Synergistic Effect of Spark Plasma Sintering Driven Solid-Solution Phases on Scratch Resistance in Two-Dimensional Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Processing
2.2. Microstructural and Phase Analysis
2.3. Wear Behavior via Scratch Test
3. Results and Discussions
3.1. Microstructural Analysis of the Powder Mixture
3.2. Phase Evolution via SPS Processing
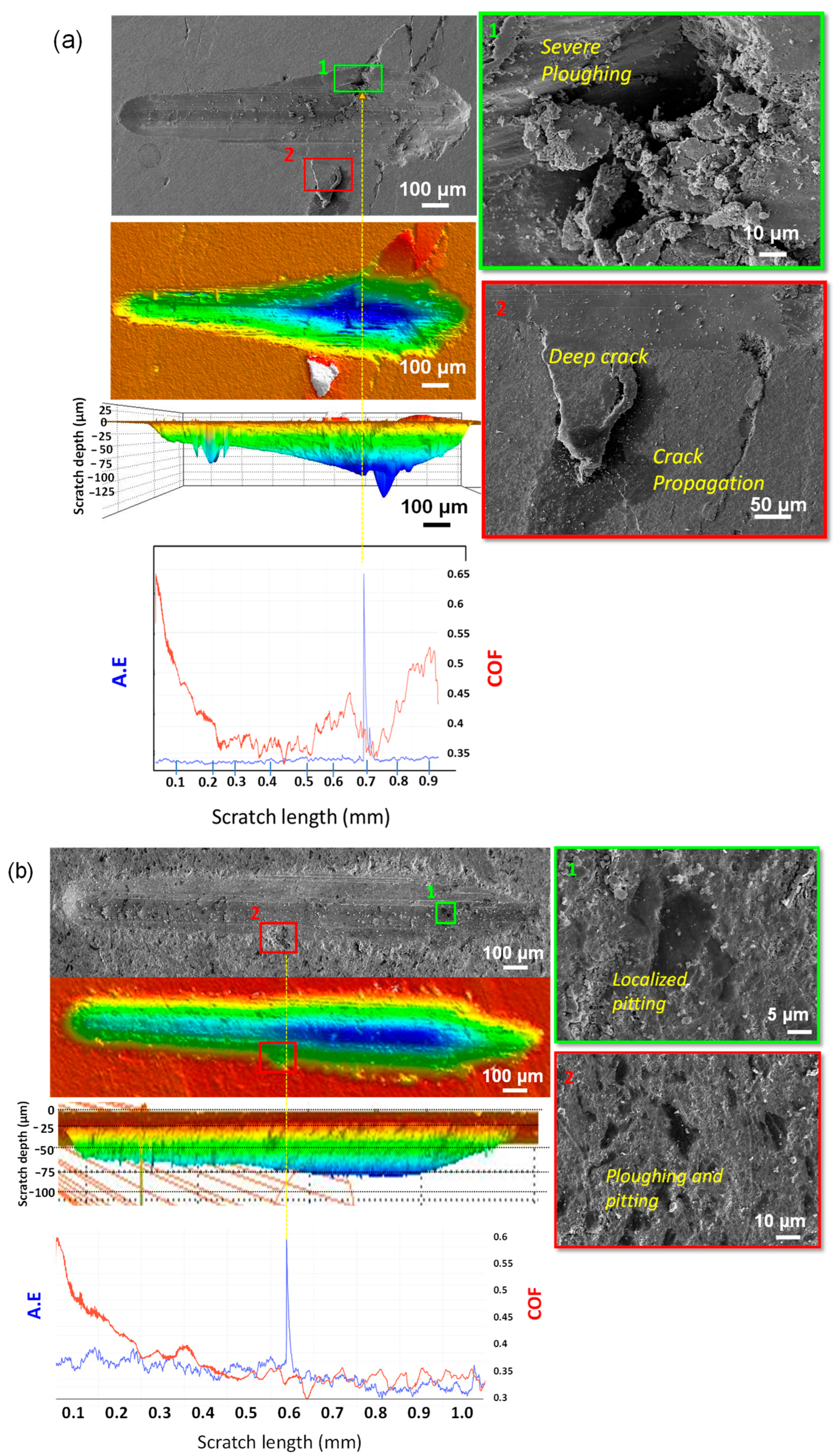
3.3. Scratch Behavior of Multicomponent 2D Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maboudian, R.; Ashurst, W.R.; Carraro, C. Tribological challenges in micromechanical systems. Tribol. Lett. 2002, 12, 95–100. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, L.; Ge, C.; Liu, R.; Guan, H.; Zhang, X. Atomically thin hydroxylation boron nitride nanosheets for excellent water-based lubricant additives. J. Am. Ceram. Soc. 2020, 103, 6951–6960. [Google Scholar] [CrossRef]
- Wu, H.; Yin, S.; Du, Y.; Wang, L.; Yang, Y.; Wang, H. Alkyl-functionalized boron nitride nanosheets as lubricant additives. ACS Appl. Nano Mater. 2020, 3, 9108–9116. [Google Scholar] [CrossRef]
- Berman, D.; Deshmukh, S.A.; Sankaranarayanan, S.K.R.S.; Erdemir, A.; Sumant, A.V. Macroscale superlubricity enabled by graphene nanoscroll formation. Science 2015, 348, 1118–1122. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, C.; Xiao, G.; Zhang, J.; Chen, Z.; Yi, M. Lubrication performance of graphene as lubricant additive in 4-n-pentyl-4′-cyanobiphyl liquid crystal (5CB) for steel/steel contacts. Materials 2018, 11, 2110. [Google Scholar] [CrossRef]
- Berman, D.; Erdemir, A.; Sumant, A.V. Reduced wear and friction enabled by graphene layers on sliding steel surfaces in dry nitrogen. Carbon 2013, 59, 167–175. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, S. 2D nanomaterials as lubricant additive: A review. Mater. Des. 2017, 135, 319–332. [Google Scholar] [CrossRef]
- Rabiei Baboukani, A.; Khakpour, I.; Drozd, V.; Wang, C. Liquid-Based Exfoliation of Black Phosphorus into Phosphorene and Its Application for Energy Storage Devices. Small Struct. 2021, 2, 2000148. [Google Scholar] [CrossRef]
