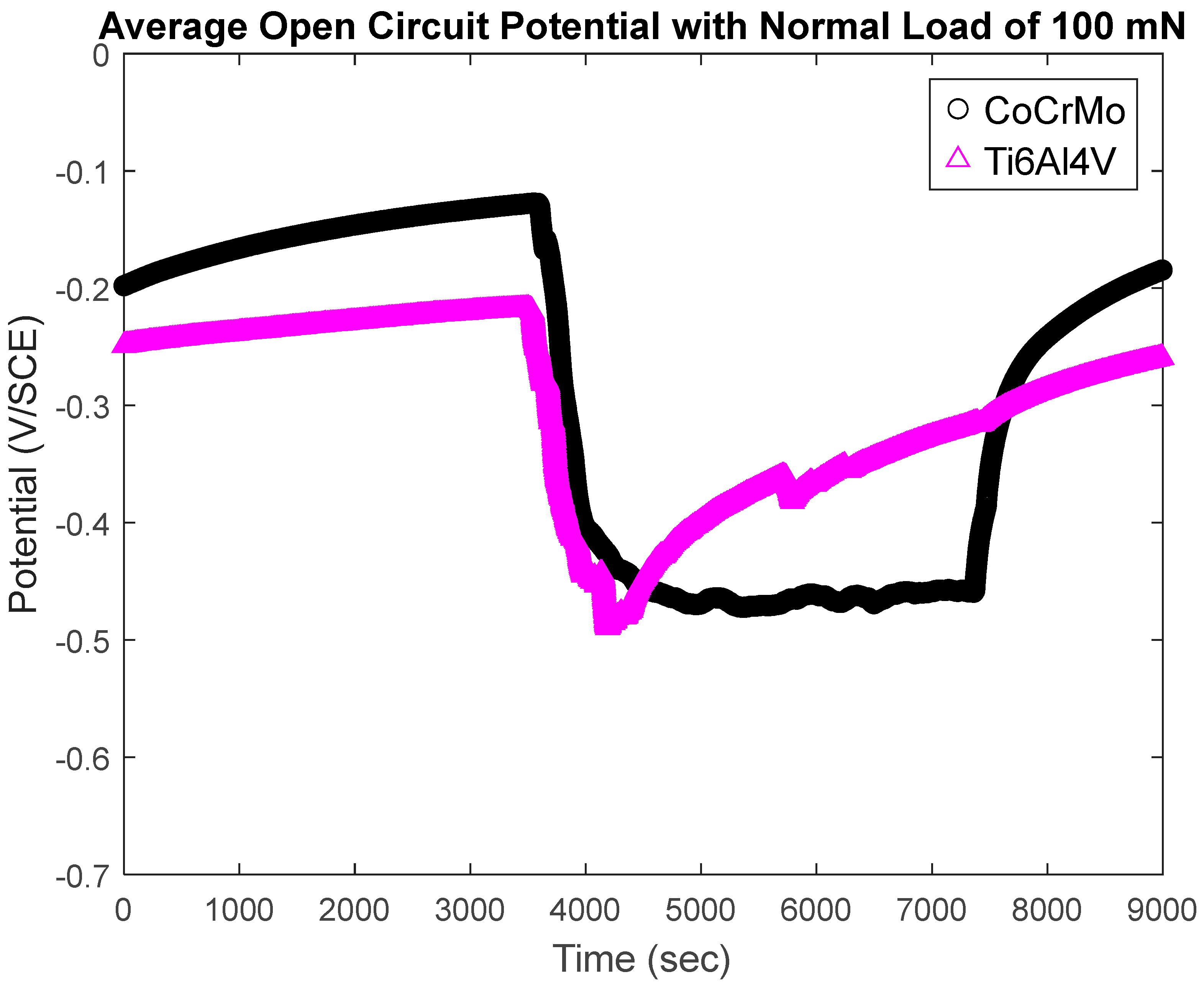
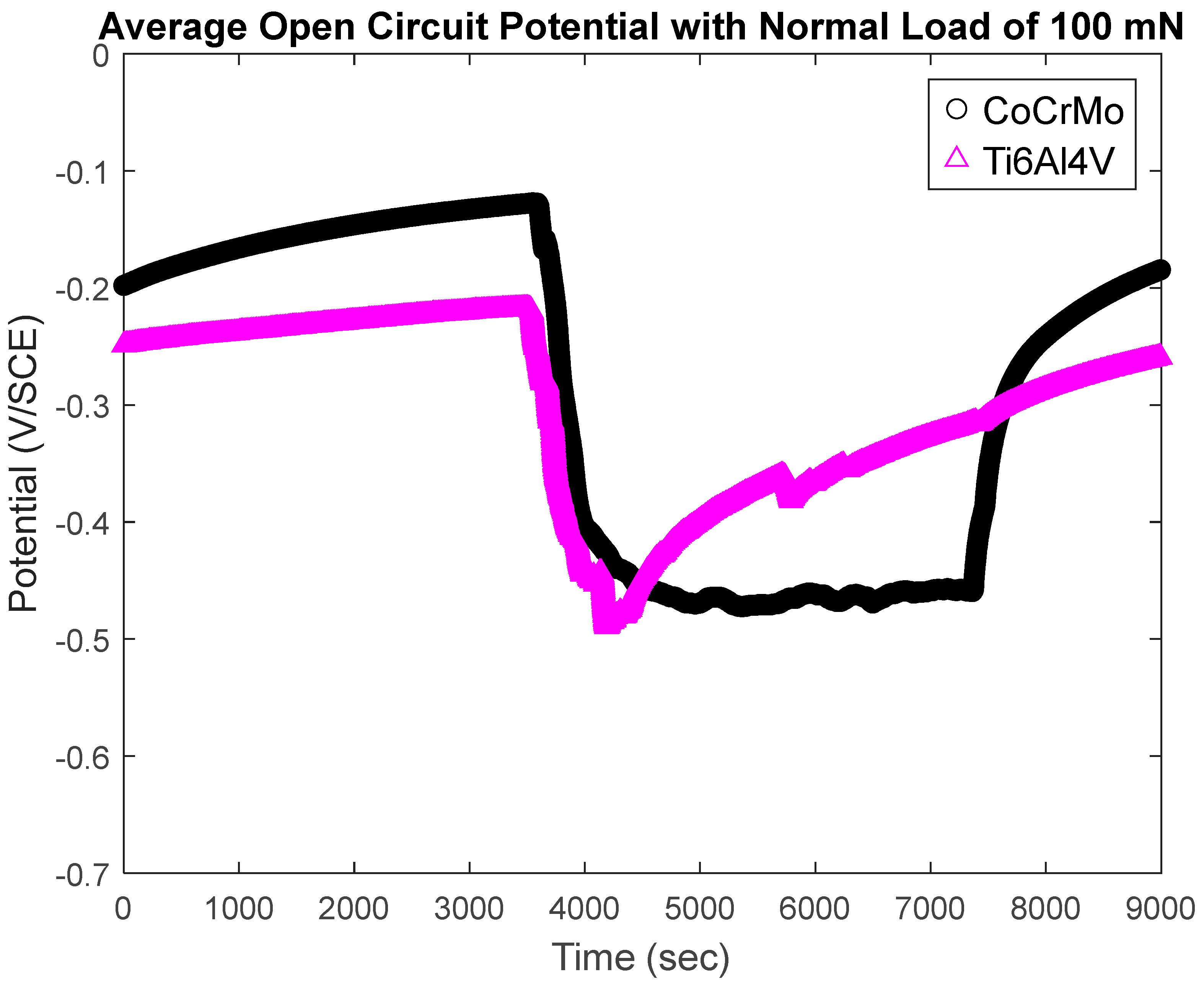
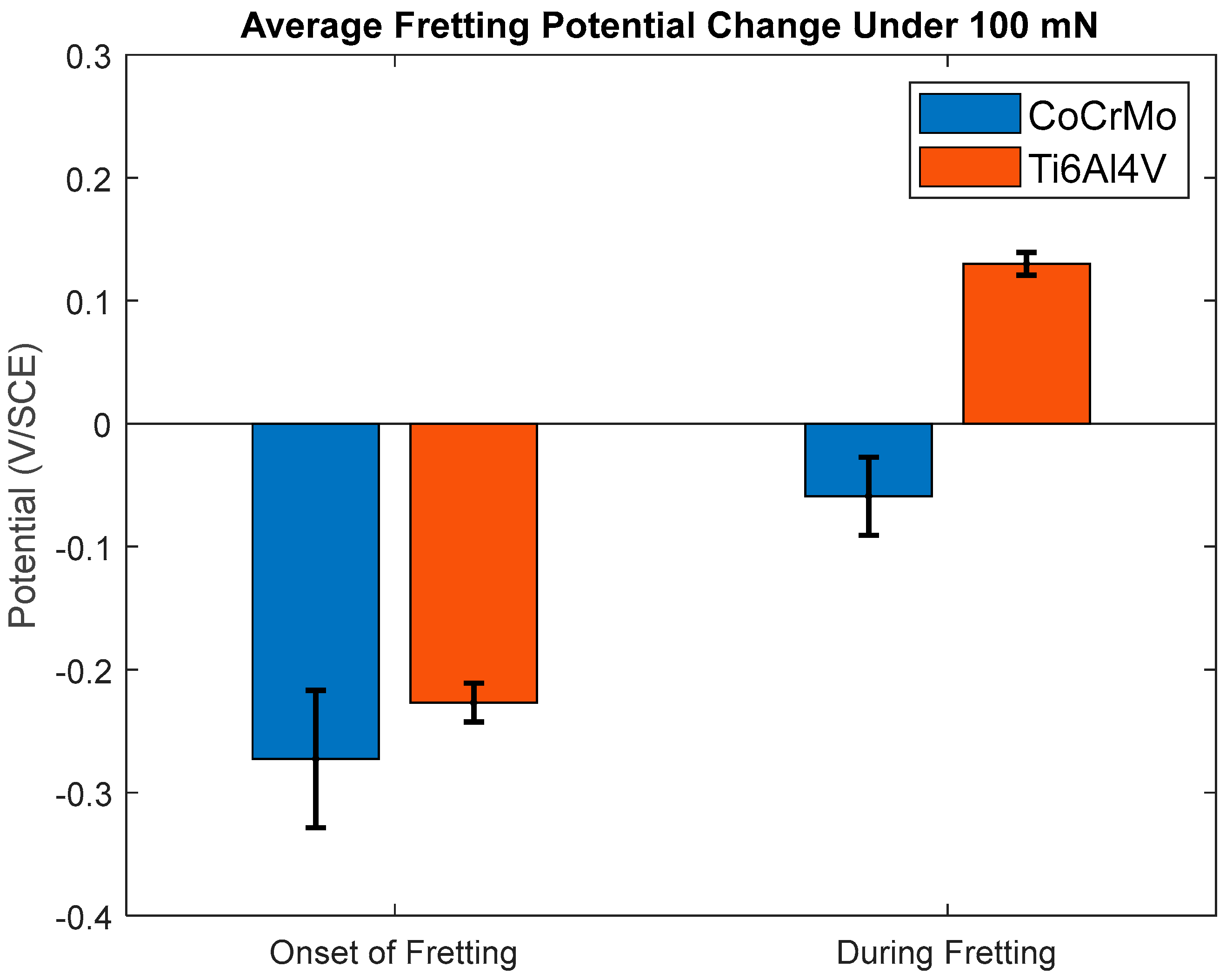
3.1.2. Open-Circuit Potential Under Normal Force 100 mN
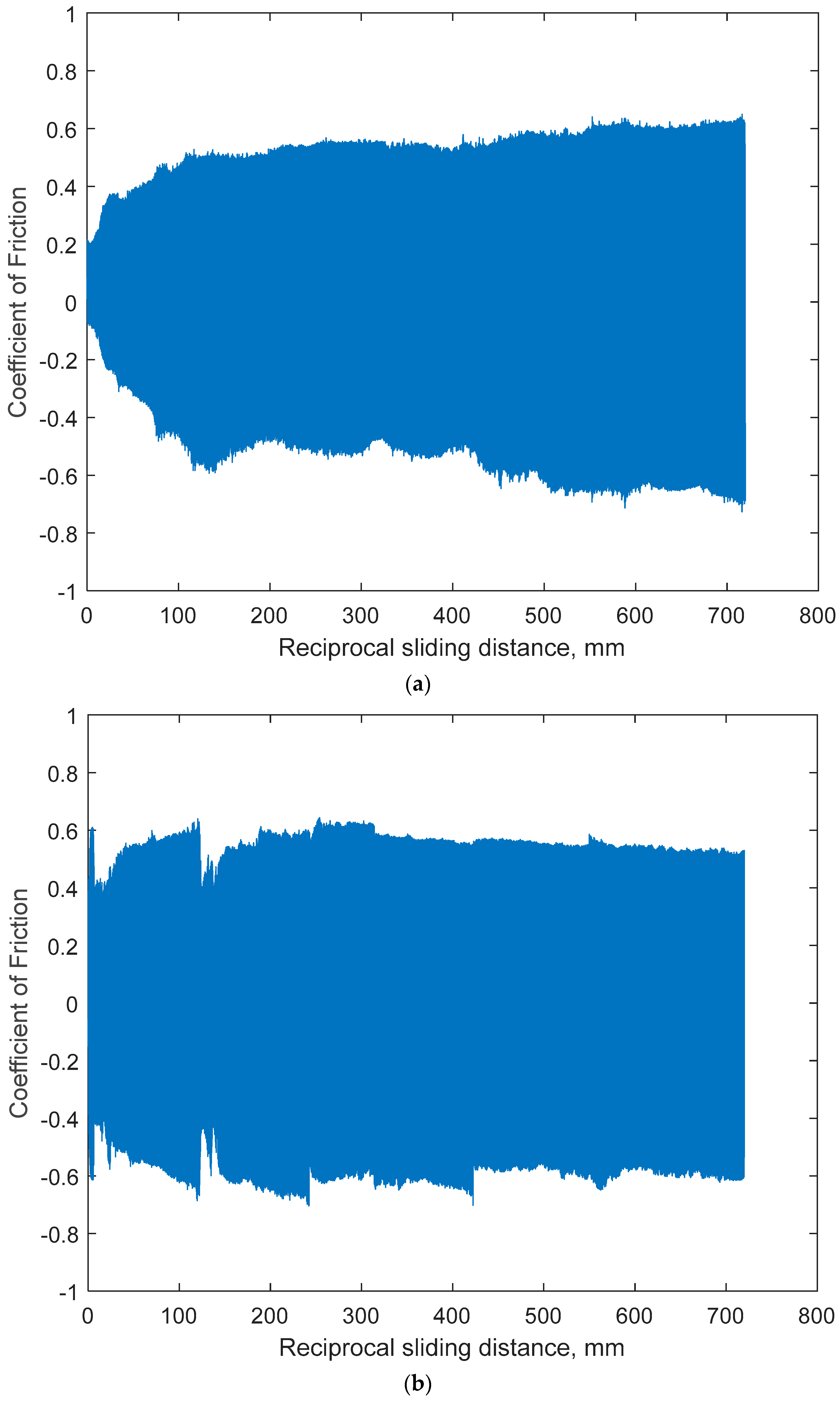
