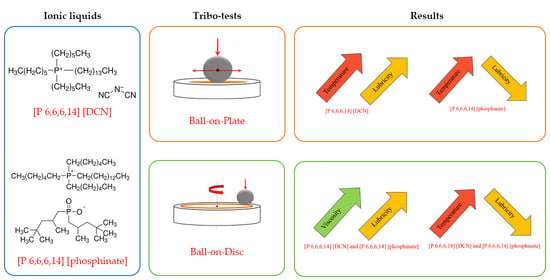
A Comparison of the Tribological Properties of Two Phosphonium Ionic Liquids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Physical Properties
2.3. Lubrication Performance
2.4. Wear Evaluation and Analysis of Worn Surface
3. Results
3.1. Physical Properties
3.2. Friction Reduction Performance
3.2.1. Friction in Reciprocating Tribo-Test
3.2.2. Friction in Continuous Sliding Tribo-Test
3.3. Wear Reduction and Analysis of Worn Surfaces
3.3.1. Wear Observed after Reciprocating Tribo-Test
3.3.2. Wear and Tribo-Film Thickness Observed in Continuous Sliding Tribo-Test
4. Discussion
5. Conclusions
- The anion undoubtedly changes the lubricity of the phosphonium ionic liquid. According to the results of the reciprocation tribo-test, the phosphinate anion-containing ionic liquid performed better at the low test temperature. At the same time, the dicyanamide anion provided phosphonion with better high-temperature lubricity. The lubricity in the continuous sliding tribo-test was governed by the viscosity, where a lower viscosity possessing a dicyanamide-containing ionic liquid was superior in both investigated temperatures.
- Due to the temperature-dependent lubricity, the investigated phosphonium ionic liquids can outperform the reference imidazolium ionic liquid only at specific temperatures, and the dicyanamide anion-containing ionic liquid was particularly superior at a high test temperature. In contrast, the lubricity of the phosphinate anion-containing ionic liquid was comparable at the low test temperature.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Austen Angell, C.; Ansari, Y.; Zhao, Z. Ionic Liquids: Past, Present and Future. Faraday Discuss 2012, 154, 9–27. [Google Scholar] [CrossRef]
- Ye, C.; Liu, W.; Chen, Y.; Yu, L. Room-Temperature Ionic Liquids: A Novel Versatile Lubricant. Chem. Commun. 2001, 21, 2244–2245. [Google Scholar] [CrossRef]
- Zhou, Y.; Qu, J. Ionic Liquids as Lubricant Additives: A Review. ACS Appl. Mater. Interfaces 2017, 9, 3209–3222. [Google Scholar] [CrossRef] [PubMed]
- Song, J. Research Progress of Ionic Liquids as Lubricants. ACS Omega 2021, 6, 29345–29349. [Google Scholar] [CrossRef] [PubMed]
- Mero, A.; Mezzetta, A.; Nowicki, J.; Łuczak, J.; Guazzelli, L. Betaine and L-Carnitine Ester Bromides: Synthesis and Comparative Study of Their Thermal Behaviour and Surface Activity. J. Mol. Liq. 2021, 334, 115988. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, D.; Wong, J.S.S.; Cai, M. Interactions between ZDDP and an Oil-Soluble Ionic Liquid Additive. Tribol. Int. 2021, 158, 106938. [Google Scholar] [CrossRef]
- Cao, Y.; Mu, T. Comprehensive Investigation on the Thermal Stability of 66 Ionic Liquids by Thermogravimetric Analysis. Ind. Eng. Chem. Res. 2014, 53, 8651–8664. [Google Scholar] [CrossRef]
- Piatti, E.; Guglielmero, L.; Tofani, G.; Mezzetta, A.; Guazzelli, L.; D’Andrea, F.; Roddaro, S.; Pomelli, C.S. Ionic Liquids for Electrochemical Applications: Correlation between Molecular Structure and Electrochemical Stability Window. J. Mol. Liq. 2022, 364, 120001. [Google Scholar] [CrossRef]