- Nieto, A.; Lahiri, D.; Agarwal, A. Synthesis and properties of bulk graphene nanoplatelets consolidated by spark plasma sintering. Carbon 2012, 50, 4068–4077. [Google Scholar] [CrossRef]
- Phiri, J.; Gane, P.; Maloney, T.C. General overview of graphene: Production, properties and application in polymer composites. Mater. Sci. Eng. B 2017, 215, 9–28. [Google Scholar] [CrossRef]
- Podgornik, B.; Kosec, T.; Kocijan, A.; Donik, Č. Tribological behavior and lubrication performance of hexagonal boron nitride (h-BN) as a replacement for graphite in alzumn forming. Tribol. Int. 2015, 81, 267–275. [Google Scholar] [CrossRef]
- Biswal, S.R.; Sahoo, S. Structural and mechanical properties of a novel Al-Al2O3-WS2 hybrid composites. Mater. Lett. 2022, 307, 131017. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, R.; Pu, J.; He, Z.; Xiong, L. 2D graphene and h-BN layers application in protective coatings. Corros. Rev. 2021, 39, 93–107. [Google Scholar] [CrossRef]
- Joseph, A.; Gautham, V.; Akshay, K.S.; Sajith, V. 2D MoS2-hBN hybrid coatings for enhanced corrosion resistance of solid lubricant coatings. Surf. Coat. Technol. 2022, 443, 128612. [Google Scholar] [CrossRef]
- Li, C.; Cao, Q.; Wang, F.; Xiao, Y.; Li, Y.; Delaunay, J.-J.; Zhu, H. Engineering graphene and TMDs based van der Waals heterostructures for photovoltaic and photoelectrochemical solar energy conversion. Chem. Soc. Rev. 2018, 47, 4981–5037. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, F.; Sun, M. Graphene, hexagonal boron nitride, and their heterostructures: Properties and applications. RSC Adv. 2017, 7, 16801–16822. [Google Scholar] [CrossRef]
- Wright, A.J.; Wang, Q.; Huang, C.; Nieto, A.; Chen, R.; Luo, J. From high-entropy ceramics to compositionally-complex ceramics: A case study of fluorite oxides. J. Eur. Ceram. Soc. 2020, 40, 2120–2129. [Google Scholar] [CrossRef]