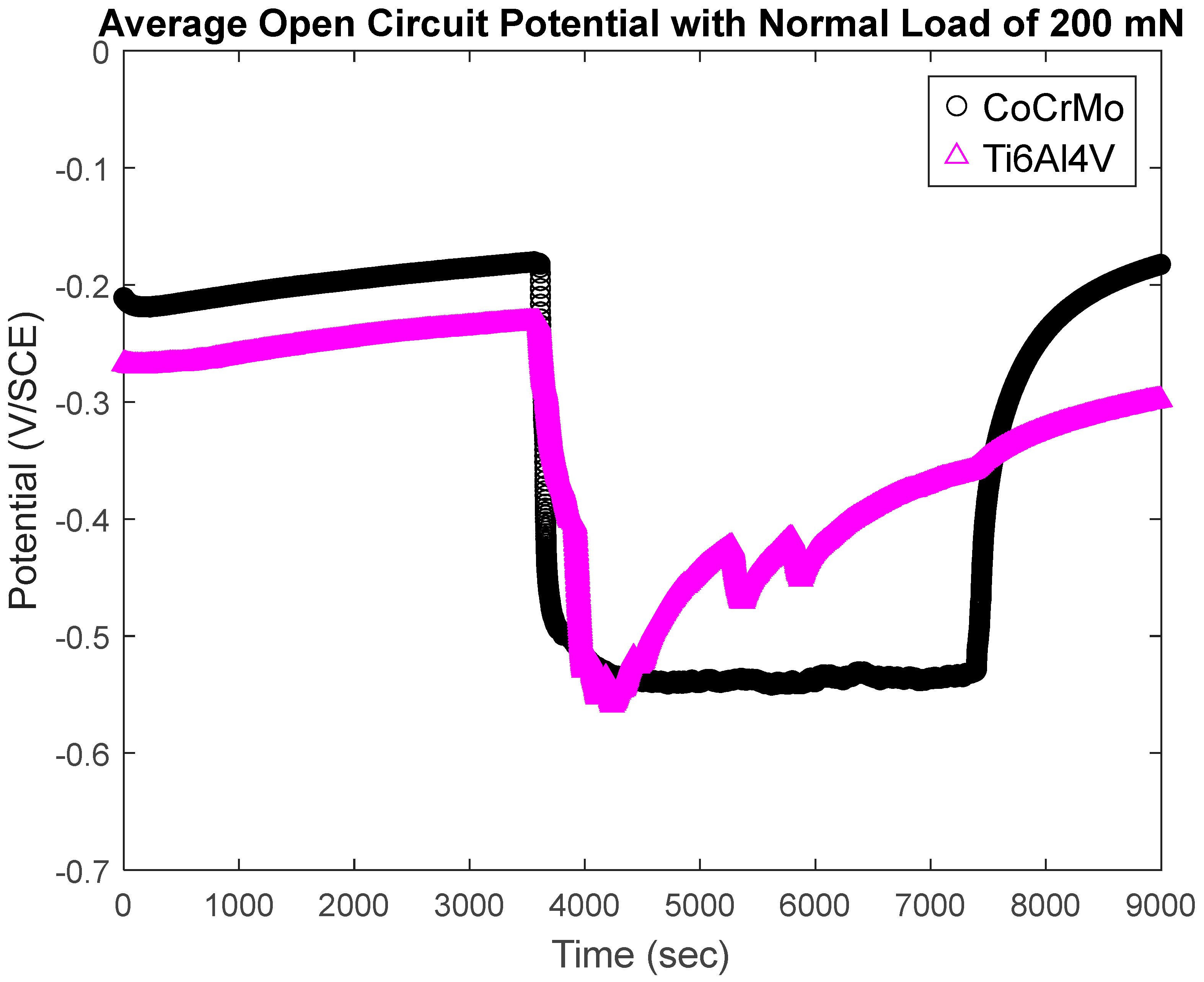
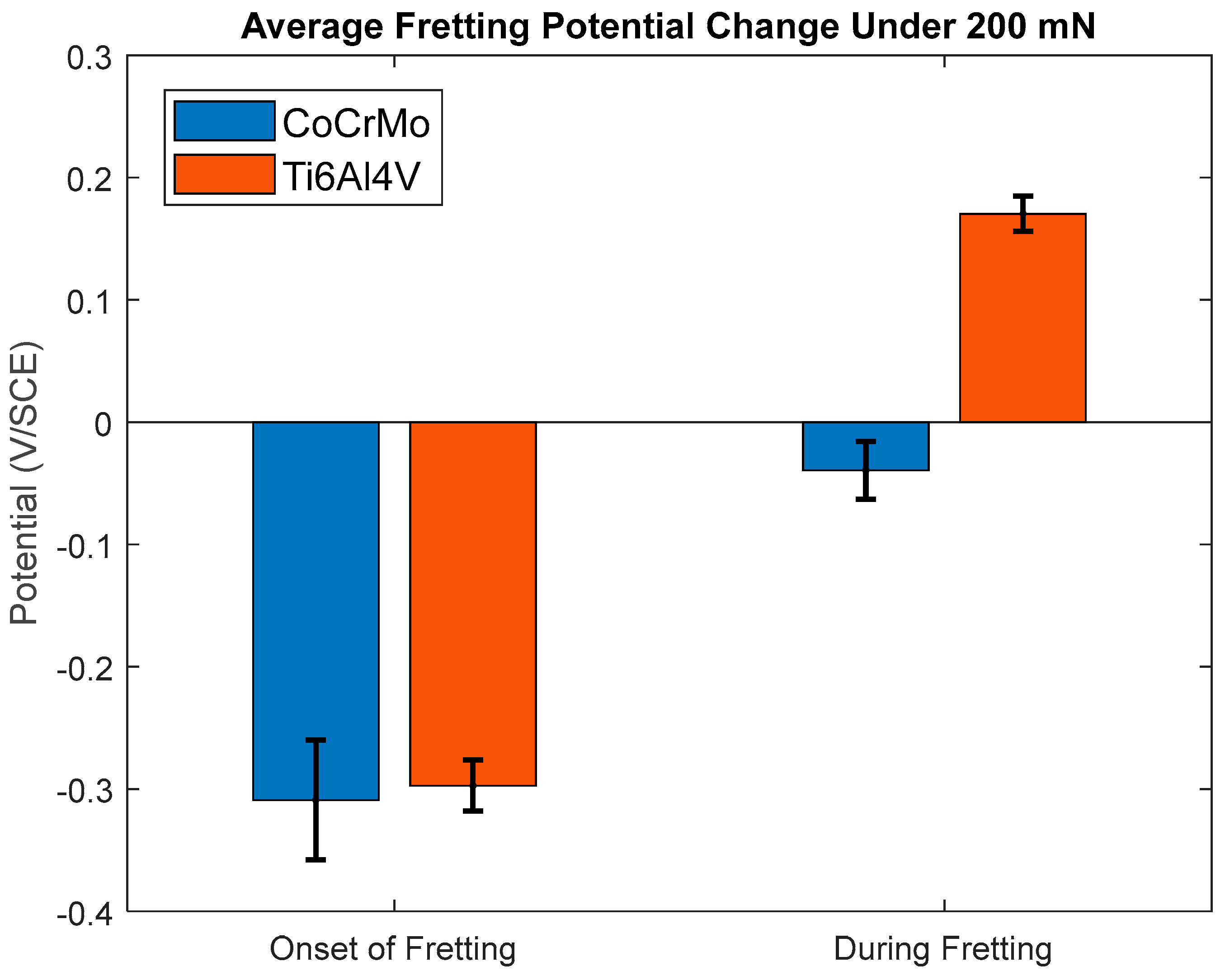
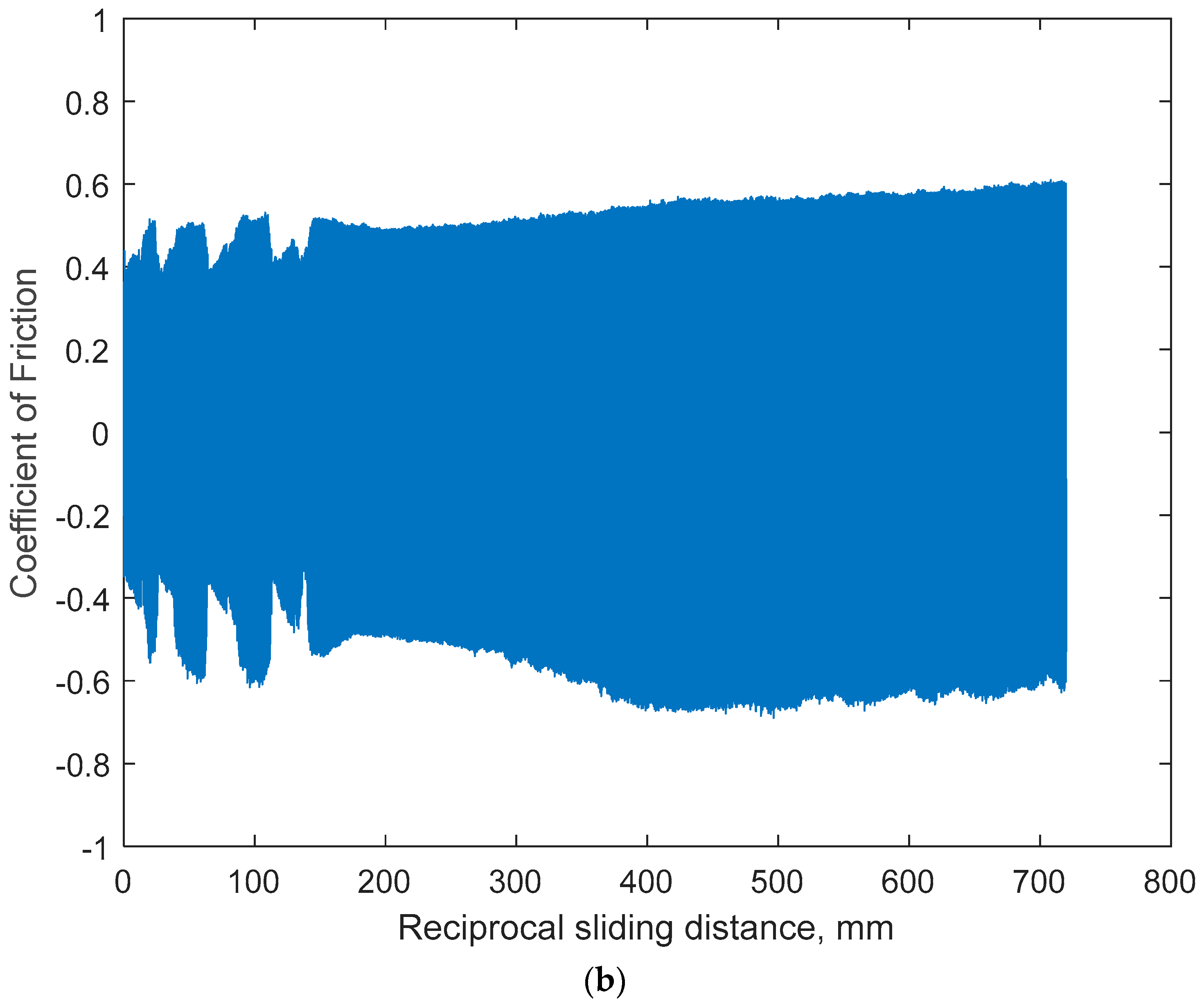
3.1.3. Open-Circuit Potential Under Normal Force of 200 mN
