Impact of Brake Wear Particles on Eukaryotic Cell Viability and Associated Oxidative Stress Responses
Abstract
1. Introduction
2. Materials and Methods
3. Results
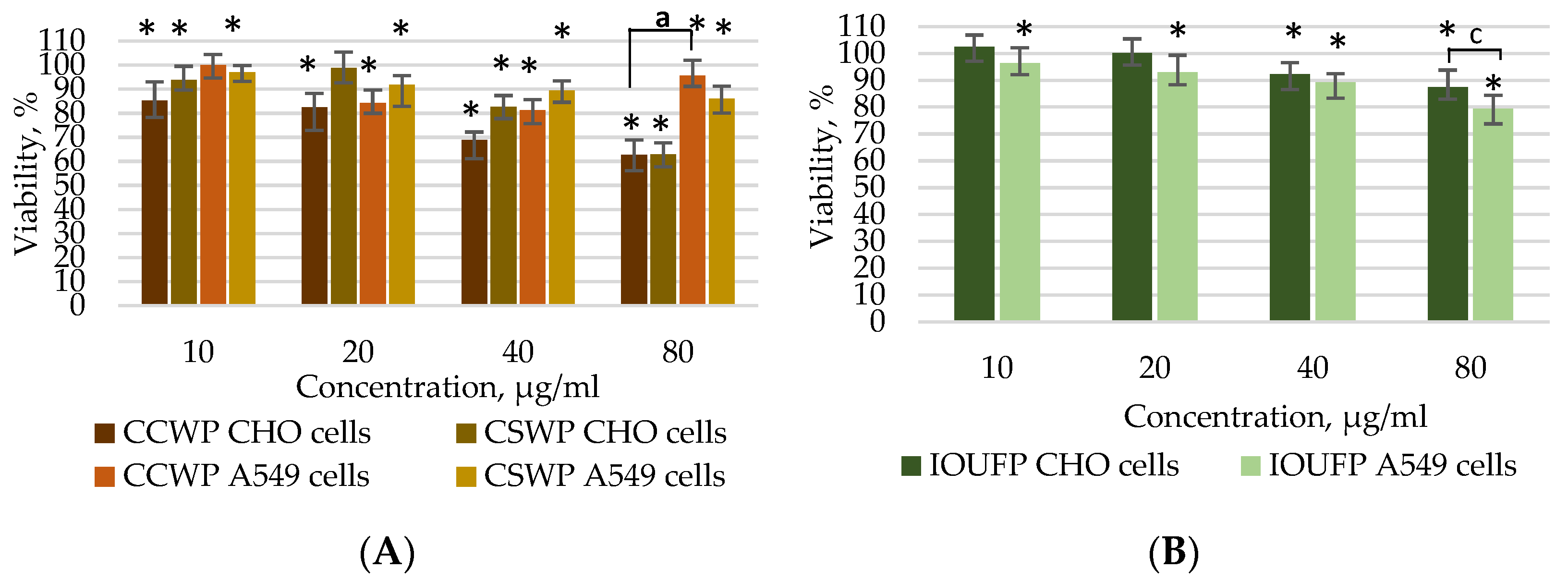
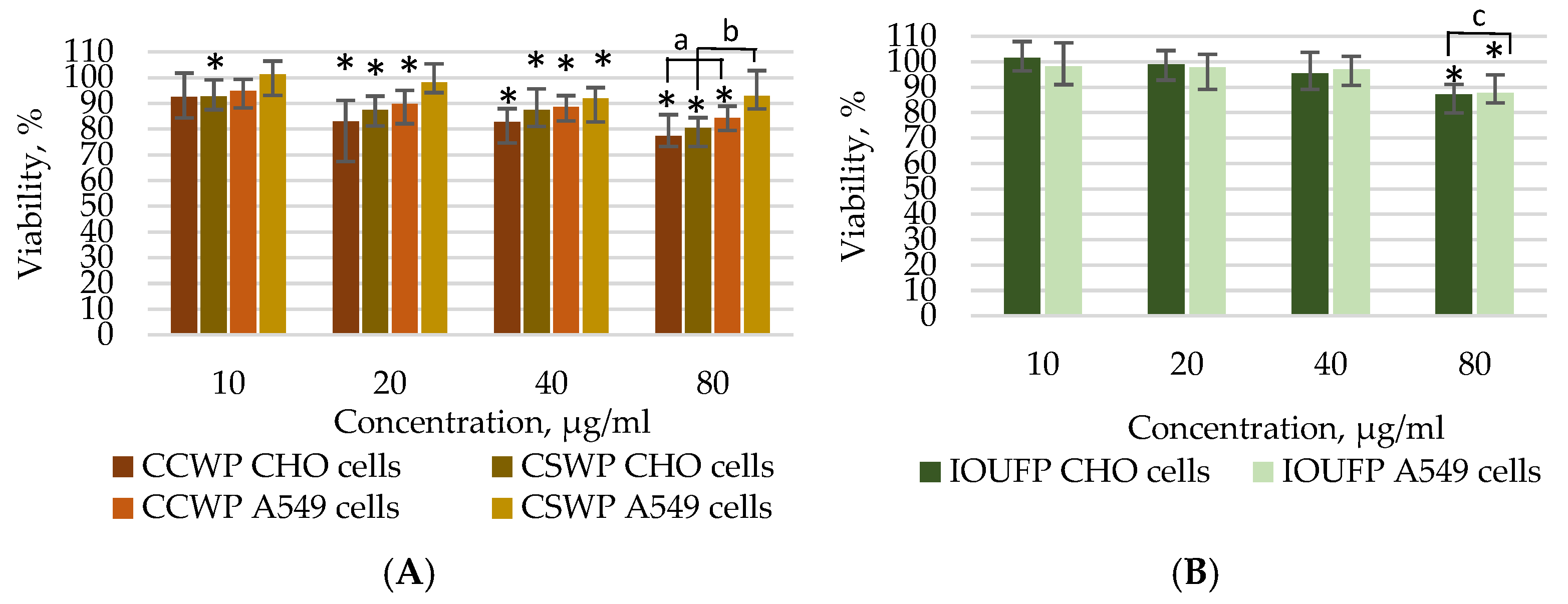
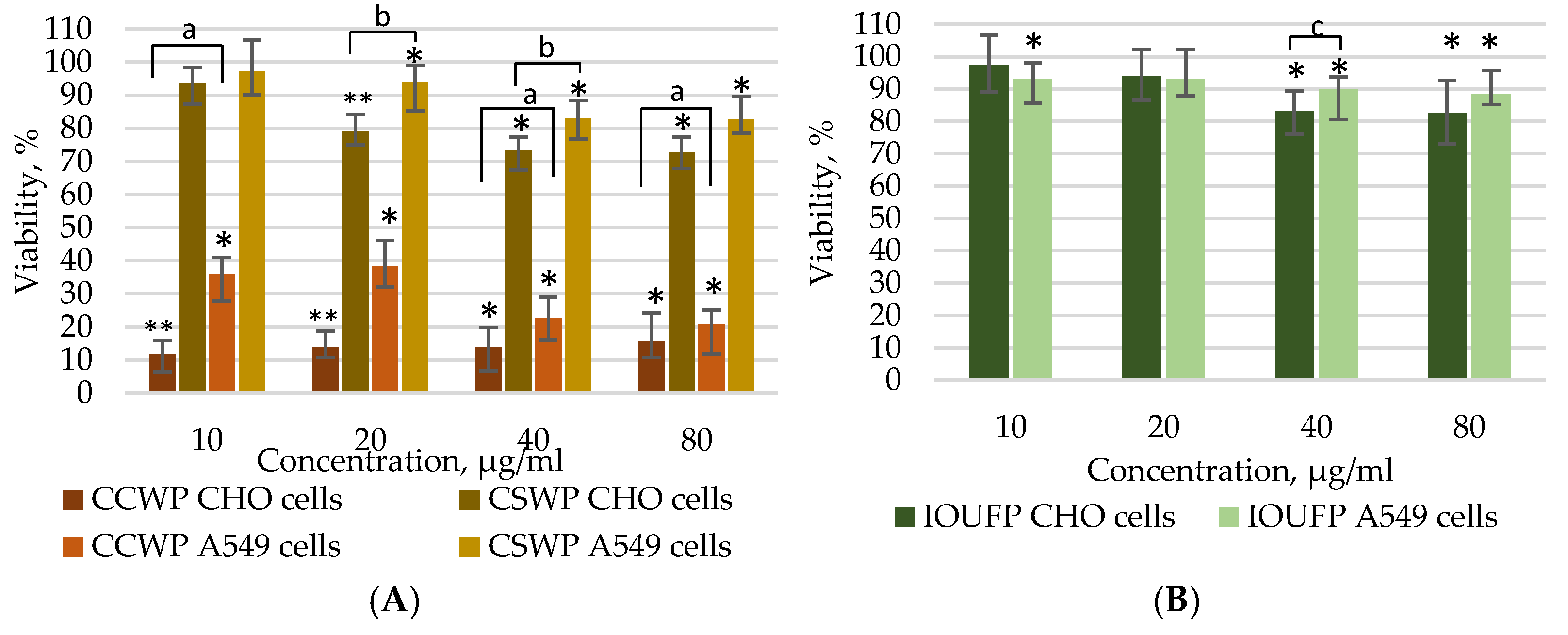