- Gild, J.; Zhang, Y.; Harrington, T.; Jiang, S.; Hu, T.; Quinn, M.C.; Mellor, W.M.; Zhou, N.; Vecchio, K.; Luo, J. High-entropy metal diborides: A new class of high-entropy materials and a new type of ultrahigh temperature ceramics. Sci. Rep. 2016, 6, 37946. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, L.; Gan, G.-Y.; Wang, W.; Tang, B.-Y. Investigation of thermodynamic properties of high entropy (TaNbHfTiZr) C and (TaNbHfTiZr) N. J. Alloys Compd. 2019, 788, 1076–1083. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, J.-X.; Li, F.; Wei, X.; Wu, H.; Zhang, G.-J. A high entropy silicide by reactive spark plasma sintering. J. Adv. Ceram. 2019, 8, 148–152. [Google Scholar] [CrossRef]
- Castle, E.; Csanádi, T.; Grasso, S.; Dusza, J.; Reece, M. Processing and properties of high-entropy ultra-high temperature carbides. Sci. Rep. 2018, 8, 8609. [Google Scholar] [CrossRef] [PubMed]
- Nisar, A.; Dolmetsch, T.; Paul, T.; Sakthivel, T.S.; Zhang, C.; Boesl, B.; Seal, S.; Agarwal, A. Unveiling enhanced oxidation resistance and mechanical integrity of multicomponent ultra-high temperature carbides. J. Am. Ceram. Soc. 2022, 105, 2500–2516. [Google Scholar] [CrossRef]
- Nisar, A.; Sakthivel, T.; Zhang, C.; Boesl, B.; Seal, S.; Agarwal, A. Quantification of complex protective surface oxide layer formed during plasma jet exposure of multicomponent ultra-high temperature carbides. Appl. Surf. Sci. 2022, 592, 153247. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, Y.; Cao, Z.; Gu, J.; Du, Z.; Li, B.; Yang, S. A perspective on high-entropy two-dimensional materials. SusMat 2022, 2, 65–75. [Google Scholar] [CrossRef]
- Quilty, C.D.; Housel, L.M.; Bock, D.C.; Dunkin, M.R.; Wang, L.; Lutz, D.M.; Abraham, A.; Bruck, A.M.; Takeuchi, E.S.; Takeuchi, K.J.; et al. Ex Situ and Operando XRD and XAS Analysis of MoS2: A Lithiation Study of Bulk and Nanosheet Materials. ACS Appl. Energy Mater. 2019, 2, 7635–7646. [Google Scholar] [CrossRef]
- Zhou, J.; Tao, Q.; Ahmed, B.; Palisaitis, J.; Persson, I.; Halim, J.; Barsoum, M.W.; Persson, P.O.Å.; Rosen, J. High-Entropy Laminate Metal Carbide (MAX Phase) and Its Two-Dimensional Derivative MXene. Chem. Mater. 2022, 34, 2098–2106. [Google Scholar] [CrossRef]