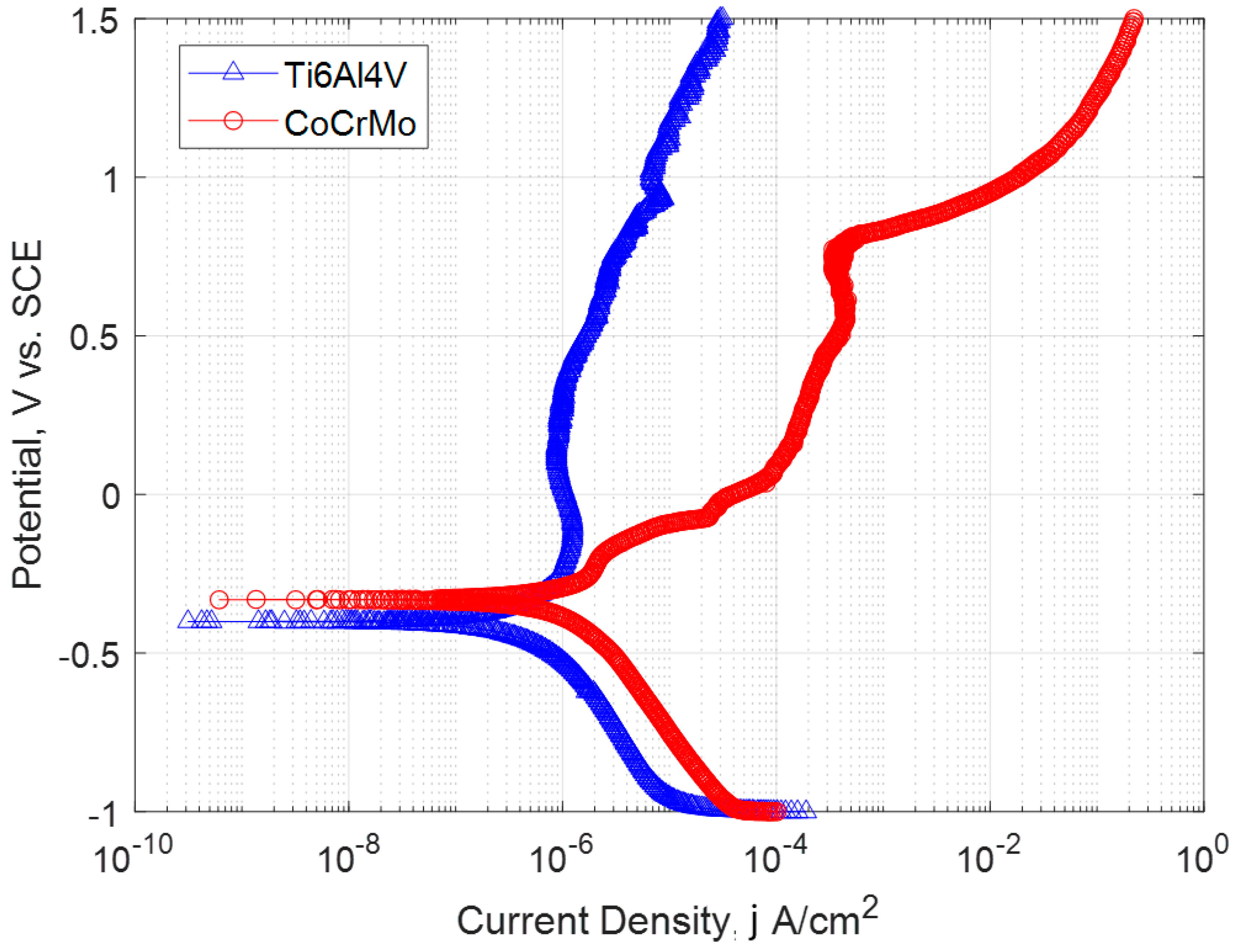
3.2. Potentiodynamic Polarization Resistance
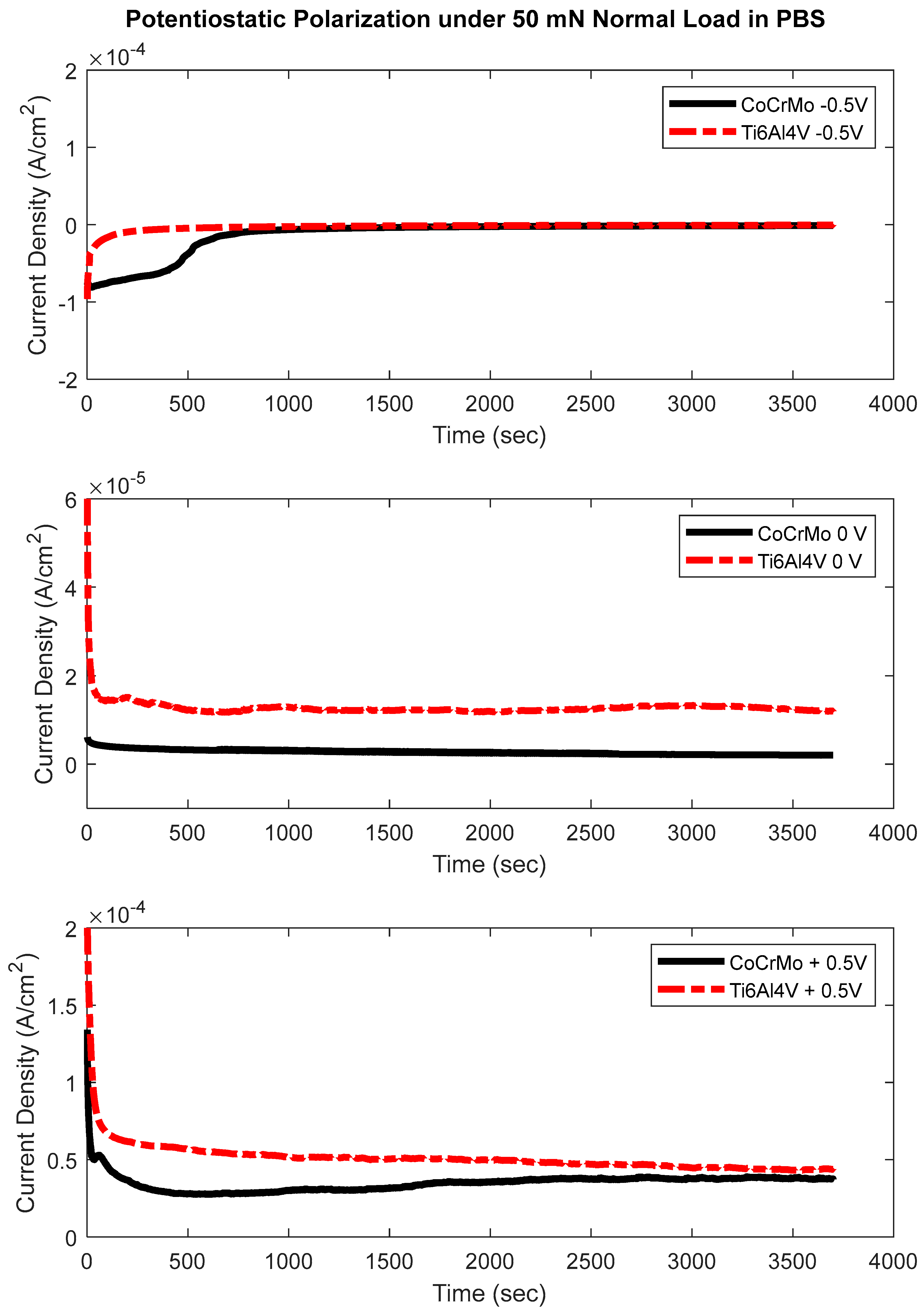
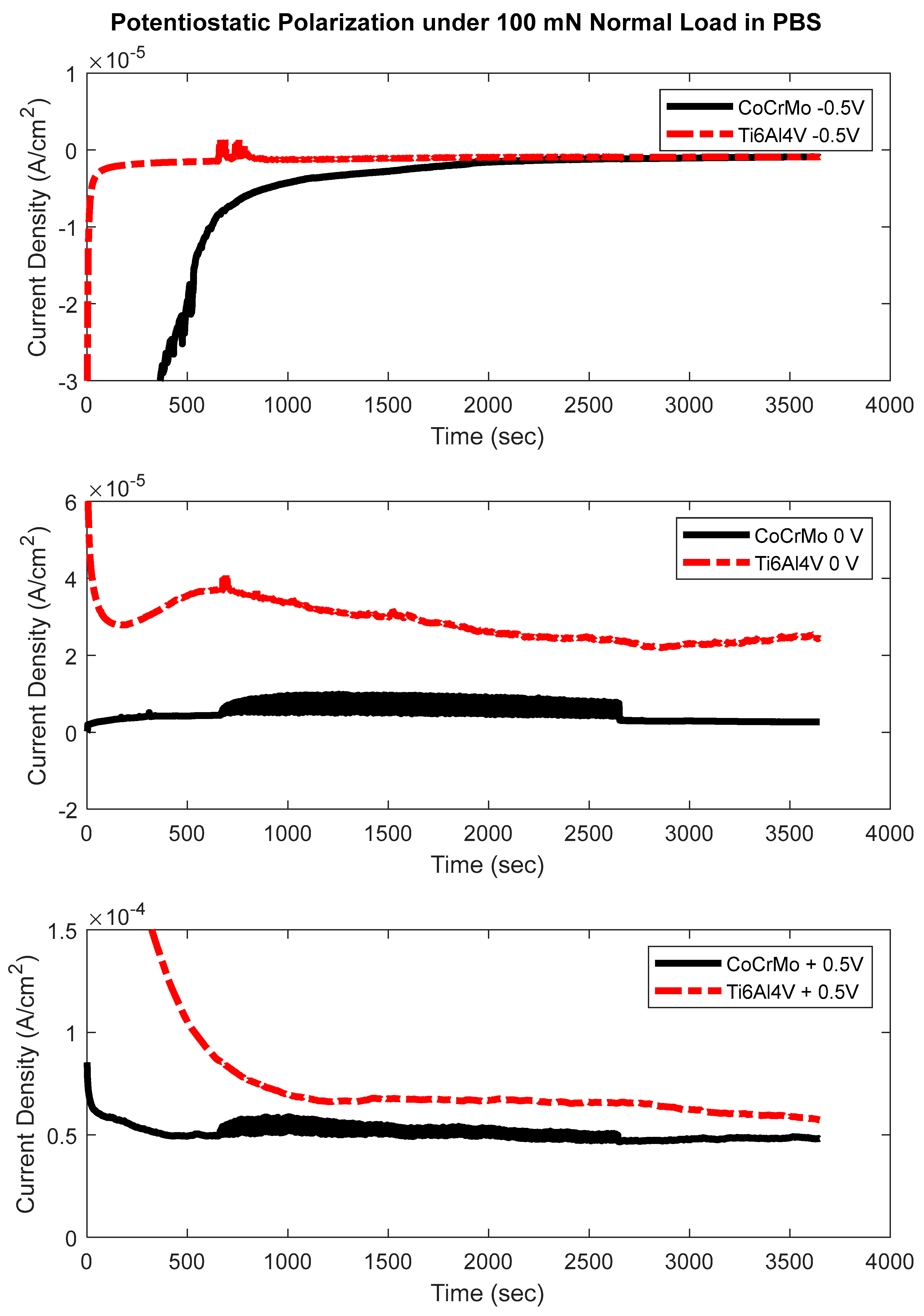
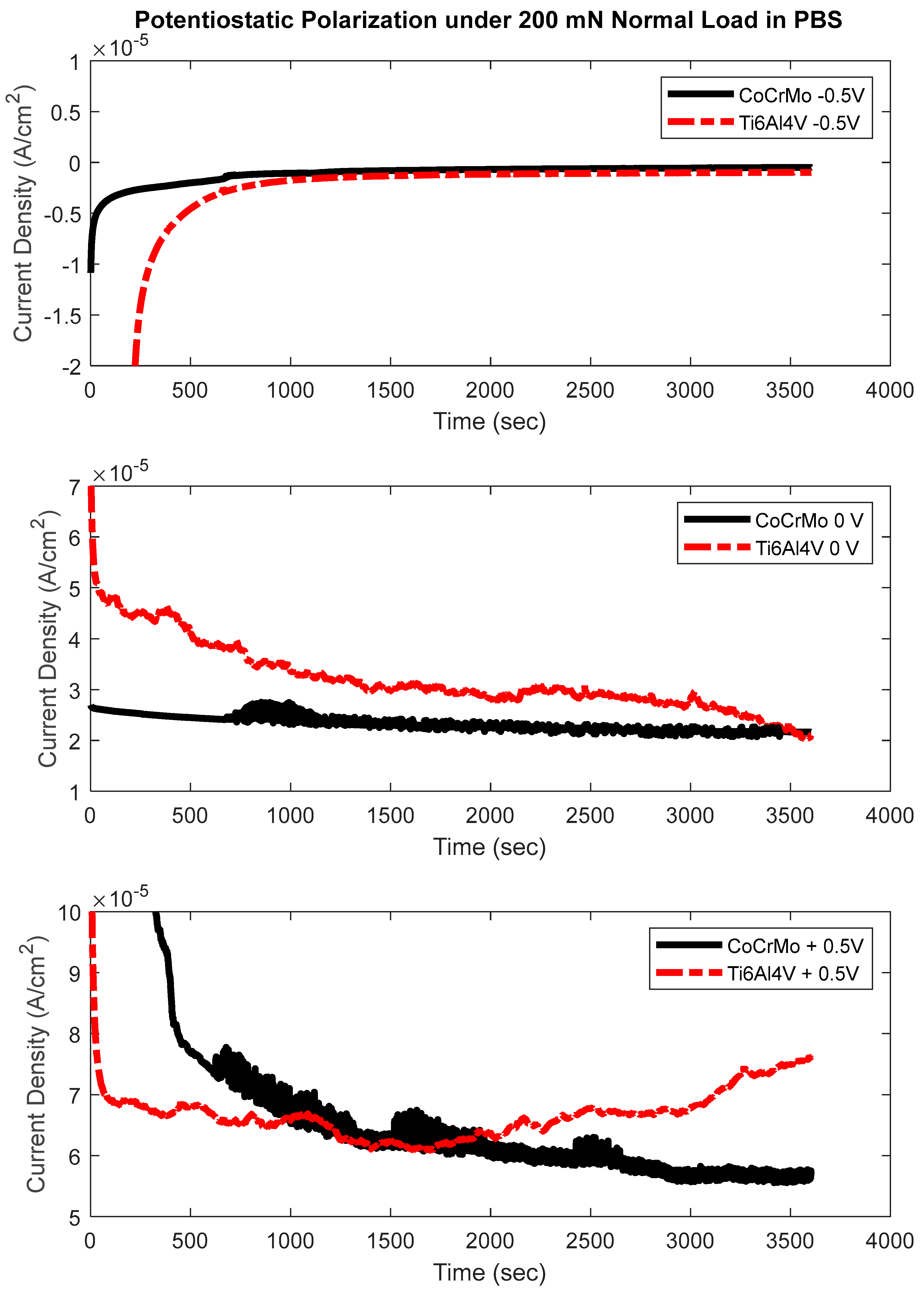
