Oxidation and Flammability Tests for Grape (Vitis vinifera L.) Seed Oil
Abstract
1. Introduction
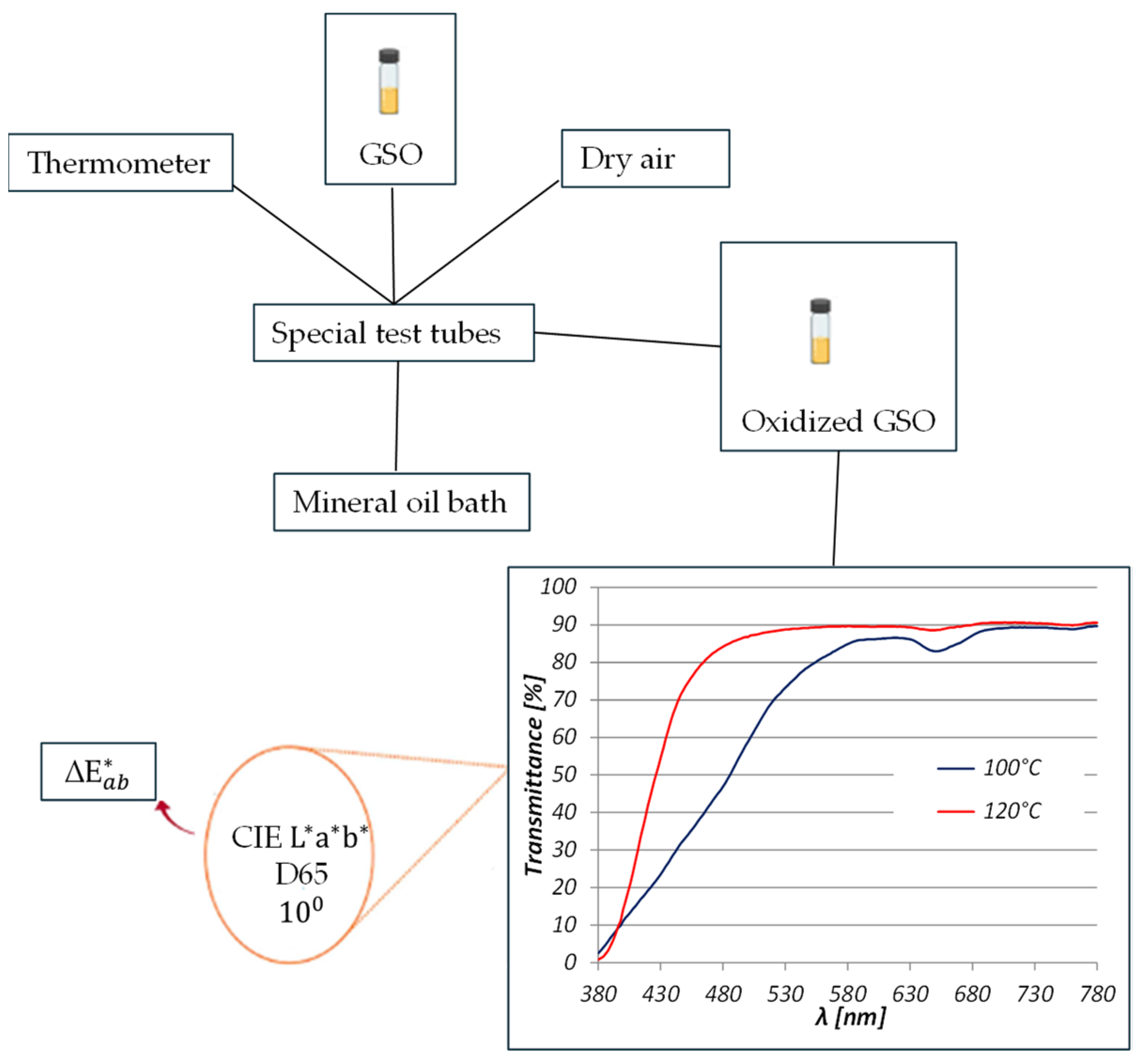
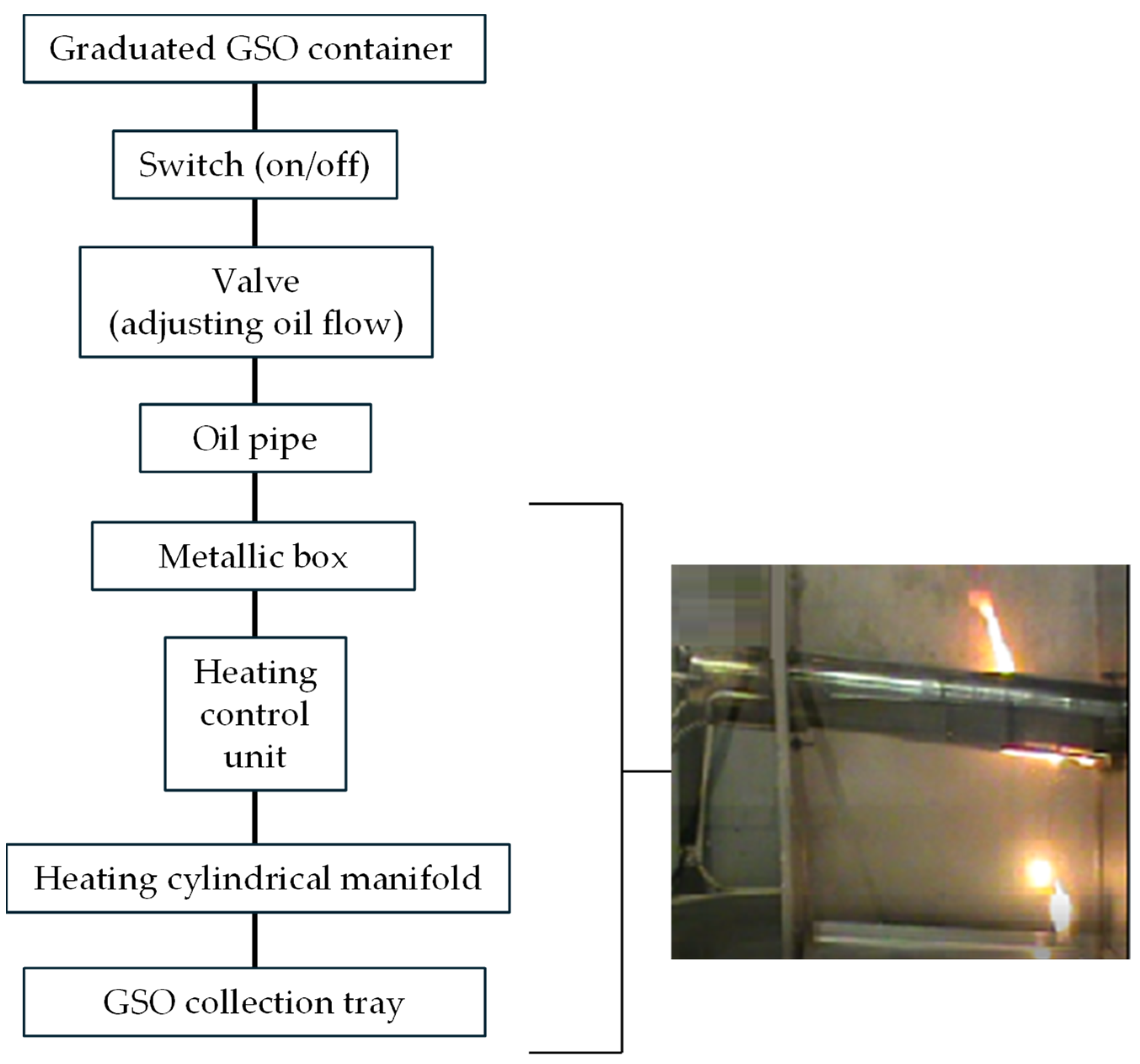
2. Materials and Methods
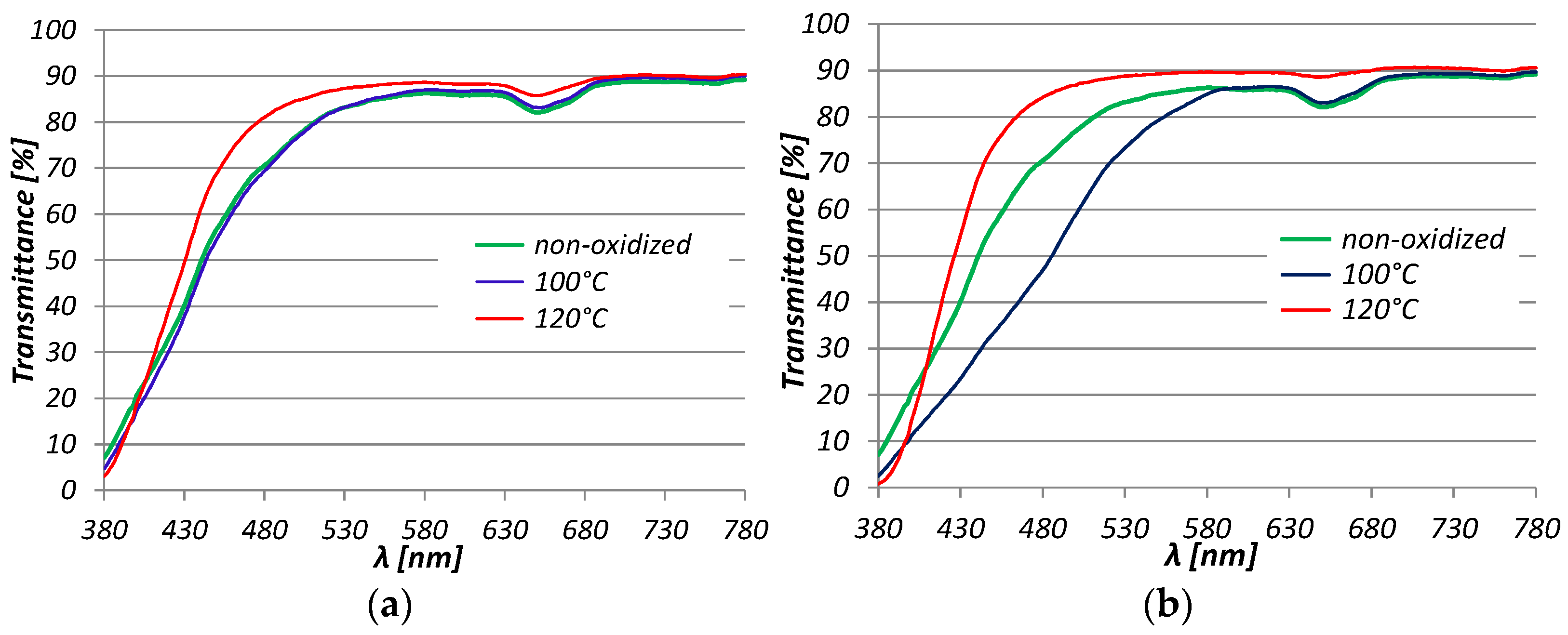
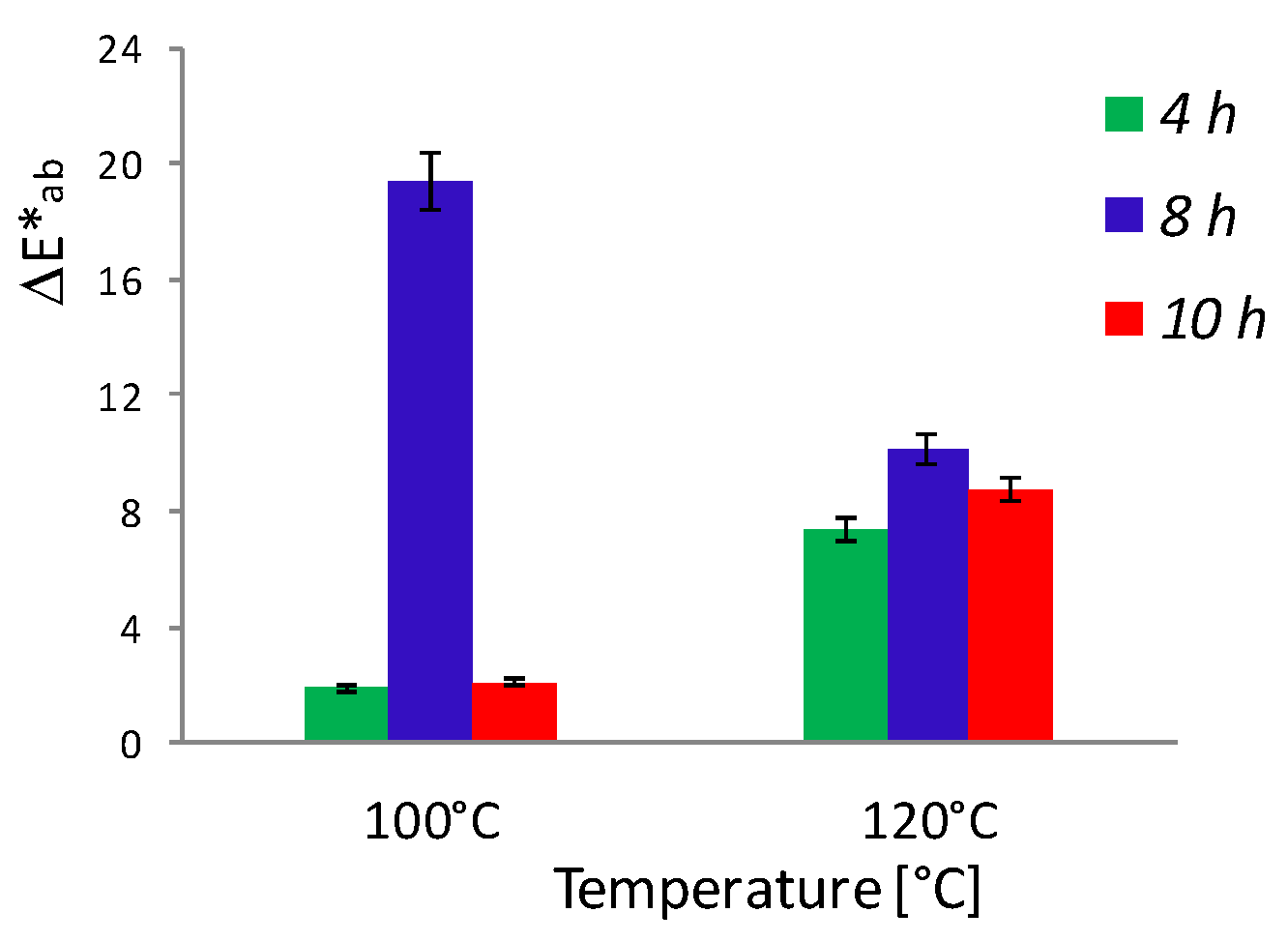
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maier, T.; Schieber, A.; Kammerer, D.R.; Carle, R. Residues of grape (Vitis vinifera L.) seed oil production as a valuable source of phenolic antioxidants. Food Chem. 2009, 112, 551–559. [Google Scholar] [CrossRef]
- Yun-Sang, C.; Ji-Hun, C.; Doo-Jeong, H.; Hack-Youn, K.; Mi-Ai, L.; Hyun-Wook, K.; Ju-Woon, L.; Hai-Jung, C.; Cheon-Jei, K. Optimization of replacing pork back fat with grape seed oil and rice bran fiber for reduced-fat meat emulsion systems. Meat Sci. 2010, 84, 212–218. [Google Scholar]
- Martin, M.E.; Grao-Cruces, E.; Millan-Linares, M.C.; Montserrat-de la Paz, S. Grape (Vitis vinifera L.) seed oil: A functional food from the winemaking industry. Foods 2020, 9, 1360. [Google Scholar] [CrossRef]
- Agriculture and Rural Development. Wine. Available online: https://agriculture.ec.europa.eu/farming/crop-productions-and-plant-based-products/wine_en (accessed on 21 January 2024).