3.1. Impact of Ceramic/Ceramic and Ceramic/Steel Wear Particles and Iron (III) Oxide Ultrafine Particles on the Viability of A549 and CHO Cells
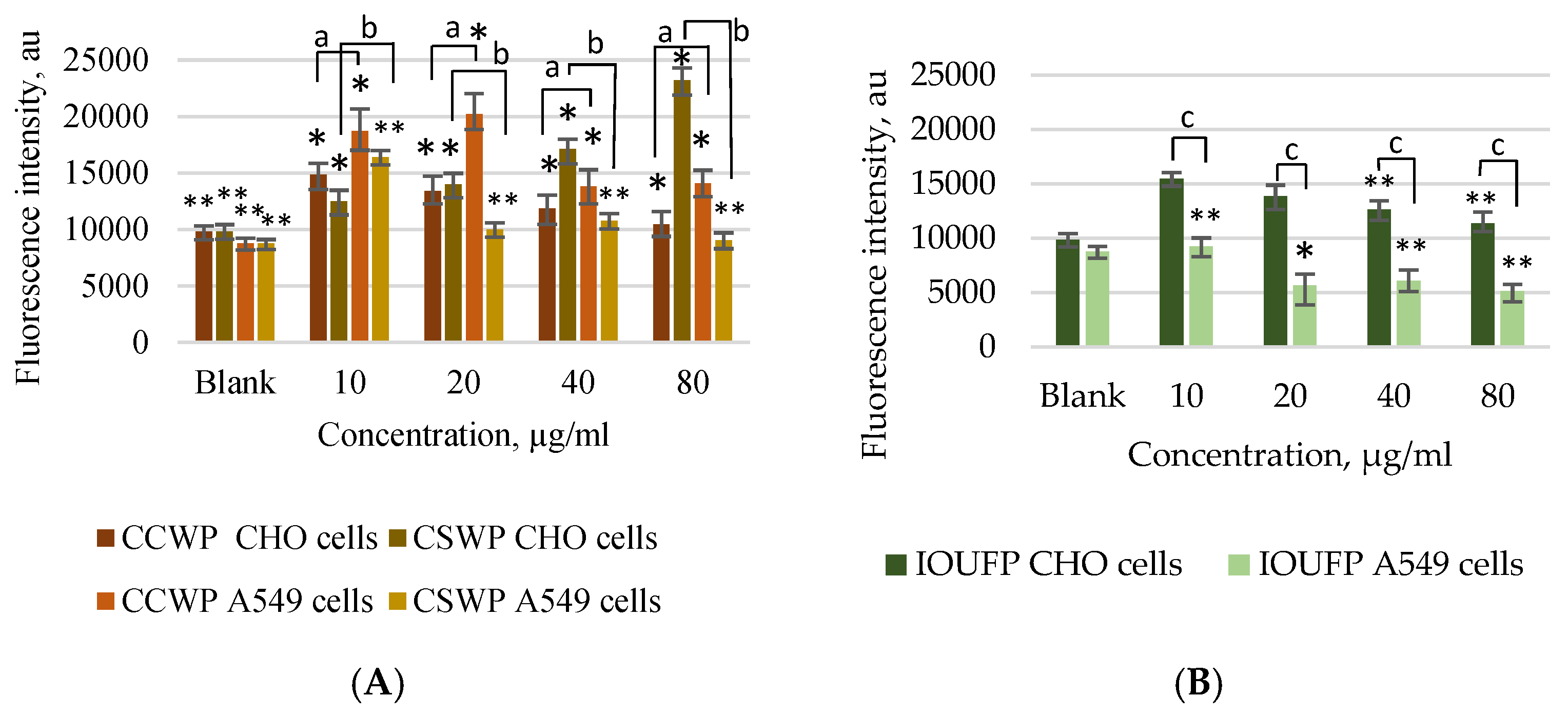
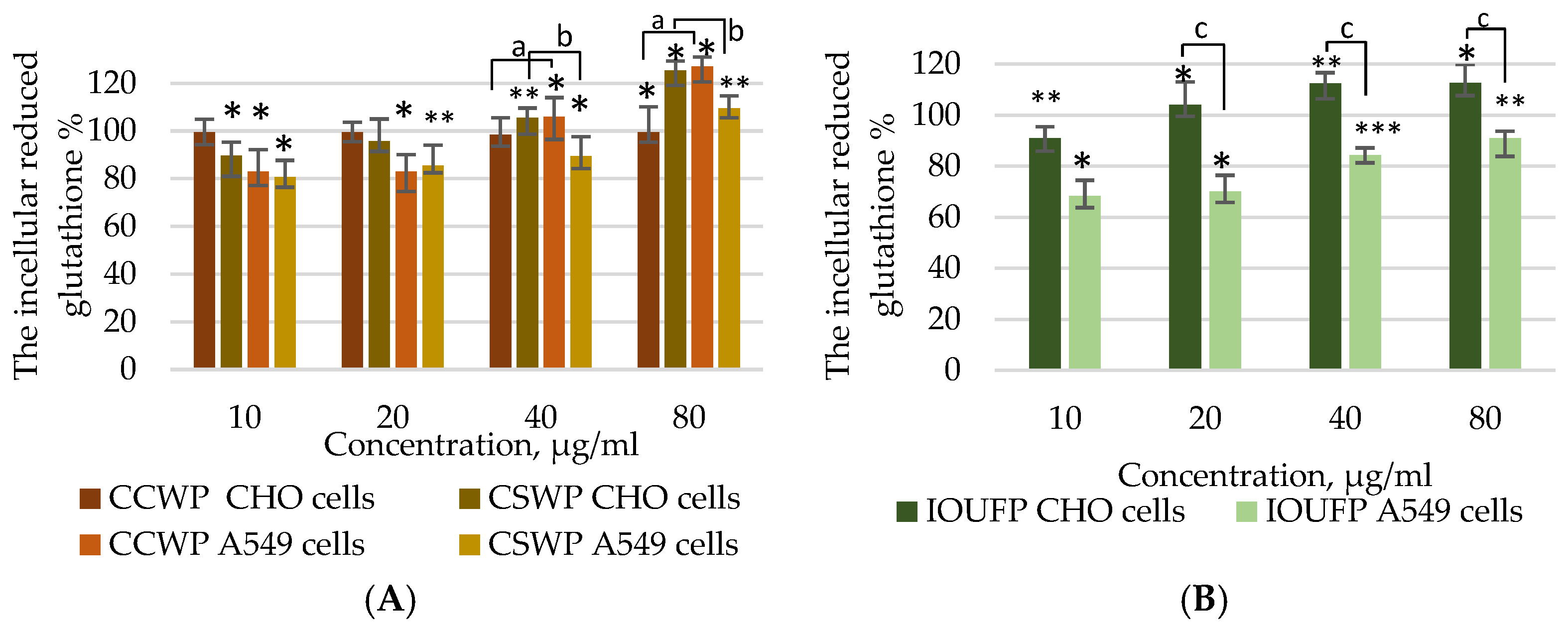
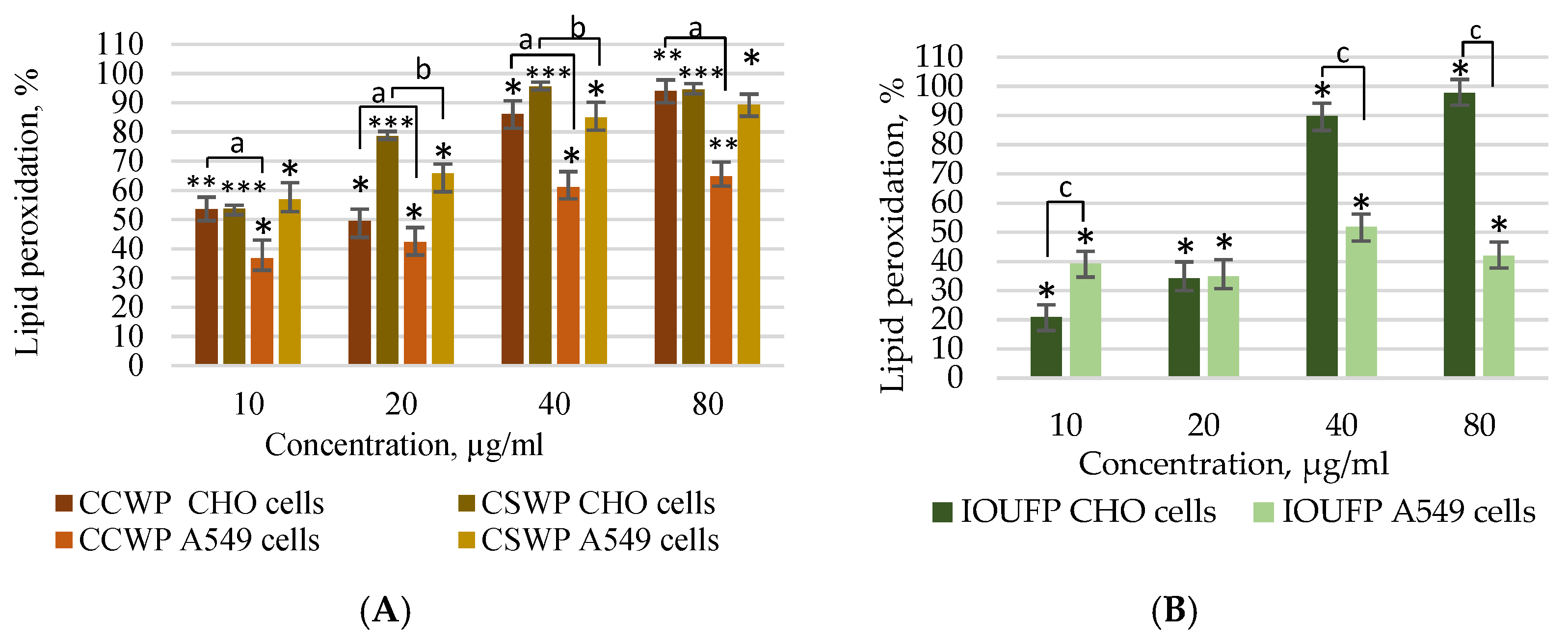
3.2. Impact of Ceramic/Ceramic and Ceramic/Steel Wear Particles and Iron (III) Oxide Ultrafine Particles on the Oxidative Stress in A549 and CHO Cells
4. Conclusions
- The viability studies using the MTT and Calcein AM assays demonstrated that the A549 cells show reduced susceptibility to CCWPs and CSWPs compared to CHO cells during both short (4 h) and prolonged (24 h) exposures. While 40 or 80 µg/mL IOUFP significantly decreased the CHO cell viability, A549 cells showed a higher sensitivity at lower concentrations. The higher viability observed in the Calcein AM assay may be attributed to the chelating effect of iron.