- Cavin, J.; Ahmadiparidari, A.; Majidi, L.; Thind, A.S.; Misal, S.N.; Prajapati, A.; Hemmat, Z.; Rastegar, S.; Beukelman, A.; Singh, M.R.; et al. 2D high-entropy transition metal dichalcogenides for carbon dioxide electrocatalysis. Adv. Mater. 2021, 33, 2100347. [Google Scholar] [CrossRef]
- Li, F.; Sun, S.-K.; Chen, Y.; Naka, T.; Hashishin, T.; Maruyama, J.; Abe, H. Bottom-up synthesis of 2D layered high-entropy transition metal hydroxides. Nanoscale Adv. 2022, 4, 2468–2478. [Google Scholar] [CrossRef]
- Nemani, S.K.; Zhang, B.; Wyatt, B.C.; Hood, Z.D.; Manna, S.; Khaledialidusti, R.; Hong, W.; Sternberg, M.G.; Sankaranarayanan, S.K.R.S.; Anasori, B. High-entropy 2D carbide mxenes: TiVNbMoC3 and TiVCrMoC3. ACS Nano 2021, 15, 12815–12825. [Google Scholar] [CrossRef]
- Samal, S.; Cibulková, J.; Čtvrtlík, R.; Tomáštík, J.; Václavek, L.; Kopeček, J.; Šittner, P. Tribological behavior of NiTi alloy produced by spark plasma sintering method. Coatings 2021, 11, 1246. [Google Scholar] [CrossRef]
- Samal, S.; Molnárová, O.; Průša, F.; Kopeček, J.; Heller, L.; Šittner, P.; Škodová, M.; Abate, L.; Blanco, I. Net-shape NiTi shape memory alloy by spark plasma sintering method. Appl. Sci. 2021, 11, 1802. [Google Scholar] [CrossRef]
- Fontoura, L.; Nautiyal, P.; Loganathan, A.; Boesl, B.; Agarwal, A. Nacre-Inspired Graphene/Metal Hybrid by In Situ Cementation Reaction and Joule Heating. Adv. Eng. Mater. 2018, 20, 1800518. [Google Scholar] [CrossRef]
- Rajesh, K.; Dubey, A.; Rangaswamy, M.K.; Lahiri, I.; Lahiri, D. Tailoring the tribological behavior of composite structures with optimized ratio of graphene and boron nitride nanosheets. Tribol. Int. 2022, 175, 107835. [Google Scholar] [CrossRef]
- Loganathan, A.; Sharma, A.; Rudolf, C.; Zhang, C.; Nautiyal, P.; Suwas, S.; Boesl, B.; Agarwal, A. In-situ deformation mechanism and orientation effects in sintered 2D boron nitride nanosheets. Mater. Sci. Eng. A 2017, 708, 440–450. [Google Scholar] [CrossRef]