- Fernández, C.M.; Ramos, M.J.; Pérez, A.; Rodríguez, J.F. Production of biodiesel from winery waste: Extraction, refining and transesterification of grape seed oil. Bioresour. Technol. 2010, 101, 7019–7024. [Google Scholar] [CrossRef]
- Ramos, M.J.; Fernández, C.M.; Casas, A.; Rodríguez, L.; Pérez, A. Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour. Technol. 2009, 100, 261–268. [Google Scholar] [CrossRef]
- Crews, C.; Hough, P.; Godward, J.; Brereton, P.; Lees, M.; Guiet, S. Quantification of the main constituents of same authentic grape-seed oils of different origin. J. Agric. Food Chem. 2006, 54, 6261–6265. [Google Scholar] [CrossRef]
- Kamel, B.S.; Dawson, H.; Kakuda, Y. Characteristics and composition of melon and grape seed oils and cakes. J. Am. Oil Chem. Soc. 1985, 62, 881–883. [Google Scholar] [CrossRef]
- Roberts, J.S.; Kidd, D.R.; Padilla-Zakour, O. Drying kinetics of grape seeds. J. Food Eng. 2008, 89, 460–465. [Google Scholar] [CrossRef]
- Chenlu, Y.; Kun, S.; Chanchan, L.; Can, W.; Xueqing, S.; Hua, W.; Hua, L. Processing technologies, phytochemical constituents, and biological activities of grape seed oil (GSO): A review. Trends Food Sci. Technol. 2021, 116, 1074–1083. [Google Scholar]
- Jokic, S.; Bijuk, M.; Aladic, K.; Bilic, M.; Molnar, M. Optimisation of superficial CO2 extraction of grape seed oil using response surface methodology. Int. J. Food Sci. Technol. 2016, 51, 403–410. [Google Scholar] [CrossRef]
- Lutterodt, H.; Slavin, M.; Whent, M.; Turner, E.; Yu, L.L. Fatty acid composition, oxidative stability, antioxidant and antiproliferative properties of selected cold-pressed grape seed oils and flours. Food Chem. 2011, 128, 391–399. [Google Scholar] [CrossRef]
- Silva, T.L.; Bernardo, E.; Nobre, B.; Mendes, R.; Reis, A. Extraction of victoria and red globe grape seed oils using supercritical carbon dioxide with and without ethanol. J. Food Lipids 2008, 15, 356–369. [Google Scholar] [CrossRef]
- Boso, S.; Gago, P.; Santiago, J.L.; Rodríguez-Canas, E.; Martínez, M.C. New monovarietal grape seed oils derived from white grape bagasse generated on an industrial scale at a winemarking plant. Food Sci. Technol. 2018, 92, 388–394. [Google Scholar]
- Tangolar, S.G.; Özogul, Y.; Tangolar, S.; Torun, A. Evaluation of fatty acid profiles and mineral content of grape seed oil of some grape genotypes. Int. J. Food Sci. Nutr. 2009, 60, 32–39. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Cruz, R.; Pereira, J.A.; Ramalhosa, E. Seed oils of ten traditional Portuguese grape varieties with interesting chemical and antioxidant properties. Food Res. Int. 2013, 50, 161–166. [Google Scholar] [CrossRef]
- Heshmati, M.K.; Jafarzadeh-Moghaddam, M.; Pezeshki, A.; Shaddel, R. The oxidative and thermal stability of optimal synergistic mixture of sesame and grapeseed oils as affected by frying process. Food Sci. Nutr. 2022, 10, 1103–1112. [Google Scholar] [CrossRef]
- Maszewska, M.; Florowska, A.; Dłużewska, E.; Wroniak, M.; Marciniak-Lukasiak, K.; Żbikowska, A. Oxidative stability of selected edible oils. Molecules 2018, 23, 1746. [Google Scholar] [CrossRef]