- This study highlights the cell-specific nature of the oxidative stress caused by CCWPs, CSWPs, and IOUFPs. A549 cells exhibited higher ROS levels in response to CCWP, whereas the higher IOUFP concentrations reduced ROS in these cells. In contrast, CHO cells displayed increased ROS regardless of the IOUFP concentration. This discrepancy may result from free iron ions released by IOUFPs, which can increase hydrogen peroxide levels, enhancing oxidative stress.
- In terms of antioxidant response, CCWPs had no effect on the GSH levels in CHO cells but significantly decreased GSH in A549 cells. Both cell types showed changes in GSH that mirrored ROS changes, indicating a higher sensitivity of A549 cells to the IOUFP ultrafine particles.
- The exposure to CCWPs and CSWPs led to an increase in the lipid peroxidation in both cell types, with a lower sensitivity of A549 cells in general. Notably, IOUFPs caused a moderate lipid peroxidation in CHO cells at the lower concentrations, reaching the levels similar to those of CCWPs and CSWPs at 80 µg/mL.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Grigoratos, T.; Martini, G. Brake wear particle emissions: A review. Environ. Sci. Pollut. Res. Int. 2015, 22, 2491. [Google Scholar] [CrossRef]
- Thorpe, A.; Harrison, R.M. Sources and properties of non-exhaust particulate matter from road traffic: A review. Sci. Total Environ. 2008, 400, 270–296. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.M.; Jones, A.M.; Gietl, J.; Yin, J.; Green, D.C. Estimation of the contributions of brake dust, tire wear, and resuspension to nonexhaust traffic particles derived from atmospheric measurements. Environ. Sci. Technol. 2012, 46, 6523–6529. [Google Scholar] [CrossRef]
- Lawrence, S.; Sokhi, R.; Ravindra, K.; Mao, H.; Prain, D.H.; Bull, D.I. Source apportionment of traffic emissions of particulate matter using tunnel measurements. Atmos. Environ. 2013, 77, 548–556. [Google Scholar] [CrossRef]
- Borawski, A. Conventional and unconventional materials used in the production of brake pads—Review. Sci. Eng. Compos. Mater. 2020, 27, 374–396. [Google Scholar] [CrossRef]
- Fahmy, H.M.; Abd El-Daim, T.M.; Mohamed, H.A.E.N.E. Multifunctional nanoparticles in stem cell therapy for cellular treating of kidney and liver diseases. Tissue Cell 2020, 66, 101371. [Google Scholar] [CrossRef]
- Cronholm, P.; Karlsson, H.L.; Hedberg, J.; Lowe, T.A.; Winnberg, L.; Elihn, K.; Wallinder, I.O.; Möller, L. Intracellular uptake and toxicity of Ag and CuO nanoparticles: A comparison between nanoparticles and their corresponding metal ions. Small 2013, 9, 970–982. [Google Scholar] [CrossRef]
- Chang, Y.N.; Zhang, M.; Xia, L.; Zhang, J.; Xing, G. The Toxic Effects and Mechanisms of CuO and ZnO Nanoparticles. Materials 2012, 5, 2850–2871. [Google Scholar] [CrossRef]
- Karlsson, H.; Cronholm, P.; Gustafsson, J.; Möller, L. Copper Oxide Nanoparticles Are Highly Toxic: A Comparison between Metal Oxide Nanoparticles and Carbon Nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Contini, C.; Schneemilch, M.; Gaisford, S.; Quirke, N. Nanoparticle–membrane interactions. J. Exp. Nanosci. 2018, 13, 62–81. [Google Scholar] [CrossRef]
- Lundqvist, M.; Stigler, J.; Elia, G.; Lynch, I.; Cedervall, T.; Dawson, K.A. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. USA 2008, 105, 14265–14270. [Google Scholar] [CrossRef]
- Lee, J.; Yang, J.; Kwon, S.G.; Hyeon, T. Nonclassical nucleation and growth of inorganic nanoparticles. Nat. Rev. Mater. 2016, 1, 16034. [Google Scholar] [CrossRef]
- Naz, S.; Gul, A.; Zia, M. Toxicity of copper oxide nanoparticles: A review study. IET Nanobiotechnol. 2020, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Heinz, H.; Pramanik, C.; Heinz, O.; Ding, Y.; Mishra, R.K.; Marchon, D.; Flatt, R.J.; Estrela-Lopis, I.; Llop, J.; Moya, S.; et al. Nanoparticle decoration with surfactants: Molecular interactions, assembly, and applications. Surf. Sci. Rep. 2017, 72, 1–58. [Google Scholar] [CrossRef]
- Yun, Y.; Cho, Y.W.; Park, K. Nanoparticles for oral delivery: Targeted nanoparticles with peptidic ligands for oral protein delivery. Adv. Drug Deliv. Rev. 2013, 65, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.H.; Jiang, Y.; Fang, J.C.; Zhang, L. Cell membrane-derived nanomaterials for biomedical applications. Biomaterials 2017, 128, 69–83. [Google Scholar] [CrossRef]