- Chen, Y.; Han, X.; Liu, Z.; Li, Y.; Sun, H.; Wang, H.; Wang, J. Thermal Decomposition and Volatility of Ionic Liquids: Factors, Evaluation and Strategies. J. Mol. Liq. 2022, 366, 120336. [Google Scholar] [CrossRef]
- He, Y.; Li, H.; Qu, C.; Cao, W.; Ma, M. Recent Understanding of Solid-Liquid Friction in Ionic Liquids. Green Chem. Eng. 2020, 2, 145–157. [Google Scholar] [CrossRef]
- Santhosh Kumar, S.; Ramesh Kumar, S. Ionic Liquids as Environmental Friendly Cutting Fluids—A Review. Mater. Today Proc. 2020, 37, 2121–2125. [Google Scholar] [CrossRef]
- Xiao, H. Ionic Liquid Lubricants: Basics and Applications. Tribol. Trans. 2017, 60, 20–30. [Google Scholar] [CrossRef]
- Kreivaitis, R.; Kupčinskas, A.; Žunda, A.; Ta, T.N.; Horng, J.H. Effect of Temperature on the Lubrication Ability of Two Ammonium Ionic Liquids. Wear 2022, 492–493, 204217. [Google Scholar] [CrossRef]
- Kreivaitis, R.; Gumbytė, M.; Kupčinskas, A.; Kazancev, K.; Ta, T.N.; Horng, J.H. Investigation of Tribological Properties of Two Protic Ionic Liquids as Additives in Water for Steel–Steel and Alumina–Steel Contacts. Wear 2020, 456–457, 203390. [Google Scholar] [CrossRef]
- Donato, M.T.; Colaço, R.; Branco, L.C.; Saramago, B. A Review on Alternative Lubricants: Ionic Liquids as Additives and Deep Eutectic Solvents. J. Mol. Liq. 2021, 333, 116004. [Google Scholar] [CrossRef]
- Reeves, C.J.; Kasar, A.K.; Menezes, P.L. Tribological Performance of Environmental Friendly Ionic Liquids for High-Temperature Applications. J. Clean Prod. 2021, 279, 123666. [Google Scholar] [CrossRef]
- Del Sol, I.; Gámez, A.J.; Rivero, A.; Iglesias, P. Tribological Performance of Ionic Liquids as Additives of Water-Based Cutting Fluids. Wear 2019, 426–427, 845–852. [Google Scholar] [CrossRef]
- Nasser, K.I.; Liñeira del Río, J.M.; López, E.R.; Fernández, J. Hybrid Combinations of Graphene Nanoplatelets and Phosphonium Ionic Liquids as Lubricant Additives for a Polyalphaolefin. J. Mol. Liq. 2021, 336, 116266. [Google Scholar] [CrossRef]
- Yu, B.; Bansal, D.G.; Qu, J.; Sun, X.; Luo, H.; Dai, S.; Blau, P.J.; Bunting, B.G.; Mordukhovich, G.; Smolenski, D.J. Oil-Miscible and Non-Corrosive Phosphonium-Based Ionic Liquids as Candidate Lubricant Additives. Wear 2012, 289, 58–64. [Google Scholar] [CrossRef]
- Somers, A.E.; Khemchandani, B.; Howlett, P.C.; Sun, J.; Macfarlane, D.R.; Forsyth, M. Ionic Liquids as Antiwear Additives in Base Oils: Influence of Structure on Miscibility and Antiwear Performance for Steel on Aluminum. ACS Appl. Mater. Interfaces 2013, 5, 11544–11553. [Google Scholar] [CrossRef] [PubMed]
- Barnhill, W.C.; Qu, J.; Luo, H.; Meyer, H.M.; Ma, C.; Chi, M.; Papke, B.L. Phosphonium-Organophosphate Ionic Liquids as Lubricant Additives: Effects of Cation Structure on Physicochemical and Tribological Characteristics. ACS Appl. Mater. Interfaces 2014, 6, 22585–22593. [Google Scholar] [CrossRef]
- Arora, H.; Cann, P.M. Lubricant Film Formation Properties of Alkyl Imidazolium Tetrafluoroborate and Hexafluorophosphate Ionic Liquids. Tribol. Int. 2010, 43, 1908–1916. [Google Scholar] [CrossRef]