- Ovcharova, T.N.; Zlatanov, M.D. Oxidative stability and stabilization of grape seed oil. Bulg. Chem. Commun. 2014, 46, 30–33. [Google Scholar]
- Nateghi, L.; Hosseini, E. Investigating the oxidative stability of grape seed oil using aqueous extract of pistachio green hull. J. Food Meas. Charact. 2023, 17, 4434–4447. [Google Scholar] [CrossRef]
- Dragancea, V.; Gurev, A.; Sturza, R.; Haritonov, S.; Netreba, N.; Boeștean, O. The effect of grape seed oil fortification with extracts of natural antioxidants. J. Eng. Sci. 2023, 30, 173–184. [Google Scholar]
- Malicanin, M.; Rac, V.; Antic, V.; Antic, M.; Palade, L.M.; Kefalas, P.; Rakic, V. Content of antioxidants, antioxidant capacity and oxidative stability of grape seed oil obtained by ultrasound-assisted extraction. J. Am. Oil Chem. Soc. 2014, 91, 989–999. [Google Scholar] [CrossRef]
- Di Stefano, V.; Buzzanca, C.; Melilli, M.G.; Indelicato, S.; Mauro, M.; Vazzana, M.; Arizza, V.; Lucarini, M.; Durazzo, A.; Bongiorno, D. Polyphenol characterization and antioxidant activity of grape seeds and skins from Sicily: A Preliminary Study. Sustainability 2022, 14, 6702. [Google Scholar] [CrossRef]
- Konuskan, D.B.; Kamiloglu, O.; Demirkeser, O. Fatty acid composition, total phenolic content and antioxidant activity of grape seed oils obtained by cold-pressed and solvent extraction. Indian J. Pharm. Educ. 2019, 53, 144–150. [Google Scholar] [CrossRef]
- Sochorova, L.; Prusova, B.; Jurikova, T.; Mlcek, J.; Adamkova, A.; Baron, M.; Sochor, J. The study of antioxidant components in grape seeds. Molecules 2020, 25, 3736. [Google Scholar] [CrossRef]
- Fanigliulo, R.; Bondioli, P.; Biocca, M.; Grilli, R.; Gallo, P.; Fornaciari, L.; Folegatti, L.; Benigni, S.; Calderari, I.; Gallucci, F.; et al. Olive Pomace Oil as a Chainsaw Lubricant: First Results of Tests on Performance and Safety Aspects. Lubricants 2023, 11, 494. [Google Scholar] [CrossRef]
- Ugolini, L.; Matteo, R.; Lazzeri, L.; Malaguti, L.; Folegatti, L.; Bondioli, P.; Pochi, D.; Grilli, R.; Fornaciari, L.; Benigni, S.; et al. Technical Performance and Chemical–Physical Property Assessment of Safflower Oil Tested in an Experimental Hydraulic Test Rig. Lubricants 2023, 11, 39. [Google Scholar] [CrossRef]
- Sanahuja-Parejo, O.; Veses, A.; Navarro, M.V.; López, J.M.; Murillo, R.; Callén, M.S.; García, T. Drop-in biofuels from the co-pyrolysis of grape seeds and polystyrene. Chem. Eng. J. 2019, 377, 120296. [Google Scholar] [CrossRef]
- Azad, M.; Rasul, M. Performance and combustion analysis of diesel engine fueled with grape seed and waste cooking biodiesel. Energy Procedia 2019, 160, 340–347. [Google Scholar] [CrossRef]
- Sridhar, R.; Shaisundaram, V.S.; Baskar, S.; Ramasubramanian, S.; Sathiskumar, G.; Sivabalan, S. Investigation of grape seed oil biodiesel with cerium oxide nanoparticle in CI engine. Mater. Today Proc. 2021, 37, 1399–1402. [Google Scholar] [CrossRef]
- Rodrigues, M.A.V.; Bertolo, M.R.V.; Marangon, C.A.; Martins, V.C.A.; Plepis, A.M.G. Chitostan and gelatin material incorporated with phenolic extracts of grape seed and jabutiga peel: Rheological, physical-chemical, antioxidant, antimicrobial and barrier properties. Int. J. Biol. Macromol. 2020, 160, 769–779. [Google Scholar] [CrossRef]