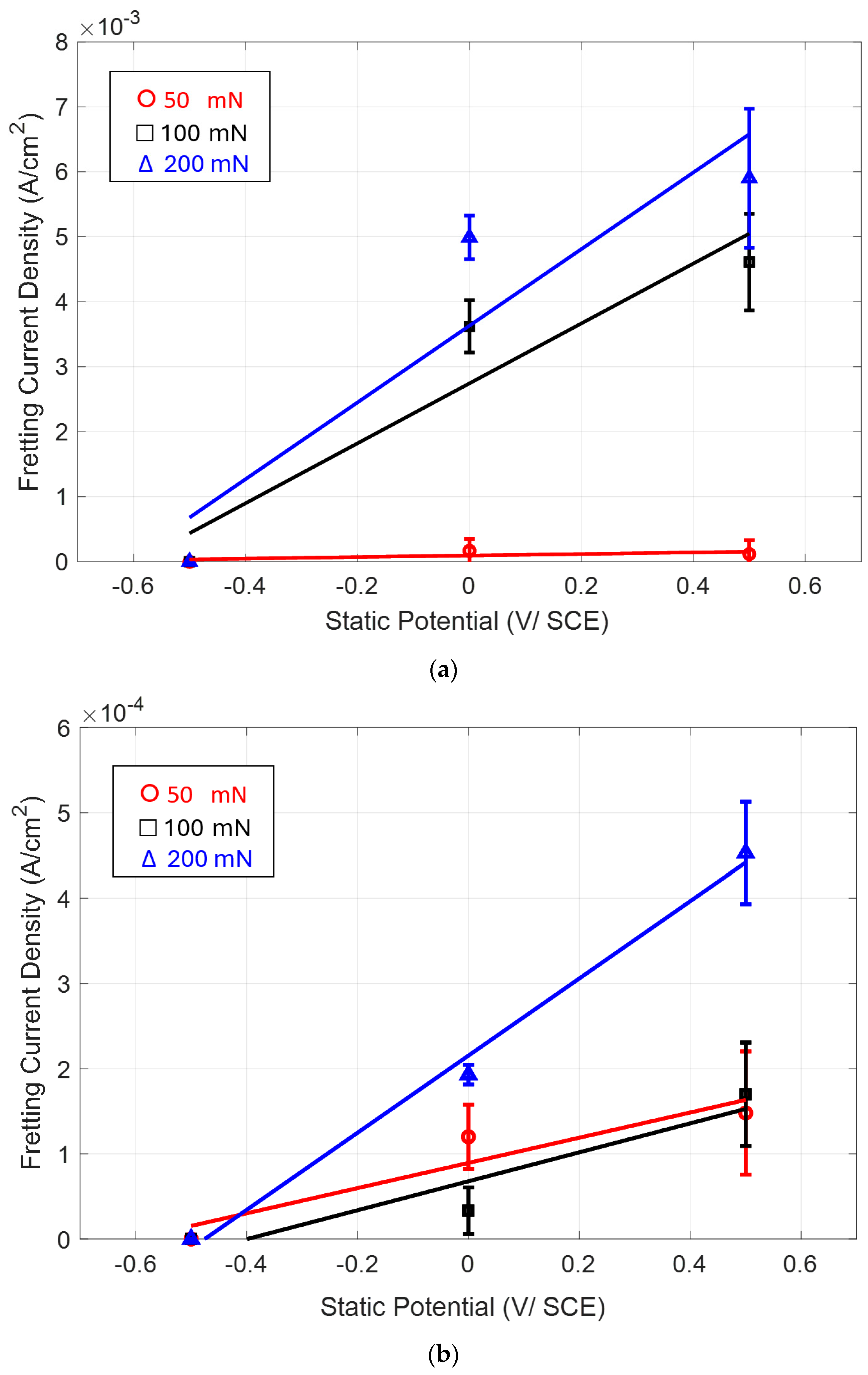
3.3. Potentiostatic Polarization Test
4. Conclusions
- This study compared the modified electrochemical responses on medical grade CoCrMo and Ti6Al4V surfaces when the active sliding fatigue initiated oxide damages. Reciprocal sliding motions were applied at varying normal contact loads in the elastic range to comprehend the multi-factorial behaviors of implant materials utilizing OCP, potentiodynamic polarization, and potentiostatic polarization measurements.
- The nominally elastic contact stress significantly accelerates electrochemical responses for both implant materials.