- Qu, J.; Blau, P.J.; Dai, S.; Luo, H.; Meyer, H.M. Ionic Liquids as Novel Lubricants and Additives for Diesel Engine Applications. Tribol. Lett. 2009, 35, 181–189. [Google Scholar] [CrossRef]
- Qu, J.; Blau, P.J.; Dai, S.; Luo, H.; Meyer, H.M.; Truhan, J.J. Tribological Characteristics of Aluminum Alloys Sliding against Steel Lubricated by Ammonium and Imidazolium Ionic Liquids. Wear 2009, 267, 1226–1231. [Google Scholar] [CrossRef]
- Somers, A.E.; Biddulph, S.M.; Howlett, P.C.; Sun, J.; MacFarlane, D.R.; Forsyth, M. A Comparison of Phosphorus and Fluorine Containing IL Lubricants for Steel on Aluminium. Phys. Chem. Chem. Phys. 2012, 14, 8224–8231. [Google Scholar] [CrossRef]
- Somers, A.E.; Howlett, P.C.; Sun, J.; MacFarlane, D.R.; Forsyth, M. Transition in Wear Performance for Ionic Liquid Lubricants under Increasing Load. Tribol. Lett. 2010, 40, 279–284. [Google Scholar] [CrossRef]
- Minami, I.; Inada, T.; Sasaki, R.; Nanao, H. Tribo-Chemistry of Phosphonium-Derived Ionic Liquids. Tribol. Lett. 2010, 40, 225–235. [Google Scholar] [CrossRef]
- Monge, R.; González, R.; Hernández Battez, A.; Fernández-González, A.; Viesca, J.L.; García, A.; Hadfield, M. Ionic Liquids as an Additive in Fully Formulated Wind Turbine Gearbox Oils. Wear 2015, 328–329, 50–63. [Google Scholar] [CrossRef]
- Kawada, S.; Sasaki, S. Tribological Properties of Cyano-Based Ionic Liquids under Different Environments. Tribol. Online 2018, 13, 152–156. [Google Scholar] [CrossRef]
- Kawada, S.; Sasaki, S.; Miyatake, M. In-Situ Observation of Tribo-Decomposition Behavior of Ionic Liquids Composed of Phosphonium-Cation and Cyano-Anion Using Quadrupole Mass Spectrometer. Tribol. Int. 2021, 153, 106547. [Google Scholar] [CrossRef]
- Kawada, S.; Sasaki, S.; Miyatake, M. Effect of Surrounding Atmosphere on Friction Properties of Hydrophobic and Hydrophilic Ionic Liquids. Tribol. Online 2019, 14, 285–292. [Google Scholar] [CrossRef]
- Okubo, H.; Kawada, S.; Watanabe, S.; Sasaki, S. Tribological Performance of Halogen-Free Ionic Liquids in Steel–Steel and DLC–DLC Contacts. Tribol. Trans. 2018, 61, 71–79. [Google Scholar] [CrossRef]
- Kawada, S.; Watanabe, S.; Tadokoro, C.; Tsuboi, R.; Sasaki, S. Lubricating Mechanism of Cyano-Based Ionic Liquids on Nascent Steel Surface. Tribol. Int. 2018, 119, 474–480. [Google Scholar] [CrossRef]
- Kawada, S.; Watanabe, S.; Tsuboi, R.; Sasaki, S.; Prakash, B. Lubrication Mechanism of Halogen-Free Ionic Liquids. Tribol. Online 2017, 12, 155–161. [Google Scholar] [CrossRef]
- Pejaković, V.; Tomastik, C.; Dörr, N.; Kalin, M. Influence of Concentration and Anion Alkyl Chain Length on Tribological Properties of Imidazolium Sulfate Ionic Liquids as Additives to Glycerol in Steel–Steel Contact Lubrication. Tribol. Int. 2016, 97, 234–243. [Google Scholar] [CrossRef]
- Huang, G.; Fan, S.; Ba, Z.; Cai, M.; Qiao, D. Insight into the Lubricating Mechanism for Alkylimidazolium Phosphate Ionic Liquids with Different Alkyl Chain Length. Tribol. Int. 2019, 140, 105886. [Google Scholar] [CrossRef]