- Serra, A.; Paixao, T.; Ferreira, I.; Brasil, J.; Ribeiro, F.P. Study of grape seed oil in the proposal of biolubricants in open systems. In Proceedings of the 24th ABCM International Congress of Mechanical Engineering, Curitiba, PR, Brazil, 3–8 December 2017; Volume 12, pp. 3–8. [Google Scholar]
- Liu, J.; Zhang, Y.; Yi, C.; Zhang, R.; Yang, S.; Liu, T.; Jia, D.; Yang, Q.; Peng, S. Evaluation of antioxidant properties and molecular design of lubricant antioxidants based on QSPR Model. Lubricants 2024, 12, 3. [Google Scholar] [CrossRef]
- Garcés, R.; Martínez-Force, E.; Salas, J.J. Vegetable oil basestocks for lubricants. Grasas Aceites 2011, 62, 21–28. [Google Scholar] [CrossRef]
- Owuna, F.J. Stability of vegetable based oils used in the formulation of ecofriendly lubricants—A review. Egypt. J. Petrol. 2020, 29, 251–256. [Google Scholar] [CrossRef]
- Becker, R.; Knorr, A. An evaluation of antioxidants for vegetable oils at elevated temperatures. Lubr. Sci. 1996, 8, 95–117. [Google Scholar] [CrossRef]
- Fadda, A.; Sanna, D.; Sakar, E.H.; Gharby, S.; Mulas, M.; Medda, S.; Yesilcubuk, N.S.; Karaca, A.C.; Gozukirmizi, C.K.; Lucarini, M.; et al. Innovative and sustainable technologies to enhance the oxidative stability of vegetable oils. Sustainability 2022, 14, 849. [Google Scholar] [CrossRef]
- Nagendramma, P.; Kaul, S. Development of ecofriendly/biodegradable lubricants: An overview. Renew. Sustain. Energy Rev. 2012, 16, 764–774. [Google Scholar] [CrossRef]
- Masripan, N.A.; Salim, M.A.; Omar, G.; Mansor, M.R.; Saad, A.M.; Hamid, N.A.; Syakir, M.I.; Dai, F. Vegetable oil as bio-lubricant and natural additive in lubrication: A review. Int. J. Nanoelectron. Mater. 2020, 13, 161–176. [Google Scholar]
- Javier, A.O.; Vicente, C.; Dario, R.; Victoria, M. Investigating the rheological behavior and lubrication performance of grapeseed oil modified with SiO2 and TiO2 nanoparticles for industrial applications. J. Mater. Sci. 2022, 7, 555706. [Google Scholar]
- Mohammed, H.; Abdulmunem, R.A.; Hussain, S.A. As a potential hydraulic fluid: Corn oil behavior characteristics examination. J. Therm. Eng. 2021, 7, 215–221. [Google Scholar]
- Javier, A.O.; Biswas, M.A.S.; Rahman, M.M.; Martinez, V.; Peña-Parás, L.; Maldonado-Cortés, D. Investigating the lubrication performance of vegetable oils reinforced with HNT and MMT nanoclays as green lubricant additives. Wear 2023, 523, 204859. [Google Scholar]
- Quinchia, L.A.; Delgado, M.A.; Reddyhoff, T.; Gallegos, C.; Spikes, H.A. Tribological studies of potential vegetable oil-based lubricants containing environmentally friendly viscosity modifiers. Tribol. Int. 2014, 69, 110–117. [Google Scholar] [CrossRef]
- Filho, P.R.C.; Paixão, T.; Serra, A.; Ferreira, I. Study of lubrication of castor/linseed vegetable oils through drip lubricated contact wear test. In Proceedings of the 24th ABCM International Congress of Mechanical Engineering, Curitiba, PR, Brazil, 3–8 December 2017. [Google Scholar]