- Mohanraj, J.; Chen, Y. Nanoparticles—A Review. Trop. J. Pharm. Res. 2006, 5, 561–573. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Cronholm, P.; Hedberg, Y.; Tornberg, M.; De Battice, L.; Svedhem, S.; Wallinder, I.O. Cell membrane damage and protein interaction induced by copper containing nanoparticles—Importance of the metal release process. Toxicology 2013, 313, 59–69. [Google Scholar] [CrossRef]
- Wang, Z.; Li, N.; Zhao, J.; White, J.C.; Qu, P.; Xing, B. CuO nanoparticle interaction with human epithelial cells: Cellular uptake, location, export, and genotoxicity. Chem. Res. Toxicol. 2012, 25, 1512–1521. [Google Scholar] [CrossRef]
- Hillaireau, H.; Couvreur, P. Nanocarriers’ entry into the cell: Relevance to drug delivery. Cell. Mol. Life Sci. 2009, 66, 2873–2896. [Google Scholar] [CrossRef] [PubMed]
- Panariti, A.; Miserocchi, G.; Rivolta, I. The effect of nanoparticle uptake on cellular behaviour: Disrupting or enabling functions? Nanotechnol. Sci. Appl. 2012, 5, 87–100. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Bouallegui, Y.; Ben Younes, R.; Turki, F.; Mezni, A.; Oueslati, R. Effect of exposure time, particle size and uptake pathways in immune cell lysosomal cytotoxicity of mussels exposed to silver nanoparticles. Drug Chem. Toxicol. 2018, 41, 169–174. [Google Scholar] [CrossRef]
- Lee, J.; Twomey, M.; Machado, C.; Gomez, G.; Doshi, M.; Gesquiere, A.J.; Moon, J.H. Caveolae-Mediated Endocytosis of Conjugated Polymer Nanoparticles. Macromol. Biosci. 2013, 13, 913–920. [Google Scholar] [CrossRef]
- Privalova, L.I.; Katsnelson, B.A.; Loginova, N.V.; Gurvich, V.B.; Shur, V.Y.; Valamina, I.E.; Makeyev, O.H.; Sutunkova, M.P.; Minigalieva, I.A.; Kireyeva, E.P.; et al. Subchronic Toxicity of Copper Oxide Nanoparticles and Its Attenuation with the Help of a Combination of Bioprotectors. Int. J. Mol. Sci. 2014, 15, 12379–12406. [Google Scholar] [CrossRef]
- Gupta, G.; Cappellini, F.; Farcal, L.; Gornati, R.; Bernardini, G.; Fadeel, B. Copper oxide nanoparticles trigger macrophage cell death with misfolding of Cu/Zn superoxide dismutase 1 (SOD1). Part. Fibre Toxicol. 2022, 19, 33. [Google Scholar] [CrossRef]
- Yao, K.; Ge, W. Differential regulation of Kit Ligand A (kitlga) expression in the zebrafish ovarian follicle cells—Evidence for the existence of a cyclic adenosine 3′, 5′ monophosphate-mediated binary regulatory system during folliculogenesis. Mol. Cell. Endocrinol. 2015, 402, 21–31. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Reth, M. Hydrogen peroxide as second messenger in lymphocyte activation. Immunology 2002, 3, 1129–1134. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 1984, 219, 1–14. [Google Scholar] [CrossRef]
- Repetto, M.; Semprine, J.; Boveris, A. Lipid Peroxidation: Chemical Mechanism, Biological Implications and Analytical Determination. In Lipid Peroxidation; Catala, A., Ed.; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Franco, R.; Cidlowski, J.A. Glutathione Efflux and Cell Death. Antioxid. Redox Signal. 2012, 17, 1694–1713. [Google Scholar] [CrossRef]
- Mateos, R.; Goya, L.; Bravo, L. Determination of malondialdehyde by liquid chromatography as the 2,4-dinitrophenylhydrazone derivative: A marker for oxidative stress in cell cultures of human hepatoma HepG2. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004, 805, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Camandola, S.; Poli, G.; Mattson, M.P. The Lipid Peroxidation Product 4-Hydroxy-2,3-Nonenal Increases AP-1-Binding Activity Through Caspase Activation in Neurons. J. Neurochem. 2000, 74, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H. Estimation of peroxidative damage. A critical review. Pathol. Biol. 1996, 44, 25–28. [Google Scholar]
- Barosova, H.; Chortarea, S.; Peikertova, P.; Clift, M.J.D.; Petri-Fink, A.; Kukutschova, J.; Rothen-Rutishauser, B. Biological Response of an in Vitro Human 3D Lung Cell Model Exposed to Brake Wear Debris Varies Based on Brake Pad Formulation. Arch. Toxicol. 2018, 92, 2339–2351. [Google Scholar] [CrossRef]