- Bartolomé, M.; Gonçalves, D.; Tuero, A.G.; González, R.; Battez, A.H.; Seabra, J.H.O. Greases Additised with Phosphonium-Based Ionic Liquids—Part I: Rheology, Lubricant Film Thickness and Stribeck Curves. Tribol. Int. 2021, 156, 106851. [Google Scholar] [CrossRef]
- Matczak, L.; Johanning, C.; Gil, E.; Guo, H.; Smith, T.W.; Schertzer, M.; Iglesias, P. Effect of Cation Nature on the Lubricating and Physicochemical Properties of Three Ionic Liquids. Tribol. Int. 2018, 124, 23–33. [Google Scholar] [CrossRef]
- Lu, R.; Mori, S.; Kobayashi, K.; Nanao, H. Study of Tribochemical Decomposition of Ionic Liquids on a Nascent Steel Surface. Appl. Surf. Sci. 2009, 255, 8965–8971. [Google Scholar] [CrossRef]
- Minami, I.; Kita, M.; Kubo, T.; Nanao, H.; Mori, S. The Tribological Properties of Ionic Liquids Composed of Trifluorotris(Pentafluoroethyl) Phosphate as a Hydrophobic Anion. Tribol. Lett. 2008, 30, 215–223. [Google Scholar] [CrossRef]
- Han, T.; Yi, S.; Zhang, C.; Li, J.; Chen, X.; Luo, J.; Banquy, X. Superlubrication Obtained with Mixtures of Hydrated Ions and Polyethylene Glycol Solutions in the Mixed and Hydrodynamic Lubrication Regimes. J. Colloid. Interface Sci. 2020, 579, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, T.; Jiménez, M.; Sanes, J.; Jiménez, A.-E.; Iglesias, M.; Bermúdez, M.-D. Ultra-Low Friction with a Protic Ionic Liquid Boundary Film at the Water-Lubricated Sapphire–Stainless Steel Interface. Tribol. Lett. 2014, 53, 1–9. [Google Scholar] [CrossRef]
- Hua, J.; Björling, M.; Larsson, R.; Shi, Y. Controllable Superlubricity Achieved with Mixtures of Green Ionic Liquid and Glycerol Aqueous Solution via Humidity. J. Mol. Liq. 2022, 345, 117860. [Google Scholar] [CrossRef]
- Huddleston, J.G.; Visser, A.E.; Reichert, W.M.; Willauer, H.D.; Broker, G.A.; Rogers, R.D. Characterization and Comparison of Hydrophilic and Hydrophobic Room Temperature Ionic Liquids Incorporating the Imidazolium Cation. Green Chem. 2001, 3, 156–164. [Google Scholar] [CrossRef]
- Jiménez, A.E.; Bermúdez, M.D.; Iglesias, P.; Carrión, F.J.; Martínez-Nicolás, G. 1-N-Alkyl -3-Methylimidazolium Ionic Liquids as Neat Lubricants and Lubricant Additives in Steel–Aluminium Contacts. Wear 2006, 260, 766–782. [Google Scholar] [CrossRef]
- Luiz, J.F.; Spikes, H. Tribofilm Formation, Friction and Wear-Reducing Properties of Some Phosphorus-Containing Antiwear Additives. Tribol. Lett. 2020, 68, 75. [Google Scholar] [CrossRef]
- Dawczyk, J.; Morgan, N.; Russo, J.; Spikes, H. Film Thickness and Friction of ZDDP Tribofilms. Tribol. Lett. 2019, 67, 34. [Google Scholar] [CrossRef]
- Seddon, K.R.; Stark, A.; Torres, M.-J. Influence of Chloride, Water, and Organic Solvents on the Physical Properties of Ionic Liquids. Pure Appl. Chem. 2000, 72, 2275–2287. [Google Scholar] [CrossRef]
- Marták, J.; Schlosser, Š. Density, Viscosity, and Structure of Equilibrium Solvent Phases in Butyric Acid Extraction by Phosphonium Ionic Liquid. J. Chem. Eng. Data 2017, 62, 3025–3035. [Google Scholar] [CrossRef]