- Adigilli, H.K.; Murugan, K.; Srinivas, P.V.V.; Basha, D.N.; Karati, A.; Pandey, A.K.; Joardar, J. Spark plasma sintering behavior and structural stability of 2D-WS2 nanosheets. Ceram. Int. 2022, 48, 25151–25158. [Google Scholar] [CrossRef]
- Jin, Z.; Chao, M.A.; Xiao, K.; Zhang, L.; Liu, X.-L. Effect of WS2 particle size on mechanical properties and tribological behaviors of Cu-WS2 composites sintered by SPS. Trans. Nonferrous Met. Soc. 2018, 28, 1176–1185. [Google Scholar]
- Chen, Z.; Liu, X.; Liu, Y.; Gunsel, S.; Luo, J. Ultrathin MoS2 nanosheets with superior extreme pressure property as boundary lubricants. Sci. Rep. 2015, 5, 12869. [Google Scholar] [CrossRef]
- Loganathan, A. Spark Plasma Sintering of 2D Nitride and Carbide Based Ceramics. Ph.D. Thesis, Florida International University, Miami, FL, USA, 2019. [Google Scholar]
- Azimi, S.; Rastgoo, A.; Sattari, S.; Rashidi, A. Defects and Structural Analysis of Multi-Wall Carbon Nano Tubes via Ball milling and Cryo-milling. J. Comput. Appl. Mech. 2016, 47, 1–9. [Google Scholar]
- Li, T.; Yin, Z.; Wu, G. Study on heat transfer behavior and thermal breakage characteristic of the charge in ball mills. Adv. Mech. Eng. 2021, 13, 1687814021994964. [Google Scholar] [CrossRef]
- Jacobson, N.S.; Morscher, G.N.; Bryant, D.R.; Tressler, R.E. High-temperature oxidation of boron nitride: II, boron nitride layers in composites. J. Am. Ceram. Soc. 1999, 82, 1473–1482. [Google Scholar] [CrossRef]
- Nisar, A.; Ariharan, S.; Venkateswaran, T.; Sreenivas, N.; Balani, K. Effect of carbon nanotube on processing, microstructural, mechanical and ablation behavior of ZrB2-20SiC based ultra-high temperature ceramic composites. Carbon 2017, 111, 269–282. [Google Scholar] [CrossRef]
- Song, L.; Liu, Z.; Reddy, A.L.M.; Narayanan, N.T.; Taha-Tijerina, J.; Peng, J.; Gao, G.; Lou, J.; Vajtai, R.; Ajayan, P.M. Binary and ternary atomic layers built from carbon, boron, and nitrogen. Adv. Mater. 2012, 24, 4878–4895. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.J.; Sun, J.; Ling, H.; Xu, N.; Ying, Z.F.; Wu, J.D. Preparation of thin films of carbon-based compounds. Appl. Surf. Sci. 2003, 218, 298–305. [Google Scholar] [CrossRef]