- Gasser, M.; Riediker, M.; Mueller, L.; Perrenoud, A.; Blank, F.; Gehr, P.; Rothen-Rutishauser, B. Toxic Effects of Brake Wear Particles on Epithelial Lung Cells In Vitro. Part. Fibre Toxicol. 2009, 6, 1–13. [Google Scholar] [CrossRef]
- Puisney-Dakhli, C.; Oikonomou, E.K.; Tharaud, M.; Sivry, Y.; Berret, J.F.; Baeza-Squiban, A. Effects of Brake Wear Nanoparticles on the Protection and Repair Functions of the Airway Epithelium. Environ. Pollut. 2023, 327, 121554. [Google Scholar] [CrossRef]
- Alves, C.A.; Soares, M.; Figueiredo, D.; Oliveira, H. Effects of Particle-Bound Polycyclic Aromatic Hydrocarbons and Plasticisers from Different Traffic Sources on the Human Alveolar Epithelial Cell Line A549. Atmos. Environ. 2023, 303, 119736. [Google Scholar] [CrossRef]
- Melzi, G.; Nozza, E.; Frezzini, M.A.; Canepari, S.; Vecchi, R.; Cremonesi, L.; Potenza, M.; Marinovich, M.; Corsini, E. Toxicological Profile of PM from Different Sources in the Bronchial Epithelial Cell Line BEAS-2B. Toxics 2023, 11, 413. [Google Scholar] [CrossRef]
- Puisney, C.; Oikonomou, E.K.; Nowak, S.; Chevillot, A.; Casale, S.; Baeza-Squiban, A.; Berret, J.F. Brake Wear (Nano)particle Characterization and Toxicity on Airway Epithelial Cells In Vitro. Environ. Sci. Nano 2018, 5, 1036–1044. [Google Scholar] [CrossRef]
- Zhao, J.; Lewinski, N.; Riediker, M. Physico-Chemical Characterization and Oxidative Reactivity Evaluation of Aged Brake Wear Particles. Aerosol Sci. Technol. 2015, 49, 65–74. [Google Scholar] [CrossRef]
- Tarasiuk, W.; Golak, K.; Tsybrii, Y.; Nosko, O. Correlations between the wear of car brake friction materials and airborne wear particle emissions. Wear 2020, 456–457, 203361. [Google Scholar] [CrossRef]
- ISO 19007:2018; Nanotechnologies—In Vitro MTS Assay for Measuring the Cytotoxic Effect of Nanoparticles. International Organization for Standardization: Geneva, Switzerland, 2018; Last reviewed and confirmed 2023.
- Kamiloglu, S.; Sari, G.; Ozdal, T.; Capanoglu, E. Guidelines for Cell Viability Assays. Food Front. 2020, 1, 332–349. [Google Scholar] [CrossRef]
- Zhao, K.; Li, M.; Zhao, L.; Sang, N.; Guo, L.H. The Identification of the Major Contributors in Atmospheric Particulate Matter to Oxidative Stress Using Surrogate Particles. Environ. Sci. Nano 2021, 8, 527–542. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Potter, T.M.; Neun, B.W.; Stern, S.T. Assay to Detect Lipid Peroxidation upon Exposure to Nanoparticles. In Characterization of Nanoparticles Intended for Drug Delivery; McNeil, S., Ed.; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2011; Volume 697, pp. 299–310. [Google Scholar] [CrossRef]
- Boucher, R.C. Regulation of airway surface liquid volume. J. Clin. Investig. 2002, 109, 863–868. [Google Scholar] [CrossRef]
- Giard, D.J.; Aaronson, S.A.; Todaro, G.J.; Arnstein, P.; Kersey, J.H.; Dosik, H.; Parks, W.P. In Vitro Cultivation of Human Tumors: Establishment of Cell Lines Derived From a Series of Solid Tumors. J. Natl. Cancer Inst. 1973, 51, 1417–1423. [Google Scholar] [CrossRef]
- Stearns, R.C.; Paulauskis, J.D.; Godleski, J.J. Endocytosis of Ultrafine Particles by A549 Cells. Am. J. Respir. Cell Mol. Biol. 2001, 24, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Dumont, J.; Euwart, D.; Mei, B.; Estes, S.; Kshirsagar, R. Human cell lines for biopharmaceutical manufacturing: History, status, and future perspectives. Crit. Rev. Biotechnol. 2016, 36, 1110–1122. [Google Scholar] [CrossRef]
- Stach, C.S.; McCann, M.G.; O’Brien, C.M.; Le, T.S.; Somia, N.; Chen, X.; Lee, K.; Fu, H.-Y.; Daoutidis, P.; Zhao, L.; et al. Model-Driven Engineering of N-Linked Glycosylation in Chinese Hamster Ovary Cells. ACS Synth. Biol. 2019, 8, 2524–2535. [Google Scholar] [CrossRef] [PubMed]
- Forest, V.; Pourchez, J. Biological Effects of Brake Wear Particles in Mammalian Models: A Systematic Review. Sci. Total Environ. 2023, 905, 167266. [Google Scholar] [CrossRef]
- Ragelienė, L.; Trečiokaitė, L.; Tučkutė, S. Analysis of Elemental and Mineral Composition of Car Brake Pads. Chemistry and Chemical Technology 2017. Available online: https://www.vdu.lt/ (accessed on 8 July 2024).