- Irina, L.B.; Dragos, C.; Constantin, T. Improving the oxidation stability end biodegradability of environmentally friendly lubricants. Rev. Chim. 2010, 61, 1003–1007. [Google Scholar]
- Jayadasa, N.H.; Prabhakaran, K.N. Coconut oil as base oil for industrial lubricants—Evaluation and modification of thermal, oxidative and low temperature properties. Tribol. Int. 2006, 39, 873–878. [Google Scholar] [CrossRef]
- Biswas, M.A.S.; Rahman, M.M.; Javier, A.O.; Peña-Parás, L.; Maldonado-Cortés, D.; González, J.A.; Cantú, R.; Campos, A.; Flores, E. Lubrication performance of sunflower oil reinforced with halloysite clay nanotubes (HNT) as lubricant additives. Lubricants 2022, 10, 139. [Google Scholar] [CrossRef]
- Kabuya, A.; Bozet, J.L. Comparative analysis of the lubricating power between a pure mineral oil and biodegradable oils of the same mean iso grade. In Lubricants and Lubrication; Dowson, D., Taylor, C.M., Childs, T.H.C., Dalmaz, G., Eds.; Tribology Series; Elsevier: Amsterdam, The Netherlands, 1995; Volume 30, pp. 25–30. [Google Scholar]
- Ozioko, F.U. Extraction and characterization of soybean oil based bio-lubricant. AU J. Technol. 2012, 15, 260–264. [Google Scholar]
- CIE. Recommendation Officielles de la Commission Internationale de l’Eclairage Proceedings, Colourimetrie; Compte Rendu des Seances Huitieme Session; University Press: Cambridge, UK, 1931; pp. 19–29. [Google Scholar]
- CIE. The Supplementary Standard Colourimetric Observer; Proceedings of the CIE Vienna Session, B (Committee Report E-1.4.1); CIE Central Bureau: Vienna, Austria, 1964; pp. 209–220. [Google Scholar]
- CIE (International Commission on Illumination). CIE 15: Technical Report, Colourimetry, 3rd ed.; CIE Central Bureau: Vienna, Austria, 2004; pp. 9–66. [Google Scholar]
- Șolea, L.C.; Deleanu, L. Flammability tests on hot surface for castor oil. Mech. Test. Diagn. 2020, 4, 24–28. [Google Scholar] [CrossRef]
- Georgescu, C.; Cristea, G.C.; Șolea, L.C.; Deleanu, L.; Sandu, I.G. Flammability of some vegetal oils on hot surface. Rev. Chim. 2018, 69, 668–673. [Google Scholar] [CrossRef]
- Deleanu, L.; Buzoianu, D.; Rîpă, M.; Crăciunoiu, S.; Drug, A. Flammability tests on hot surfaces for industrial fluids. Ann. Univ. “Dunărea De Jos” Galaţi Fascicle VIII Tribol. 2007, 22–31. [Google Scholar]
- Șolea, L.C.; Deleanu, L. Flammability tests for hemp seed oil. Mech. Test. Diagn. 2021, 2, 5–9. [Google Scholar] [CrossRef]
- Crețu, R.; Șolea, L.C. Effect of the temperature and oxidation time on some physicochemical characteristics of the rice bran oil. Ann. “Dunarea De Jos” Univ. Galati. Fascicle IX Metall. Mater. Sci. 2022, 45, 5–10. [Google Scholar] [CrossRef]
- Șolea, L.C.; Crețu, R. Research on the rice bran oil—Flammability tests on a high-temperature surface. Ann. “Dunarea De Jos” Univ. Galati. Fascicle IX Metall. Mater. Sci. 2023, 46, 10–15. [Google Scholar] [CrossRef]
- Cobzaru, C.; Matcabojă (Gherasim), M.A.; Marinoiu, A.; Apostolescu, G.A.; Tataru, F.R.E.; Cernătescu, C. Characteristics of oil obtained from grape seeds separated from dried and fresh marc. Bul. Inst. Politeh. Iaşi 2017, 63, 23–28. [Google Scholar]