- Zeng, H.; Kan, Y.-M.; Zhang, G.-J. Synthesis of boron carbide powder from hexagonal boron nitride. Mater. Lett. 2010, 64, 2000–2002. [Google Scholar] [CrossRef]
- Zeng, Y.; Domask, A.C.; Mohney, S.E. Condensed phase diagrams for the metal–W–S systems and their relevance for contacts to WS2. Mater. Sci. Eng. B 2016, 212, 78–88. [Google Scholar] [CrossRef]
- Reich, S.; Ferrari, A.C.; Arenal, R.; Loiseau, A.; Bello, I.; Robertson, J. Resonant Raman scattering in cubic and hexagonal boron nitride. Phys. Rev. B 2005, 71, 205201. [Google Scholar] [CrossRef]
- Zhou, K.-G.; Withers, F.; Cao, Y.; Hu, S.; Yu, G.; Casiraghi, C. Raman modes of MoS2 used as fingerprint of van der Waals interactions in 2-D crystal-based heterostructures. ACS Nano 2014, 8, 9914–9924. [Google Scholar] [CrossRef]
- Gołasa, K.; Grzeszczyk, M.; Bożek, R.; Leszczyński, P.; Wysmołek, A.; Potemski, M.; Babiński, A. Resonant Raman scattering in MoS2—From bulk to monolayer. Solid State Commun. 2014, 197, 53–56. [Google Scholar] [CrossRef]
- Berkdemir, A.; Gutiérrez, H.R.; Botello-Méndez, A.R.; Perea-López, N.; Elías, A.L.; Chia, C.-I.; Wang, B.; Crespi, V.H.; López-Urías, F.; Charlier, J.-C. Identification of individual and few layers of WS2 using Raman Spectroscopy. Sci. Rep. 2013, 3, 1755. [Google Scholar] [CrossRef]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Bakshi, S.R.; Musaramthota, V.; Virzi, D.A.; Keshri, A.K.; Lahiri, D.; Singh, V.; Seal, S.; Agarwal, A. Spark plasma sintered tantalum carbide–carbon nanotube composite: Effect of pressure, carbon nanotube length and dispersion technique on microstructure and mechanical properties. Mater. Sci. Eng. A 2011, 528, 2538–2547. [Google Scholar] [CrossRef]
- Chen, S.; Liu, X.; Shu, X.; Yang, J.; Wang, L.; Zhang, K.; Wang, X.; Zhang, H.; Lu, X. Rapid synthesis and chemical durability of Gd2Zr2-xCexO7 via SPS for nuclear waste forms. Ceram. Int. 2018, 44, 20306–20310. [Google Scholar] [CrossRef]