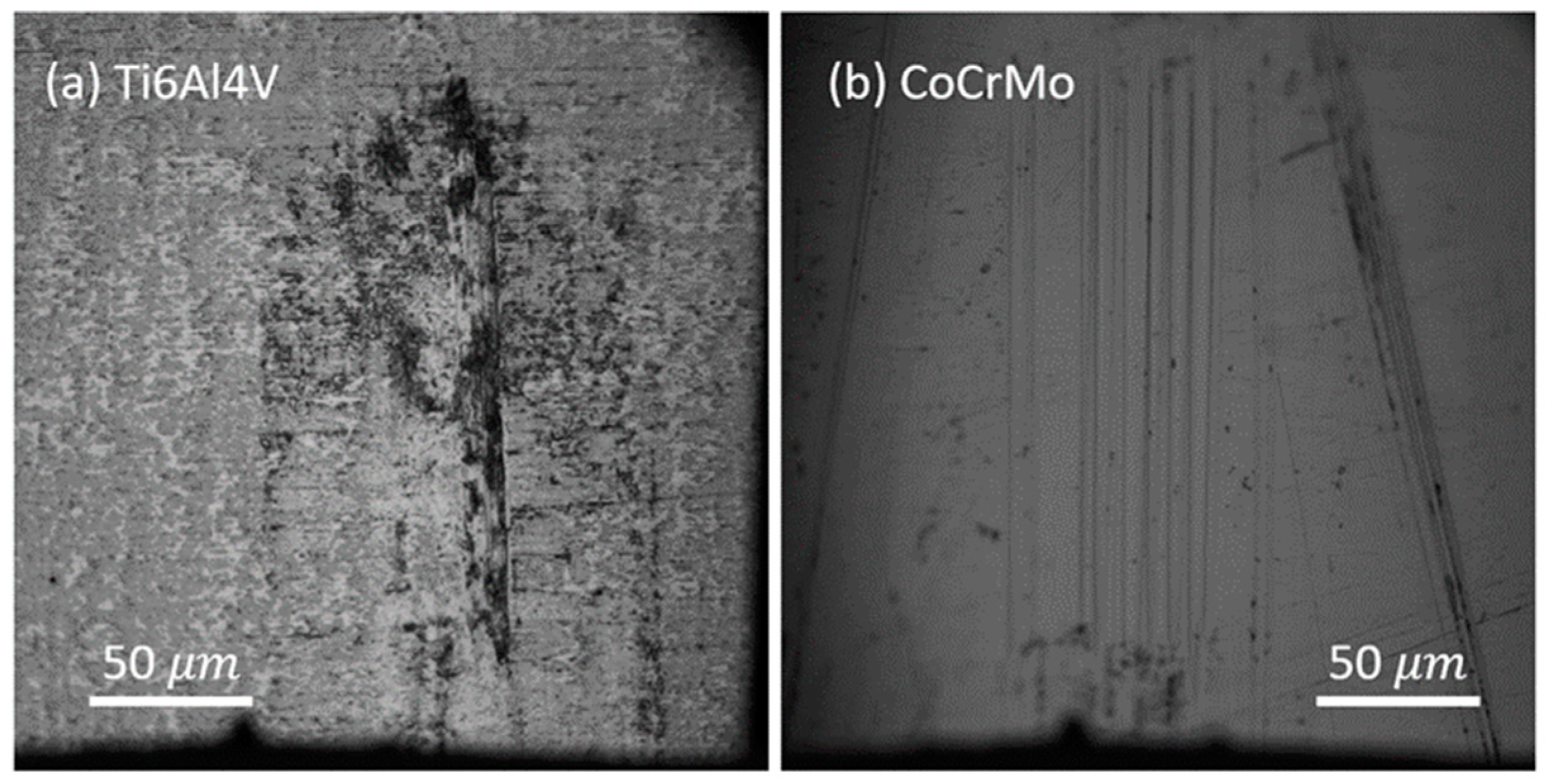
- The wear mechanism of Ti6Al4V in an aqueous environment illustrated that delamination would be the dominant wear process. The following contact cycles plastically deform and agglomerate produced wear particles that are ultimately seized on the damaged area.
- Nano-sized wear debris on CoCrMo produced during active sliding contact in PBS solution implies that immediate abrasive damage would take place on the brittle Cr oxide film at all normal loads. Wear debris were piled up surrounding the damaged zone.
- During active sliding, OCP dropped more in CoCrMo at all loads than Ti6Al4V suggesting more accelerated tribocorrosion damage on CoCrMo. OCP data illustrated prompt recovery of the titanium oxide layer that effectively protected metal matrix from anodic reactions.
- The sliding friction by COF was affected by the combination of anodic potential change and the wear damage processes of both specimens: the larger variation in OCP and COF on Ti6Al4V would be due to the alternating entrapment and ejection of the wear particle.
- The thermodynamic driving force initiated by cumulated elastic strains on Ti6Al4V played a significant role in establishing electrochemically stable titanium dioxide.
- The potentiodynamic polarization test results showed a high passive zone for Ti6Al4V. The icorr value was lower by an order of one magnitude suggesting a lower corrosion rate. The spontaneous and wide passive zone of titanium oxide represented less corrosion on higher potential. CoCrMo was more on an active anodic side and had a transpassive region which represents the dissolution of the metal ion in the electrolyte.
- The potentiostatic test concluded that oxidation chemistry on CoCrMo exhibited more sensitivity at the greater normal loads of 100 mN and 200 mN. Significant electrochemical sensitivity on Ti6Al4V was found only at the highest normal load of 200 mN.
- This experimental work generally concluded that CoCrMo is more prone to be vulnerable when mechanical stimuli is accompanied with corrosion attacks. Ti6Al4V would be a more durable metallic implant material for the load-bearing orthopedic applications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morlock, M.M.; Hube, R.; Wassilew, G.; Prange, F.; Huber, G.; Perka, C. Taper corrosion: A complication of total hip arthroplasty. EFORT Open Rev. 2020, 5, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Nečas, D.; Usami, H.; Niimi, T.; Sawae, Y.; Krupka, I.; Hartl, M. Running-in friction of hip joint replacements can be significantly reduced: The effect of surface-textured acetabular cup. Friction 2020, 8, 1137–1152. [Google Scholar] [CrossRef]
- Martin, R.B.; Burr, D.B.; Sharkey, N.A.; Fyhrie, D.P. Skeletal Tissue Mechanics; Springer: New York, NY, USA, 2015. [Google Scholar]
- Hallab, N.; Jacobs, J. Orthopedic Implant Fretting Corrosion. Corros. Rev. 2003, 21, 183–214. [Google Scholar] [CrossRef]
- Couto, M.; Vasconcelos, D.; Sousa, D.M.; Sousa, B.; Conceição, F.; Neto, E.; Lamghari, M.; Alves, C.J. The Mechanisms Underlying the Biological Response to Wear Debris in Periprosthetic Inflammation. Front. Mater. 2020, 7, 274. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, N.; Zhang, C. Characteristics of copper corrosion in simulated uterine fluid in the presence of protein. Adv. Contracept. 1999, 15, 179–190. [Google Scholar] [CrossRef]
- Liu, Y.; Gilbert, J.L. The effect of simulated inflammatory conditions and pH on fretting corrosion of CoCrMo alloy surfaces. Wear 2017, 390–391, 302–311. [Google Scholar] [CrossRef]
- Rebenda, D.; Vrbka, M.; Cípek, P.; Toropitsyn, D.; Necas, D.; Pravda, M.; Hart, M. On the Dependence of Rheology of Hyaluronic Acid Solutions and Frictional Behavior of Articular Cartilage. Materials 2020, 13, 2659. [Google Scholar] [CrossRef]
- Moharrami, N.; Langton, D.; Sayginer, O.; Bull, S. Why does titanium alloy wear cobalt chrome alloy despite lower bulk hardness: A nanoindentation study? Thin Solid Film. 2013, 549, 79–86. [Google Scholar] [CrossRef]
- Mihalko, W.M.; Haider, H.; Kurtz, S.; Marcolongo, M.; Urish, K. New materials for hip and knee joint replacement: What’s hip and what’s in kneed? J. Orthop. Res. 2019, 38, 1436–1444. [Google Scholar] [CrossRef]
- Park, J.B.; Lakes, R.S. Biomaterials: An Introduction; Springer: New York, NY, USA, 2007. [Google Scholar]
- Langton, D.J.; Sidaginamale, R.; Lord, J.K.; Nargol, A.V.F.; Joyce, T.J. Taper junction failure in large-diameter metal-on-metal bearings. Bone Jt. Res. 2012, 1, 56–63. [Google Scholar] [CrossRef]
- Compte, P. Metallurgical observations of Biomaterials. In Contemporary Biomaterials; Boretos, J.W., Eden, M., Eds.; Noyes Publications: Park Ridge, NJ, USA, 1984. [Google Scholar]
- Tardelli, J.D.C.; Bolfarini, C.; dos Reis, A.C. Comparative analysis of corrosion resistance between beta titanium and Ti-6Al-4V alloys: A systematic review. J. Trace Elem. Med. Biol. 2020, 62, 126618. [Google Scholar] [CrossRef]
- ASTM International. Standard Terminology Relating to Wear and Erosion. In Annual Book of Standards; ASTM: West Conshohocken, PA, USA, 1987; Volume 3, pp. 243–250. [Google Scholar]
- Lipsitt, H.A.; Wang, D.Y. The effects of interstitial solute atoms on the fatigue limit behavior of titanium. Trans. AIME 1961, 221, 918. [Google Scholar]
- Sonntag, R.; Reinders, J.; Gibmeier, J.; Kretzer, J.P. Fatigue Performance of Medical Ti6Al4V Alloy after Mechanical Surface Treatments. PLoS ONE 2015, 10, e0121963. [Google Scholar] [CrossRef] [PubMed]
- Ratner, B.D. Biomaterials Science: An Introduction to Materials in Medicine; Elsevier, Academic Press: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Oungoulian, S.R.; Durney, K.M.; Jones, B.K.; Ahmad, C.S.; Hung, C.T.; Ateshian, G.A. Wear and damage of articular cartilage with friction against orthopedic implant materials. J. Biomech. 2015, 48, 1957–1964. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.L. Electrochemical behavior of metals in the biological milieu. In Comprehensive Biomaterials; Healy, K.E., Ducheyne, P., Kirkpatrick, C.J., Eds.; Elsevier Press: Amsterdam, The Netherlands, 2011; Chapter 13. [Google Scholar]