- Kapcsándi, V.; Lakatos, E.H.; Sik, B.; Linka, L.Á.; Székelyhidi, R. Characterization of fatty acid, antioxidant, and polyphenol content of grape seed oil from different Vitis vinifera L. varieties. OCL Oilseeds Fats Crop. Lipids 2021, 28, 30. [Google Scholar] [CrossRef]
- Carmona-Jiménez, Y.; Igartuburu, J.M.; Guillén-Sánchez, D.A.; García-Moreno, M.V. Fatty acid and tocopherol composition of pomace and seed oil from five grape varieties southern Spain. Molecules 2022, 27, 6980. [Google Scholar] [CrossRef] [PubMed]
- Patterson, H.B.W. Chapter 4—Bleaching of important fats and oils. In Bleaching and Purifying Fats and Oils, Theory and Practice; AOCS Press: Champaign, IL, USA, 2009; pp. 97–151. [Google Scholar]
- D’Eusanio, V.; Malferrari, D.; Marchetti, A.; Roncaglia, F.; Tassi, L. Waste by-product of grape seed oil production: Chemical characterization for use as a food and feed supplement. Life 2023, 13, 326. [Google Scholar] [CrossRef] [PubMed]
- Sikorska, E.; Caponio, F.; Bilancia, M.T.; Summo, C.; Pasqualone, A.; Khmelinskii, I.; Sikorski, M. Changes in colour of extra-virgin olive oil during storage. Pol. J. Food Nutr. Sci. 2007, 57, 495–498. [Google Scholar]
- Ceballosa, C.D.; Moyanob, M.J.; Vicarioc, I.M.; Albab, J.; Herediac, F.J. Chromatic evolution of virgin olive oils submitted to an accelerated oxidation. J. Am. Oil Chem. Soc. 2003, 80, 257–262. [Google Scholar] [CrossRef]
- Arab, R.; Casal, S.; Pinho, T.; Cruz, R.; Freidja, M.L.; Lorenzo, J.M.; Hano, C.; Madani, K.; Boulekbache-Makhlouf, L. Effects of Seed Roasting Temperature on Sesame Oil Fatty Acid Composition, Lignan, Sterol and Tocopherol Contents, Oxidative Stability and Antioxidant Potential for Food Applications. Molecules 2022, 27, 4508. [Google Scholar] [CrossRef] [PubMed]
- Masan, V.; Burg, P.; Horak, M. Evaluation of grape seed oils using colour system method (CIELAB). Agric. Food Sci. 2016, 23, 883–886. [Google Scholar]
- Moyano, M.J.; Meléndez-Martínez, A.J.; Alba, J.; Heredia, F.J. A comprehensive study on the colour of virgin olive oils and its relationship with their chlorophylls and carotenoids indexes (II): CIELUV and CIELAB uniform colour spaces. Food Res. Int. 2008, 41, 513–521. [Google Scholar] [CrossRef]
- Ramos-Escudero, F.; González-Miret, M.L.; Viñas-Ospino, A.; Escudero, M.R. Quality, stability, carotenoids and chromatic parameters of commercial Sacha inchi oil originating from Peruvian cultivars. J. Food Sci. Technol. 2019, 56, 4901–4910. [Google Scholar] [CrossRef]
- Bail, S.; Stuebiger, G.; Krist, S.; Unterweger, H.; Buchbauer, G. Characterisation of various grape seed oils by volatile compounds, triacylglycerol composition, total phenols and antioxidant capacity. Food Chem. 2008, 108, 1122–1132. [Google Scholar] [CrossRef] [PubMed]
- Grebenteuch, S.; Kroh, L.W.; Drusch, S.; Rohn, S. Formation of secondary and tertiary volatile compounds resulting from the lipid oxidation of rapeseed oil. Foods 2021, 10, 2417. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ruan, S.; Hu, J.; Sun, Y.; Fei, Y.; Jiang, X.; Dong, S.; Chen, T.; Wu, N. The intrinsic relationship between color variation and performances of the deteriorated aviation lubrication oil. J. Ind. Eng. Chem. 2020, 92, 88–95. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988; 590p. [Google Scholar]