- Shibata, N.; Horigudhi, M.; Edahiro, T. Raman spectra of binary high-silica glasses and fibers containing GeO2, P2O5 and B2O3. J. Non-Cryst. Solids 1981, 45, 115–126. [Google Scholar] [CrossRef]
- Pirker, L.; Ławrowski, R.; Schreiner, R.; Remškar, M.; Višić, B. MoxWx–1S2 Nanotubes for Advanced Field Emission Application. Adv. Funct. Mater. 2023, 33, 2213869. [Google Scholar] [CrossRef]
- Zhou, Z.F.; Bello, I.; Lei, M.K.; Li, K.Y.; Lee, C.S.; Lee, S.T. Synthesis and characterization of boron carbon nitride films by radio frequency magnetron sputtering. Surf. Coat. Technol. 2000, 128–129, 334–340. [Google Scholar] [CrossRef]
- Reddy, K.M.; Liu, P.; Hirata, A.; Fujita, T.; Chen, M.W. Atomic structure of amorphous shear bands in boron carbide. Nat. Commun. 2013, 4, 2483. [Google Scholar] [CrossRef] [PubMed]
- Ilchenko, O.; Pilgun, Y.; Kutsyk, A.; Bachmann, F.; Slipets, R.; Todeschini, M.; Okeyo, P.O.; Poulsen, H.F.; Boisen, A. Fast and quantitative 2D and 3D orientation mapping using Raman microscopy. Nat. Commun. 2019, 10, 5555. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Shang, L.; Lu, Z.; Zhang, G.; Wang, L.; Xue, Q. Tailoring the mechanical and tribological properties of B4C/aC coatings by controlling the boron carbide content. Surf. Coat. Technol. 2017, 329, 11–18. [Google Scholar] [CrossRef]
- Nehate, S.D.; Saikumar, A.K.; Prakash, A.; Sundaram, K.B. A review of boron carbon nitride thin films and progress in nanomaterials. Mater. Today Adv. 2020, 8, 100106. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, Y.; Zhang, Y.; Lian, G.; Cao, Q.; Que, L. A comparative study on microstructure and tribological characteristics of Mo2FeB2/WC self-lubricating composite coatings with the addition of WS2, MoS2, and h-BN. Mater. Des. 2023, 225, 111581. [Google Scholar] [CrossRef]
- Wang, Z.; Meziani, M.J.; Patel, A.K.; Priego, P.; Wirth, K.; Wang, P.; Sun, Y.-P. Boron nitride nanosheets from different preparations and correlations with their material properties. Ind. Eng. Chem. Res. 2019, 58, 18644–18653. [Google Scholar] [CrossRef]