- Hatamleh, M.M.; Wu, X.; Alnazzawi, A.; Watson, J.; Watts, D. Surface characteristics and biocompatibility of cranioplasty titanium implants following different surface treatments. Dent. Mater. 2018, 34, 676–683. [Google Scholar] [CrossRef]
- Wimmber, M.A.; Radice, S.; Janssen, D.; Fischer, A. Fretting-corrosion of CoCr-alloys against Ti6Al4V: The importance of molybdenum in oxidative biological environments. Wear 2021, 477, 203813. [Google Scholar] [CrossRef]
- Quiram, G.; Gindri, I.M.; Kerwell, S.; Shull, K.; Mathew, M.T.; Rodrigues, D.C. Nanoscale Mechanical Evaluation of Electrochemically Generated Tribolayer on CoCrMo Alloy for Hip Joint Application. J. Bio- Tribo-Corros. 2016, 2, 15. [Google Scholar] [CrossRef]
- Sasikumar, Y.; Indira, K.; Rajendran, N. Surface Modification Methods for Titanium and Its Alloys and Their Corrosion Behavior in Biological Environment: A Review. J. Bio- Tribo-Corros. 2019, 5, 36. [Google Scholar] [CrossRef]
- Baragetti, S.; Villa, F. Corrosion Fatigue of High-Strength Titanium Alloys under Different Stress Gradients. J. Miner. Met. Mater. Soc. 2015, 67, 1154–1161. [Google Scholar] [CrossRef]
- Beevers, C.J.; Robinson, J.L. Some observations on the influence of oxygen content on the fatigue behavior of α-titanium. J. Less Common Met. 1969, 17, 345–352. [Google Scholar] [CrossRef]
- Posada, O.M.; Tate, R.J.; Meek, R.D.; Grant, M.H. In Vitro Analyses of the Toxicity, Immunological, and Gene Expression Effects of Cobalt-Chromium Alloy Wear Debris and Co Ions Derived from Metal-on-Metal Hip Implants. Lubricants 2015, 3, 539–568. [Google Scholar] [CrossRef]
- Chandra, A.; Ryu, J.; Karra, P.; Shrotriya, P.; Weik, T. Electrochemical dissolution of biomedical grade Ti6Al4V: Influence of stress and environment. CIRP Ann. 2009, 58, 499–502. [Google Scholar] [CrossRef]
- Ryu, J.; Shrotriya, P. Mechanical load assisted dissolution response of biomedical cobalt–chromium and titanium metallic alloys: Influence of in-plane stress and chemical environment. Wear 2015, 332–333, 662–668. [Google Scholar] [CrossRef]
- Ryu, J.J.; Shrotriya, P. Influence of roughness on surface instability of medical grade cobalt–chromium alloy (CoCrMo) during contact corrosion–fatigue. Appl. Surf. Sci. 2013, 273, 536–541. [Google Scholar] [CrossRef]
- Ryu, J.; Letchuman, S.; Shrotriya, P. Roughness evolution of metallic implant surfaces under contact loading and nanometer-scale chemical etching. J. Mech. Behav. Biomed. Mater. 2012, 14, 55–66. [Google Scholar] [CrossRef]
- Chattopadhyay, R. Surface Wear—Analysis, Treatment, and Prevention; ASM International: Materials Park, OH, USA, 2001. [Google Scholar]
- Kumar, S.; Sivakumar, B.; Narayanan, T.S.; Raman, S.G.S.; Seshadri, S.K. Fretting-corrosion mapping of CP-Ti in Ringer’s solution. Wear 2010, 268, 1537–1541. [Google Scholar] [CrossRef]
- Alemón, B.; Flores, M.; Ramírez, W.; Huegel, J.C.; Broitman, E. Tribocorrosion behavior and ions release of CoCrMo alloy coated with a TiAlVCN/CNx multilayer in simulated body fluid plus bovine serum albumin. Tribol. Int. 2015, 81, 159–168. [Google Scholar] [CrossRef]
- Flemming, J.R.; Suh, N.P. Mechanics of crack propagation in delamination wear. Wear 1977, 44, 9–56. [Google Scholar] [CrossRef]
- Mellado-Valero, A.; Muñoz, A.; Pina, V.; Sola-Ruiz, M. Electrochemical Behaviour and Galvanic Effects of Titanium Implants Coupled to Metallic Suprastructures in Artificial Saliva. Materials 2018, 11, 171. [Google Scholar] [CrossRef]
- Mitchell, A.; Shrotriya, P. Mechanical load-assisted dissolution of metallic implant surfaces: Influence of contact loads and surface stress state. Acta Biomater. 2008, 4, 296–304. [Google Scholar] [CrossRef]




| Ti6Al4V (F136) | ||||||||
| C (%) | N (%) | O (%) | Fe (%) | Al (%) | V (%) | Ti | ||
| 0.032 ± 0.01 | 0.0063 ± 0.001 | 0.12 ± 0.01 | 0.18 ± 0.065 | 4.67 ± 1.72 | 3.95 ± 0.11 | Balance | ||
| CoCrMo (F1537) | ||||||||
| C (%) | Si (%) | Mn (%) | Ni (%) | Cr (%) | Mo (%) | Fe (%) | N (%) | Co |
| 0.05 ± 0.01 | 0.59 ± 0.005 | 0.748 ± 0.105 | 0.133 ± 0.083 | 27.52 ± 0.439 | 5.59 ± 0.15 | 0.198 ± 0.039 | 0.168 ± 0.004 | Balance |
| Materials | Roughness (Ra, nm) | Elastic Modulus (GPa) | Yield Strength (GPa) | Hardness (GPa) |
|---|---|---|---|---|
| Ti6Al4V | 36 ± 8 | 134 ± 21 | 924 ± 19.1 | 5.07 ± 0.25 |
| CoCrMo | 40 ± 12 | 299 ± 13 | 1014 ± 19.8 | 6.96 ± 0.16 |
| Method | Parameters |
|---|---|
| Contact mode | Reciprocal sliding |
| Normal contact load | 50 mN |
| 100 mN | |
| 200 mN | |
| Sliding distance | 200 µm |
| Sliding speed | 12 mm/min |
| Sliding cycles | 1800 cycles |
| Environment | PBS pH 7.4 |
| Materials | Ecorr, V | Ep, V | ||
|---|---|---|---|---|
| CoCrMo | −0.331 | 1.351 | −0.206 | 2.176 |
| Ti6Al4V | −0.401 | 0.722 | −0.133 | 1.242 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, M.V.; Cudjoe, E.; Ryu, J.J. Sliding Contact Fatigue Damage of Metallic Implants in a Simulated Body Fluid Environment. Lubricants 2024, 12, 437. https://doi.org/10.3390/lubricants12120437
Patel MV, Cudjoe E, Ryu JJ. Sliding Contact Fatigue Damage of Metallic Implants in a Simulated Body Fluid Environment. Lubricants. 2024; 12(12):437. https://doi.org/10.3390/lubricants12120437
Chicago/Turabian StylePatel, Mihir V., Edward Cudjoe, and Jae Joong Ryu. 2024. "Sliding Contact Fatigue Damage of Metallic Implants in a Simulated Body Fluid Environment" Lubricants 12, no. 12: 437. https://doi.org/10.3390/lubricants12120437
APA StylePatel, M. V., Cudjoe, E., & Ryu, J. J. (2024). Sliding Contact Fatigue Damage of Metallic Implants in a Simulated Body Fluid Environment. Lubricants, 12(12), 437. https://doi.org/10.3390/lubricants12120437