- Shao, X.-R.; Wei, X.-Q.; Song, X.; Hao, L.-Y.; Cai, X.-X.; Zhang, Z.-R.; Peng, Q.; Lin, Y.-F. Independent effect of polymeric nanoparticle zeta potential/surface charge on their cytotoxicity and affinity to cells. Cell Prolif. 2015, 48, 465–474. [Google Scholar] [CrossRef]
- Šatkauskas, S.; Jakštys, B.; Ruzgys, P.; Jakutavičiūtė, M. Different Cell Viability Assays Following Electroporation In Vitro. In Handbook of Electroporation; Miklavcic, D., Ed.; Springer: Cham, Switzerland, 2016; pp. 1–16. [Google Scholar] [CrossRef]
- Rajeckaitė, V.; Jakštys, B.; Rafanavičius, A.; Maciulevičius, M.; Jakutavičiūtė, M.; Šatkauskas, S. Calcein Release from Cells In Vitro via Reversible and Irreversible Electroporation. J. Membr. Biol. 2018, 251, 119–130. [Google Scholar] [CrossRef]
- Tenopoulou, M.; Kurz, T.; Doulias, P.T.; Galaris, D.; Brunk, U.T. Does the calcein-AM method assay the total cellular ‘labile iron pool’ or only a fraction of it? Biochem. J. 2007, 403, 261–266. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Figliuzzi, M.; Tironi, M.; Longaretti, L.; Mancini, A.; Teoldi, F.; Sangalli, F.; Remuzzi, A. Copper-dependent biological effects of particulate matter produced by brake systems on lung alveolar cells. Arch. Toxicol. 2020, 94, 2965–2979. [Google Scholar] [CrossRef] [PubMed]
- Bielski, B.H.J.; Arudi, R.L.; Sutherland, M.W. A study of the reactivity of HO2/O2− with unsaturated fatty acids. J. Biol. Chem. 1983, 258, 4759–4761. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.K.; Hong, S.H.; Jang, M.; Kang, J.H.; Kwon, O.Y.; Han, G.M.; Shim, W.J. Large Accumulation of Micro-Sized Synthetic Polymer Particles in the Sea Surface Microlayer. Environ. Sci. Technol. 2014, 48, 9014–9021. [Google Scholar] [CrossRef] [PubMed]
- Gough, D.; Cotter, T. Hydrogen peroxide: A Jekyll and Hyde signalling molecule. Cell Death Dis. 2011, 2, e213. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, J.K.; Chu, H.H.; Wang, Y.H.; Lai, C.W.; Chou, T.P.; Hsieh, T.S.; Wang, L.J.; Liu, M.H. Macrophage physiological function after superparamagnetic iron oxide labeling. NMR Biomed. 2008, 21, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Lunova, O.; Syrovets, T.; Röcker, C.; Tron, K.; Nienhaus, U.; Rasche, V.; Mailander, V.; Landfester, K.; Simmet, T. Lysosomal degradation of the carboxydextran shell of coated superparamagnetic iron oxide nanoparticles and the fate of professional phagocytes. Biomaterials 2010, 31, 9015–9022. [Google Scholar] [CrossRef] [PubMed]
- García-Caparrós, P.; De Filippis, L.; Gul, A.; Hasanuzzaman, M.; Ozturk, M.; Altay, V.; Lao, T.M. Oxidative Stress and Antioxidant Metabolism under Adverse Environmental Conditions: A Review. Bot. Rev. 2021, 87, 421–466. [Google Scholar] [CrossRef]
- Yin, H.; Xu, L.; Porter, N.A. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef]
- Girotti, W. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J. Lipid Res. 1998, 39, 1529–1542. [Google Scholar] [CrossRef] [PubMed]
- Browne, R.W.; Armstrong, D. HPLC analysis of lipid-derived polyunsaturated fatty acid peroxidation products in oxidatively modified human plasma. Clin. Chem. 2000, 46 Pt 1, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Boeglin, W.E.; Yin, H.; Porter, N.A.; Brash, A.R. Intermolecular peroxyl radical reactions during autoxidation of hydroxy and hydroperoxy arachidonic acids generate a novel series of epoxidized products. Chem. Res. Toxicol. 2008, 21, 895–903. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trečiokaitė, L.; Tsybrii, Y.; Nosko, O.; Ragelienė, L. Impact of Brake Wear Particles on Eukaryotic Cell Viability and Associated Oxidative Stress Responses. Lubricants 2024, 12, 449. https://doi.org/10.3390/lubricants12120449
Trečiokaitė L, Tsybrii Y, Nosko O, Ragelienė L. Impact of Brake Wear Particles on Eukaryotic Cell Viability and Associated Oxidative Stress Responses. Lubricants. 2024; 12(12):449. https://doi.org/10.3390/lubricants12120449
Chicago/Turabian StyleTrečiokaitė, Lina, Yurii Tsybrii, Oleksii Nosko, and Lina Ragelienė. 2024. "Impact of Brake Wear Particles on Eukaryotic Cell Viability and Associated Oxidative Stress Responses" Lubricants 12, no. 12: 449. https://doi.org/10.3390/lubricants12120449
APA StyleTrečiokaitė, L., Tsybrii, Y., Nosko, O., & Ragelienė, L. (2024). Impact of Brake Wear Particles on Eukaryotic Cell Viability and Associated Oxidative Stress Responses. Lubricants, 12(12), 449. https://doi.org/10.3390/lubricants12120449