- Falin, A.; Cai, Q.; Santos, E.J.G.; Scullion, D.; Qian, D.; Zhang, R.; Yang, Z.; Huang, S.; Watanabe, K.; Taniguchi, T.; et al. Mechanical properties of atomically thin boron nitride and the role of interlayer interactions. Nat. Commun. 2017, 8, 15815. [Google Scholar] [CrossRef] [PubMed]

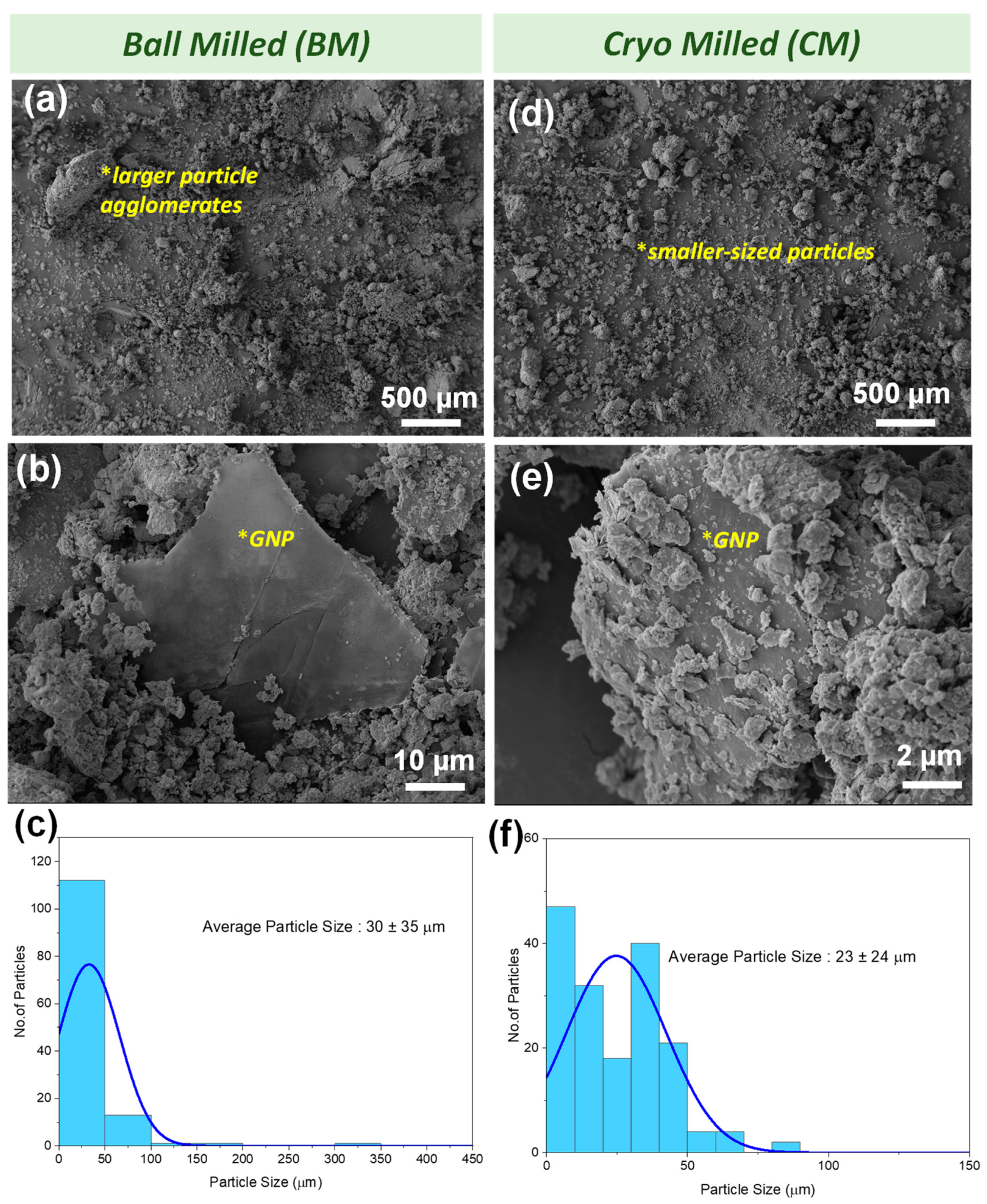
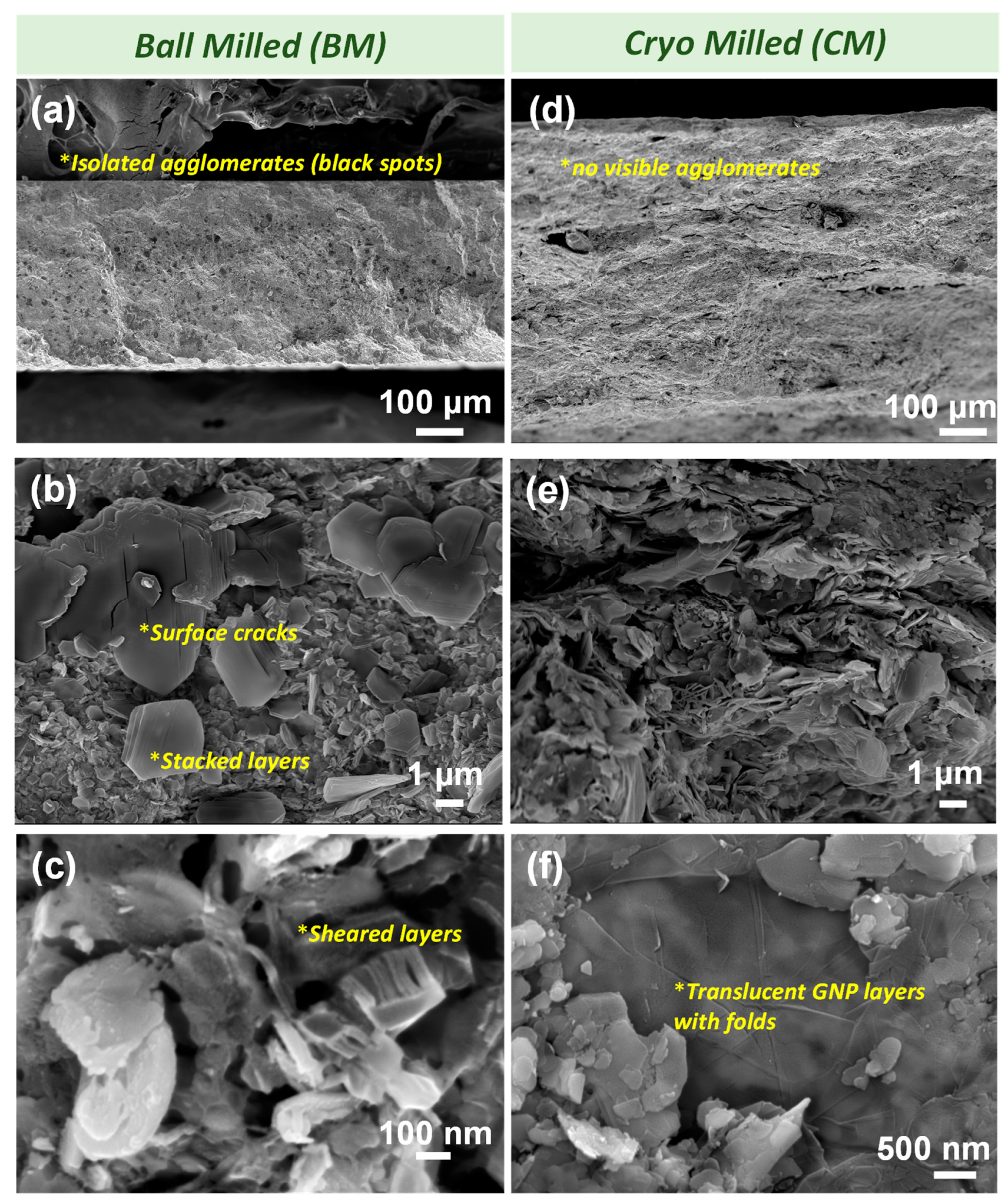

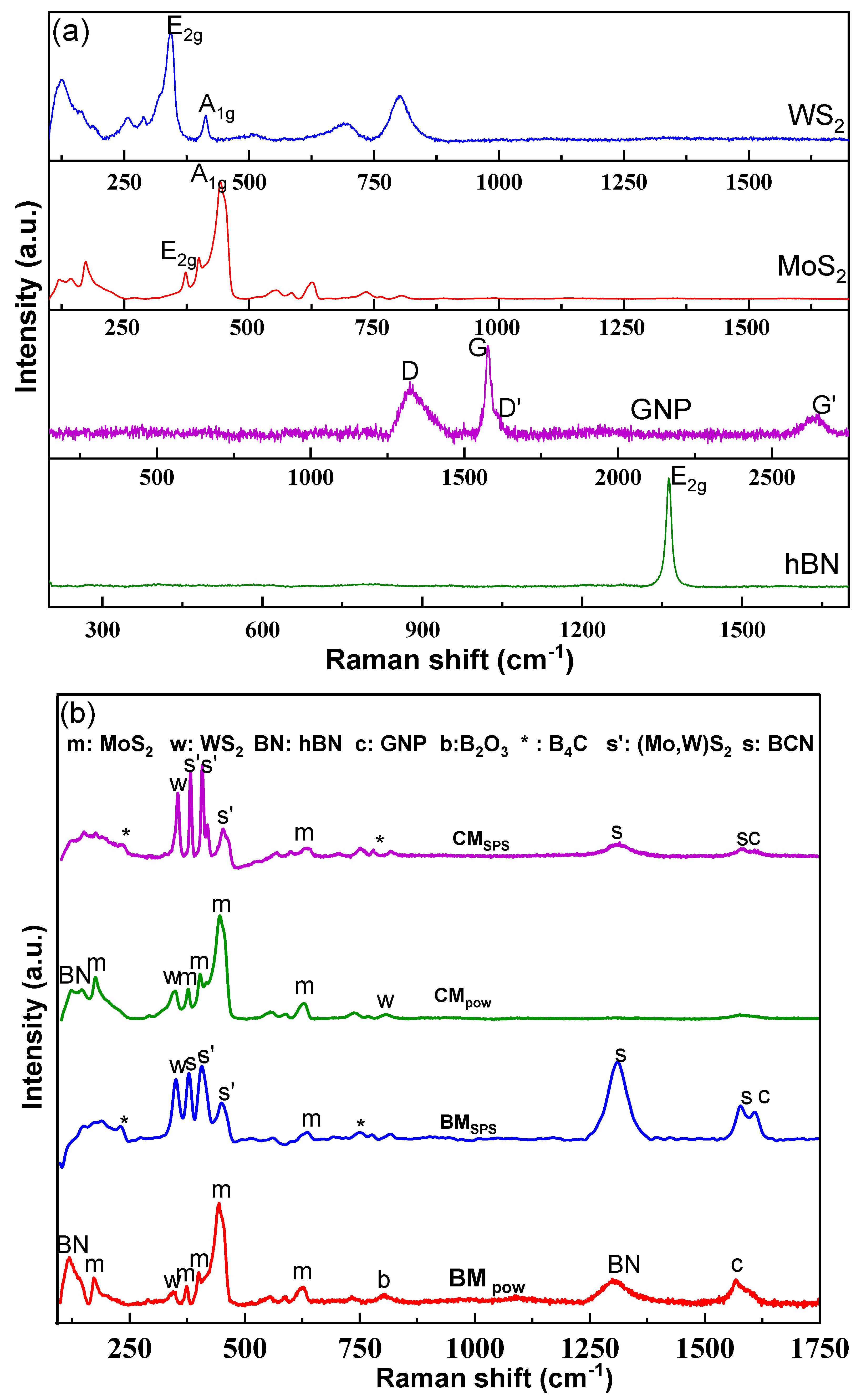
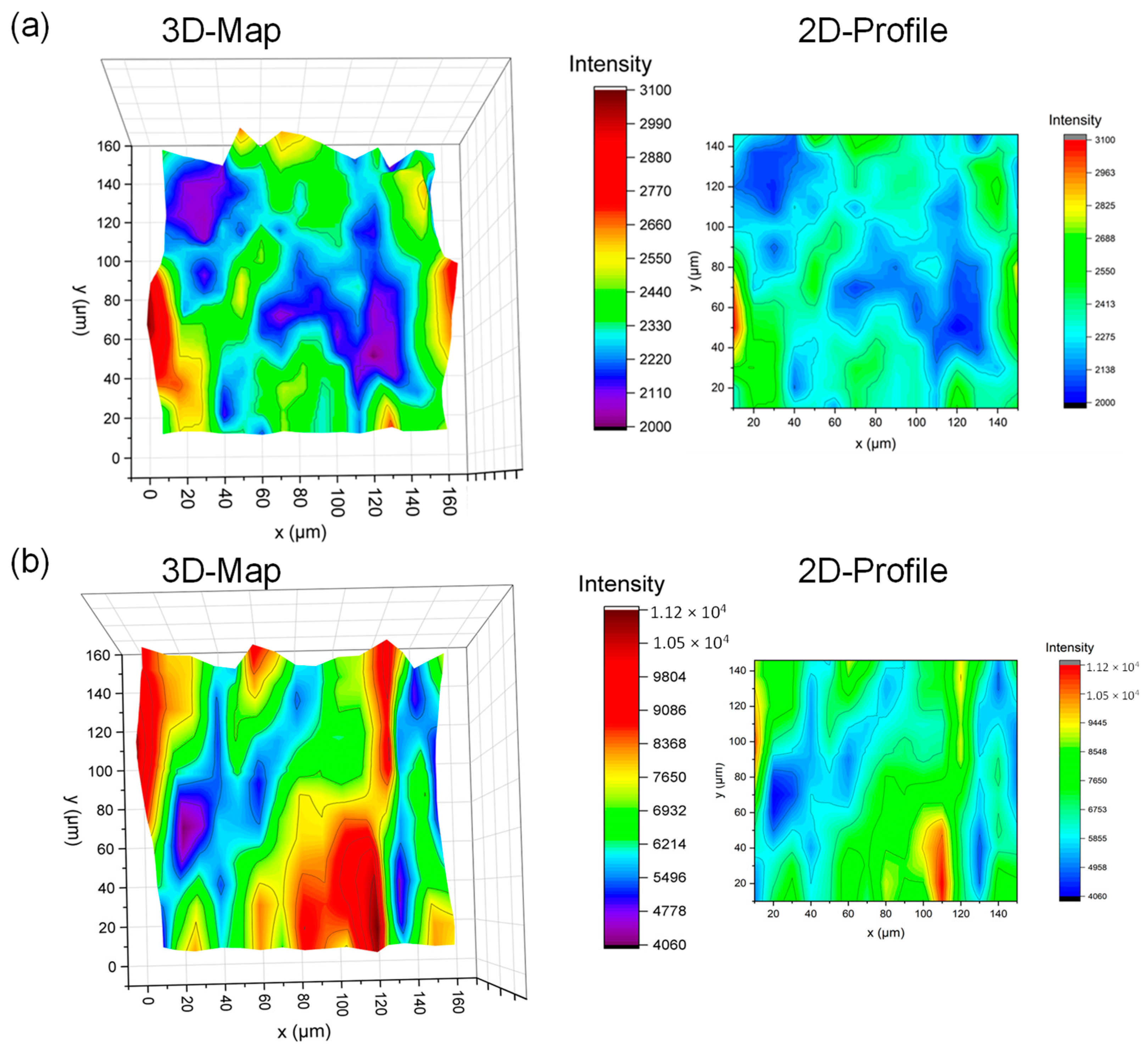

| Parameters | BM along Cross-Section | CM along Cross-Section |
|---|---|---|
| Surface Roughness (µm) | 0.288–0.366 | 0.318–0.455 |
| CoF | 0.389–0.418 | 0.338–0.350 |
| Max. Pd (µm) | 82.56 | 39.42 |
| Average Recovery | 18.31% | 38.18% |
| Wear Volume (×106 µm3) | 19.71 ± 7.67 | 9.45 ± 0.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agarwal, V.; Nisar, A.; Sukumaran, A.K.; Lou, L.; Mohammed, S.M.A.K. Synergistic Effect of Spark Plasma Sintering Driven Solid-Solution Phases on Scratch Resistance in Two-Dimensional Materials. Lubricants 2024, 12, 31. https://doi.org/10.3390/lubricants12020031
Agarwal V, Nisar A, Sukumaran AK, Lou L, Mohammed SMAK. Synergistic Effect of Spark Plasma Sintering Driven Solid-Solution Phases on Scratch Resistance in Two-Dimensional Materials. Lubricants. 2024; 12(2):31. https://doi.org/10.3390/lubricants12020031
Chicago/Turabian StyleAgarwal, Varad, Ambreen Nisar, Abhijith K. Sukumaran, Lihua Lou, and Sohail M. A. K. Mohammed. 2024. "Synergistic Effect of Spark Plasma Sintering Driven Solid-Solution Phases on Scratch Resistance in Two-Dimensional Materials" Lubricants 12, no. 2: 31. https://doi.org/10.3390/lubricants12020031
APA StyleAgarwal, V., Nisar, A., Sukumaran, A. K., Lou, L., & Mohammed, S. M. A. K. (2024). Synergistic Effect of Spark Plasma Sintering Driven Solid-Solution Phases on Scratch Resistance in Two-Dimensional Materials. Lubricants, 12(2), 31. https://doi.org/10.3390/lubricants12020031