| Ionic Liquid Lubricants | Molecular Structure | Appearance Colour | Purity, % | Water |
|---|---|---|---|---|
| Trihexyltetradecylphosphonium dicyanamide [P 6,6,6,14] [DCN] CAS No. 701921-71-3 |  | Very dark yellow | >95 * | 0.2% * |
| Trihexyltetradecylphosphonium bis(2,4,4-trimethylpentyl)phosphinate [P 6,6,6,14] [phosphinate] CAS No. 465527-59-7 |  | Light yellow | >90 * | ≤1.0% * |
| 1-Butyl-3-methylimidazolium hexafluorophosphate [BMIM] [PF6] CAS No. 174501-64-5 |  | Very faintly brownish yellow | 99.7 * | n.d. |
| Ball-on-Plate Reciprocating Mode | Ball-on-Disc Continuous Sliding | ||||
|---|---|---|---|---|---|
| Ball | Diameter—6 mm; Roughness (Ra)—0.05 μm; Hardness (HV)—750…800; Material—E 52100 | Ball | Diameter—19.05 mm; Roughness (Ra)—0.02 μm; Hardness (HV)—800…920; Material—E 52100 | ||
| Plate | Diameter—10 mm; Thickness—3 mm; Roughness (Ra)—0.02 μm; Hardness (HV)—190…200; Material—E 52100 | Disc | Diameter—46 mm; Roughness (Ra)—0.02 μm; Hardness (HV)—720…780; Material—E 52100 | ||
| Tribo-test parameters | |||||
| Reciprocation frequency, Hz | 15 | Sliding speed, m/s | 0.1 | ||
| Load, N | 4 | 40 | |||
| Contact pressure, GPa | 1.05 | 1 | |||
| Test duration, min | 120 min | 120 min | |||
| Test temperature, °C | 30 and 80 | 30 and 80 | |||
| Stroke length, mm | 1 | ||||
| Ionic Liquid | ν (mm2/s) | VI | ρ (g/cm3) at 25 °C | MW (g/mol) | |
|---|---|---|---|---|---|
| 30 | 80 | ||||
| [P 6,6,6,14] [DCN] | 358.2 | 44.2 | 147.8 | 0.8997 | 549.9 |
| [P 6,6,6,14] [phosphinate] | 677.4 | 61.9 | 139.4 | 0.8933 | 773.3 |
| [BMIM] [PF6] | 133.6 | 17.9 | 120.5 | 1.3642 | 284.2 |
| Ionic Liquid | Wear Scar Diameter [μm] | Wear Volume [μm3] × 103 | ||
|---|---|---|---|---|
| 30 °C | 80 °C | 30 °C | 80 °C | |
| [P 6,6,6,14] [DCN] | 168 | 106 | 110.20 | 0.94 |
| [P 6,6,6,14] [phosphinate] | 116 | 284 | 5.42 | 685.04 |
| [BMIM] [PF6] | 115 | 206 | 1.93 | 162.30 |
| Ionic Liquids | Ra, μm | |
|---|---|---|
| 30 °C | 80 °C | |
| [P 6,6,6,14] [DCN] | 0.033 | 0.002 |
| [P 6,6,6,14] [phosphinate] | 0.005 | 0.151 |
| [BMIM] [PF6] | 0.004 | 0.022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horng, J.-H.; Ta, T.-N.; Kreivaitis, R.; Treinytė, J.; Kupčinskas, A.; Gumbytė, M. A Comparison of the Tribological Properties of Two Phosphonium Ionic Liquids. Lubricants 2024, 12, 53. https://doi.org/10.3390/lubricants12020053
Horng J-H, Ta T-N, Kreivaitis R, Treinytė J, Kupčinskas A, Gumbytė M. A Comparison of the Tribological Properties of Two Phosphonium Ionic Liquids. Lubricants. 2024; 12(2):53. https://doi.org/10.3390/lubricants12020053
Chicago/Turabian StyleHorng, Jeng-Haur, Thi-Na Ta, Raimondas Kreivaitis, Jolanta Treinytė, Artūras Kupčinskas, and Milda Gumbytė. 2024. "A Comparison of the Tribological Properties of Two Phosphonium Ionic Liquids" Lubricants 12, no. 2: 53. https://doi.org/10.3390/lubricants12020053
APA StyleHorng, J.-H., Ta, T.-N., Kreivaitis, R., Treinytė, J., Kupčinskas, A., & Gumbytė, M. (2024). A Comparison of the Tribological Properties of Two Phosphonium Ionic Liquids. Lubricants, 12(2), 53. https://doi.org/10.3390/lubricants12020053