- Yuan, L. Ignition of hydraulic fluid sprays by open flames and hot surfaces. J. Loss Prev. Process Ind. 2006, 19, 353–361. [Google Scholar] [CrossRef]
- Sepeidnameh, M.; Fazlara, A.; Hosseini, S.M.H.; Pourmahdi Borujeni, M. Encapsulation of grape seed oil in oil-in-water emulsion using multilayer technology: Investigation of physical stability, physicochemical and oxidative properties of emulsions under the influence of the number of layers. Curr. Res. Food Sci. 2024, 16, 100771. [Google Scholar] [CrossRef]
- Qushawy, M.; Mortagi, Y.; Alshaman, R.; Mokhtar, H.I.; Hisham, F.A.; Alattar, A.; Liang, D.; Enan, E.T.; Eltrawy, A.H.; Alamrani, Z.H.; et al. Formulation and Characterization of O/W Nanoemulsions of Hemp Seed Oil for Protection from Steatohepatitis: Analysis of Hepatic Free Fatty Acids and Oxidation Markers. Pharmaceuticals 2022, 15, 864. [Google Scholar] [CrossRef]
- Li, H.; Ding, J.; Liu, C.; Huang, P.; Yang, Y.; Jin, Z.; Qin, W. Carvacrol Treatment Reduces Decay and Maintains the Postharvest Quality of Red Grape Fruits (Vitis vinifera L.) Inoculated with Alternaria alternata. Foods 2023, 12, 4305. [Google Scholar] [CrossRef]
- Sungho, J.; Junsoo, L.; Choi, W. Oxidative stability of grape seed oil by addition of grape seed extract. J. Korean Soc. Food Sci. Nutr. 2015, 44, 1813–1818. [Google Scholar]
- Garavaglia, J.; Markoski, M.M.; Oliveira, A.; Marcadent, A. Grape seed oil compounds: Biological and chemical actions for health. Nutr. Metab. Insights 2016, 9, 59–64. [Google Scholar] [CrossRef]
- Orsavova, J.; Misurcova, L.; Ambrozova, J.V.; Vicha, R.; Mlcek, J. Fatty acids composition of vegetable oils and its contribution to dietary energy intake and dependence of cardiovascular mortality on dietary intake of fatty acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef]
- Mohamed, H.B.; Duba, K.S.; Fiori, L.; Abdelgawed, H.; Tlili, I.; Tounekti, T.; Zrig, A. Bioactive compounds and antioxidant activities of different grape (Vitis vinifera L.) seed oils extracted by supercritical CO2 and organic solvent. LWT 2016, 74, 557–562. [Google Scholar] [CrossRef]
- Di Pietro Fernandes, C.; Santana, L.F.; dos Santos, J.R.; Fernandes, D.S.; Hiane, P.A.; Pott, A.; Freitas, K.C.; Bogo, D.; do Nascimento, V.A.; Filiú, W.F.O.; et al. Nutraceutical Potential of Grape (Vitis vinifera L.) Seed Oil in Oxidative Stress, Inflammation, Obesity and Metabolic Alterations. Molecules 2023, 28, 7811. [Google Scholar] [CrossRef] [PubMed]


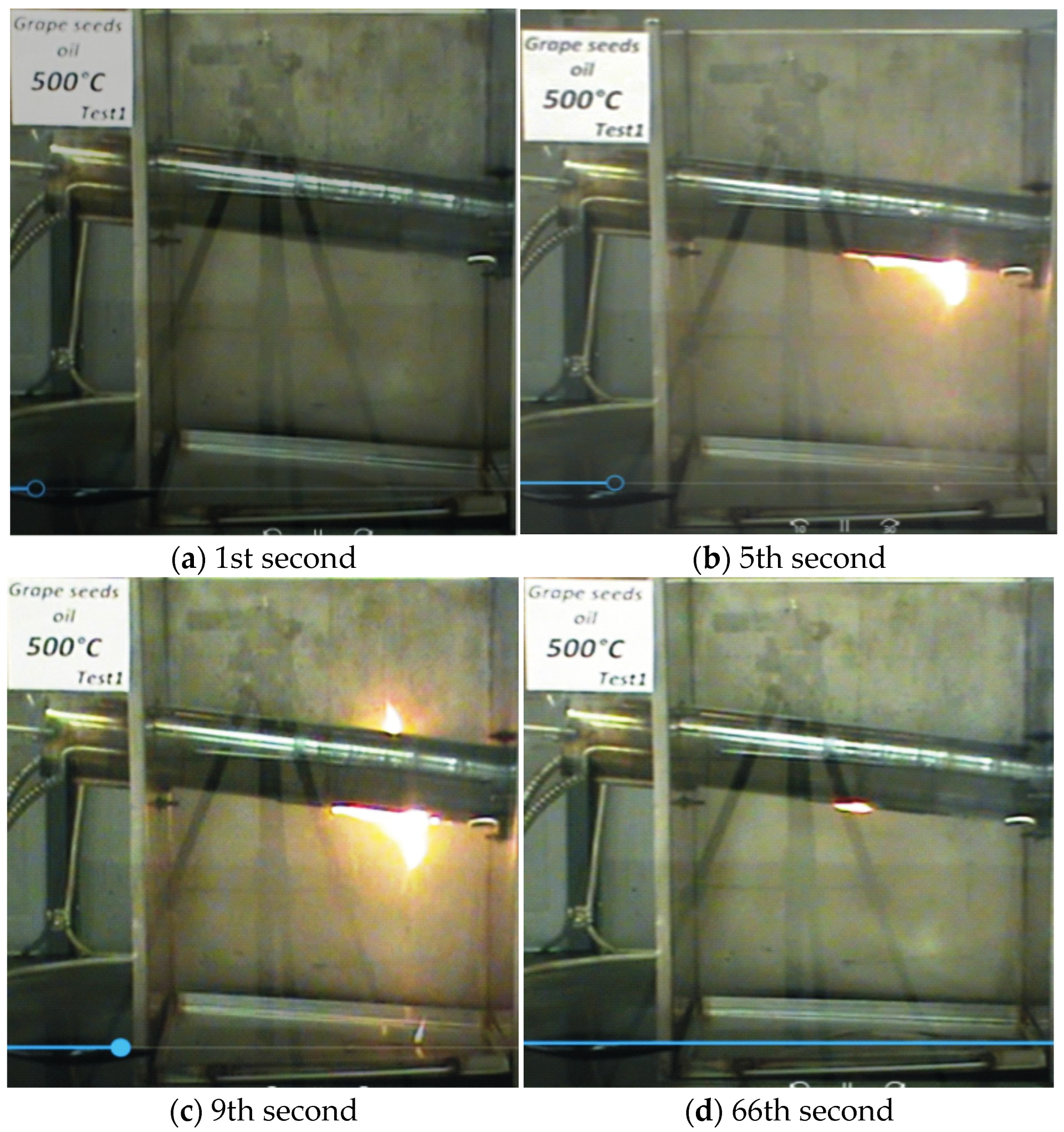
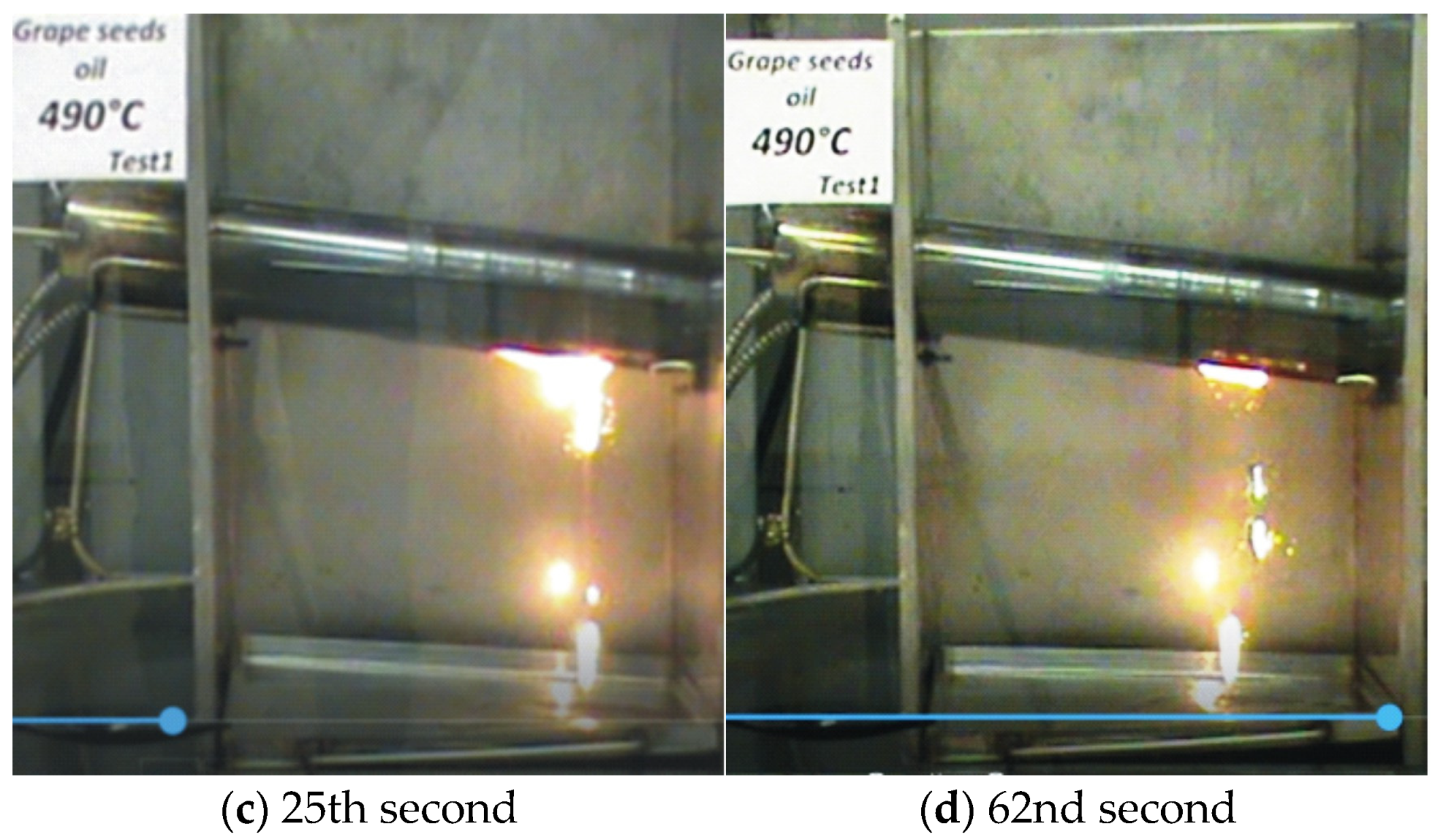
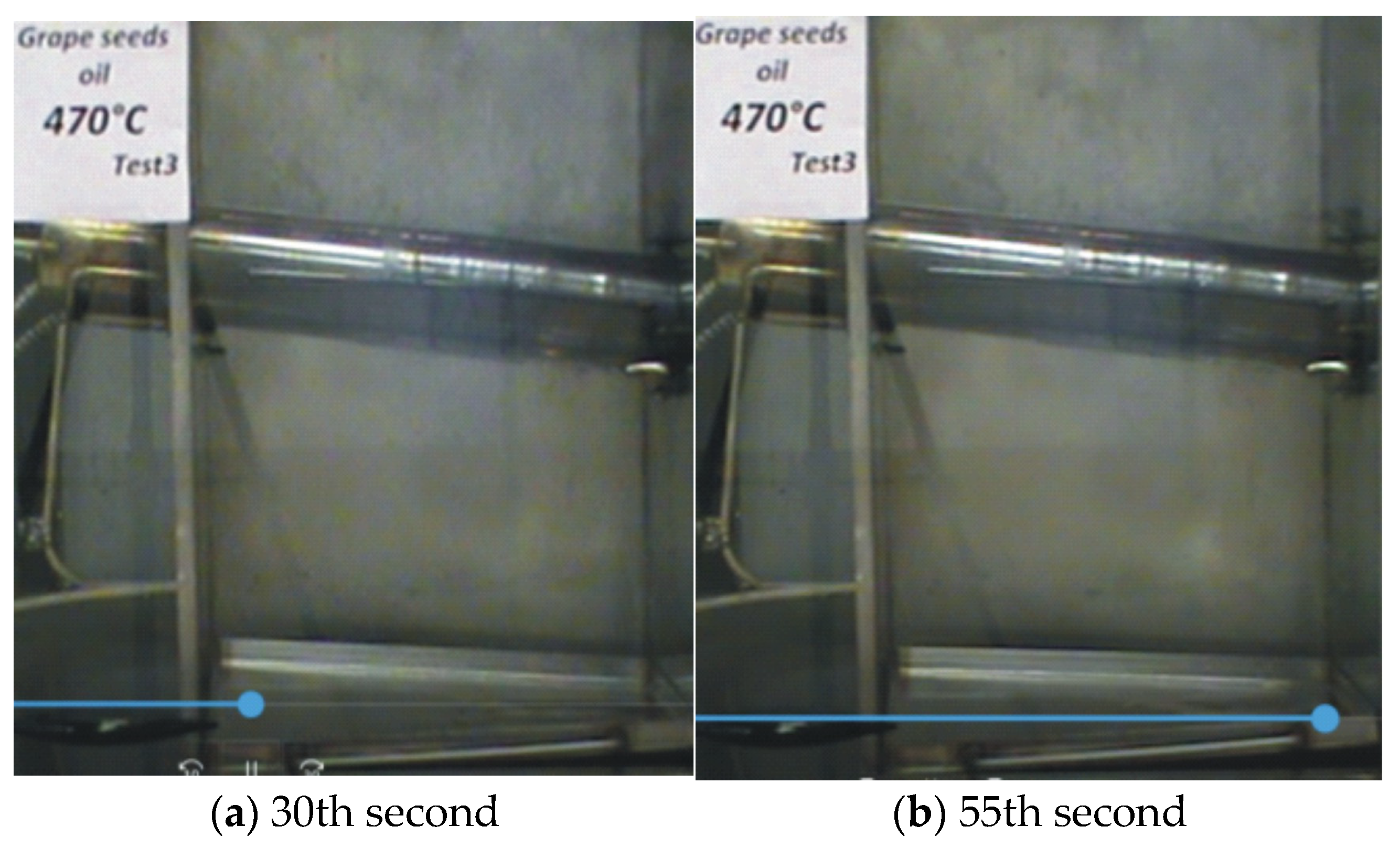
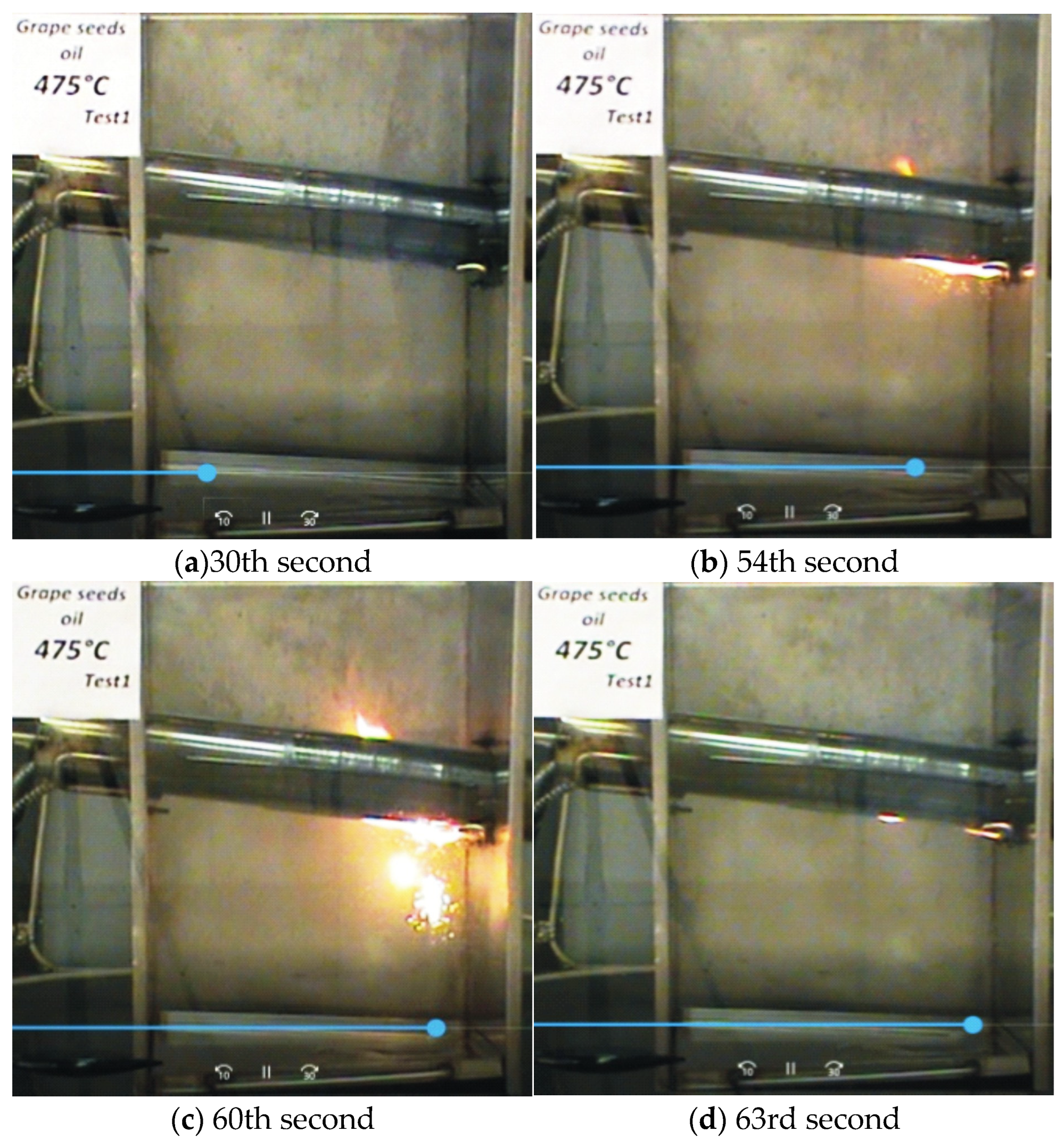
 —it ignites;
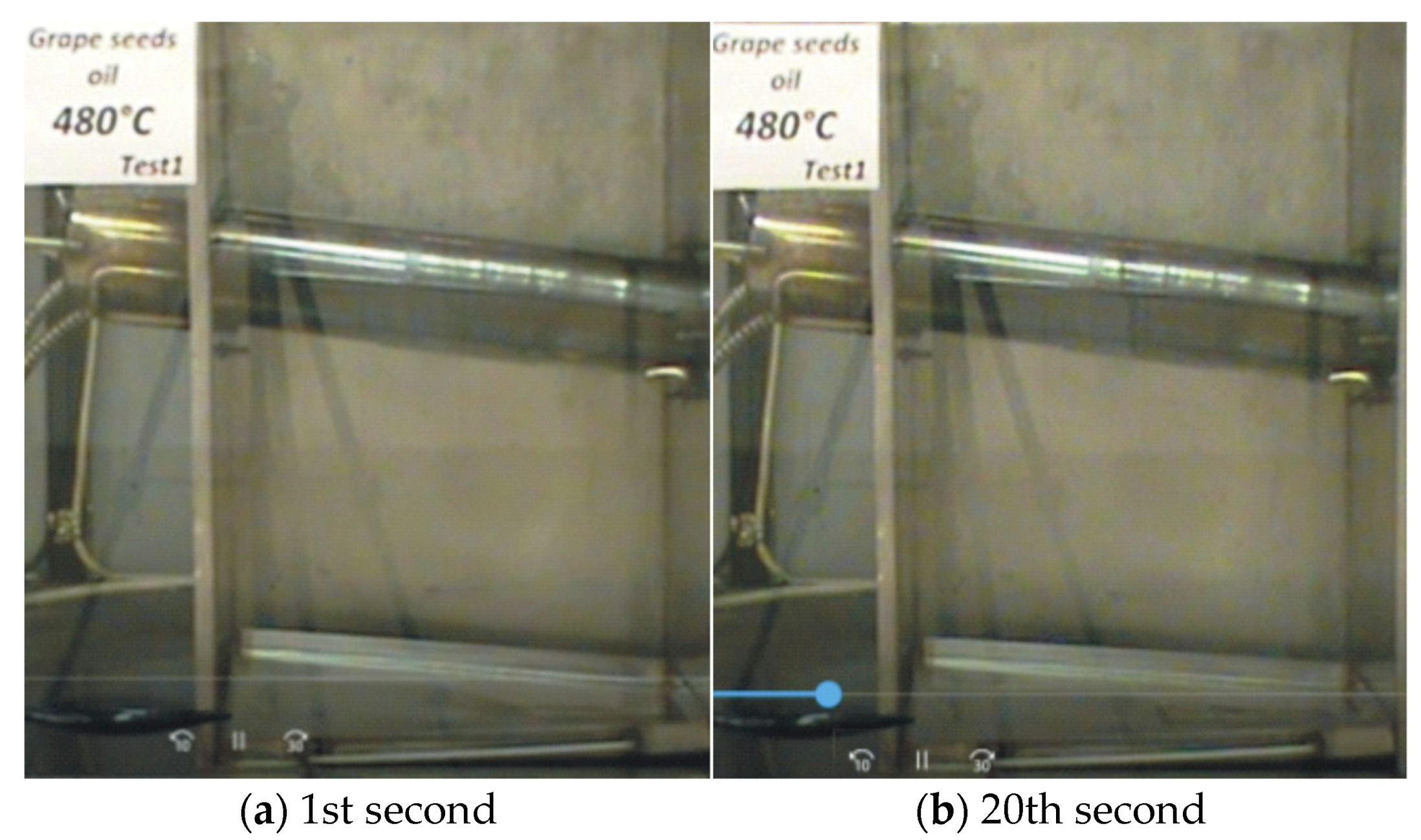
—it ignites;  —it does not ignite).
—it does not ignite).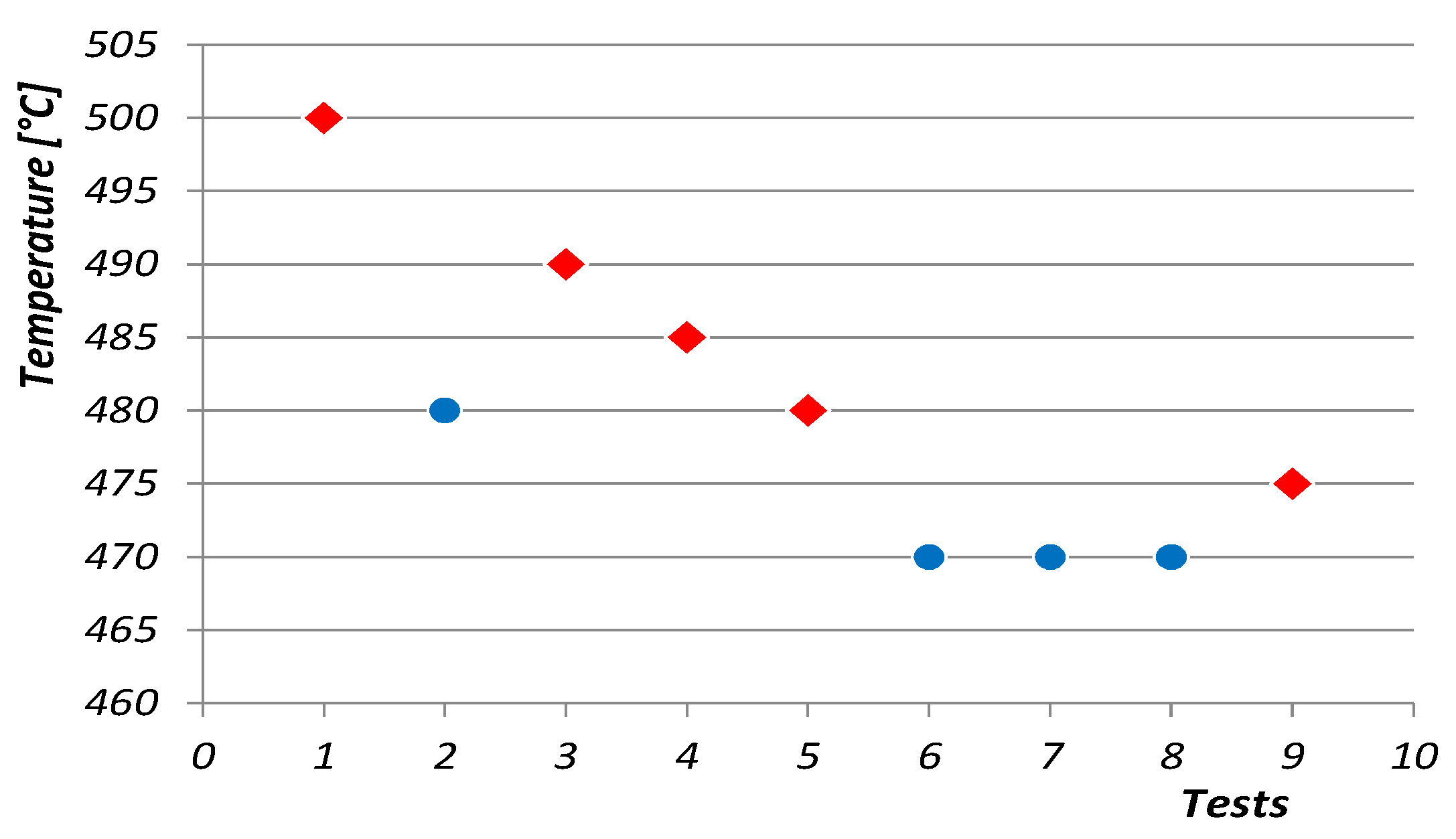


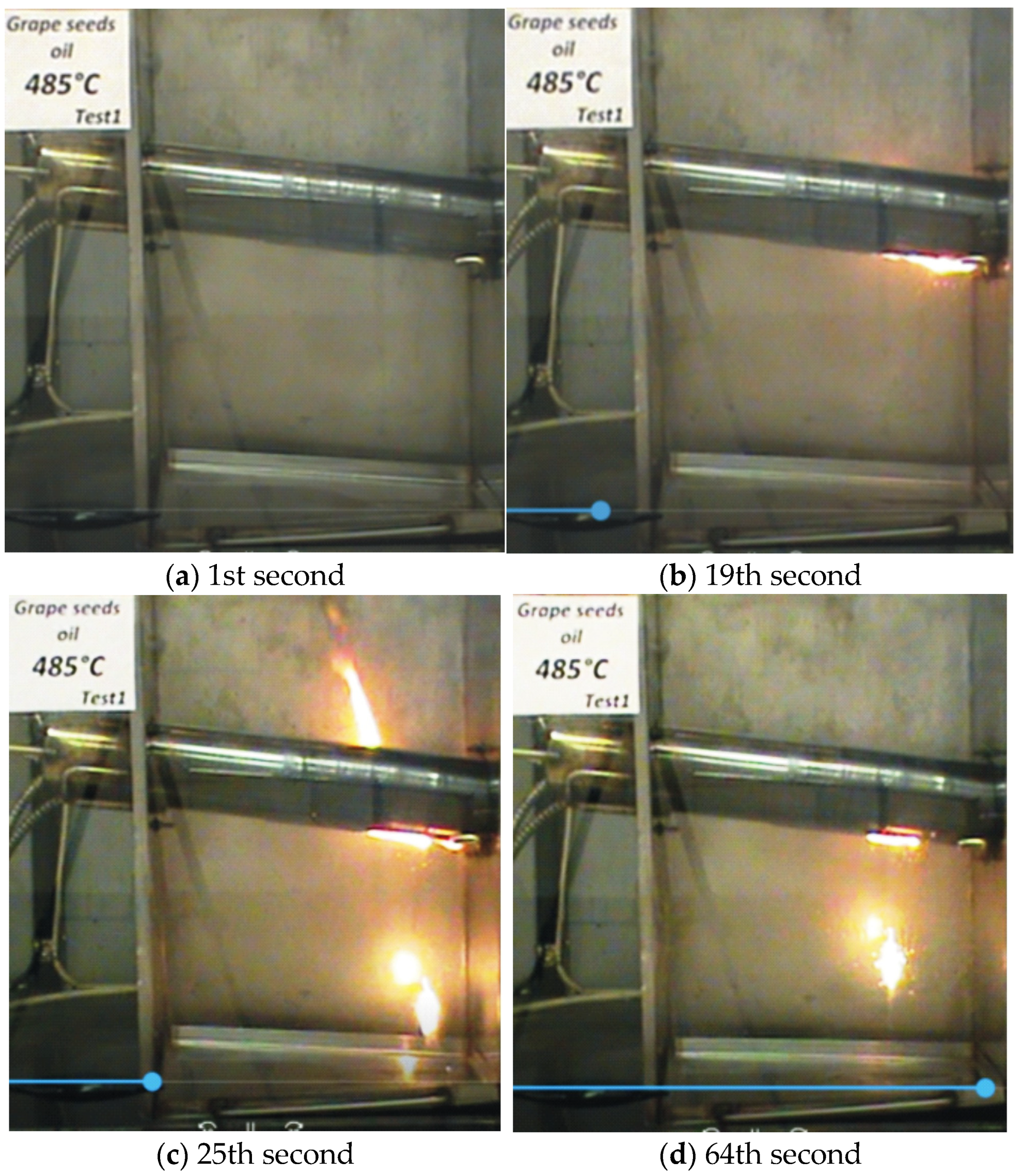
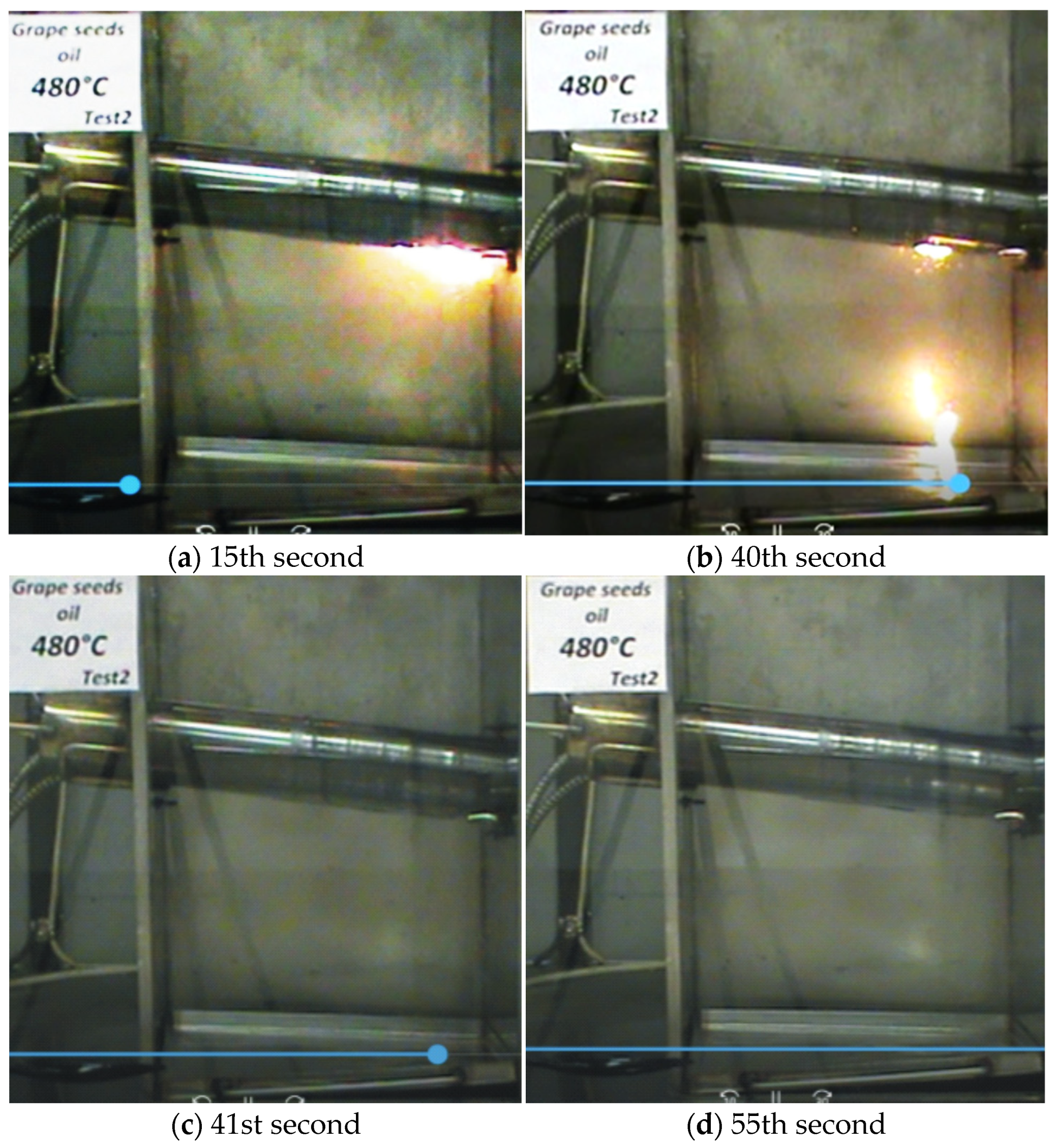
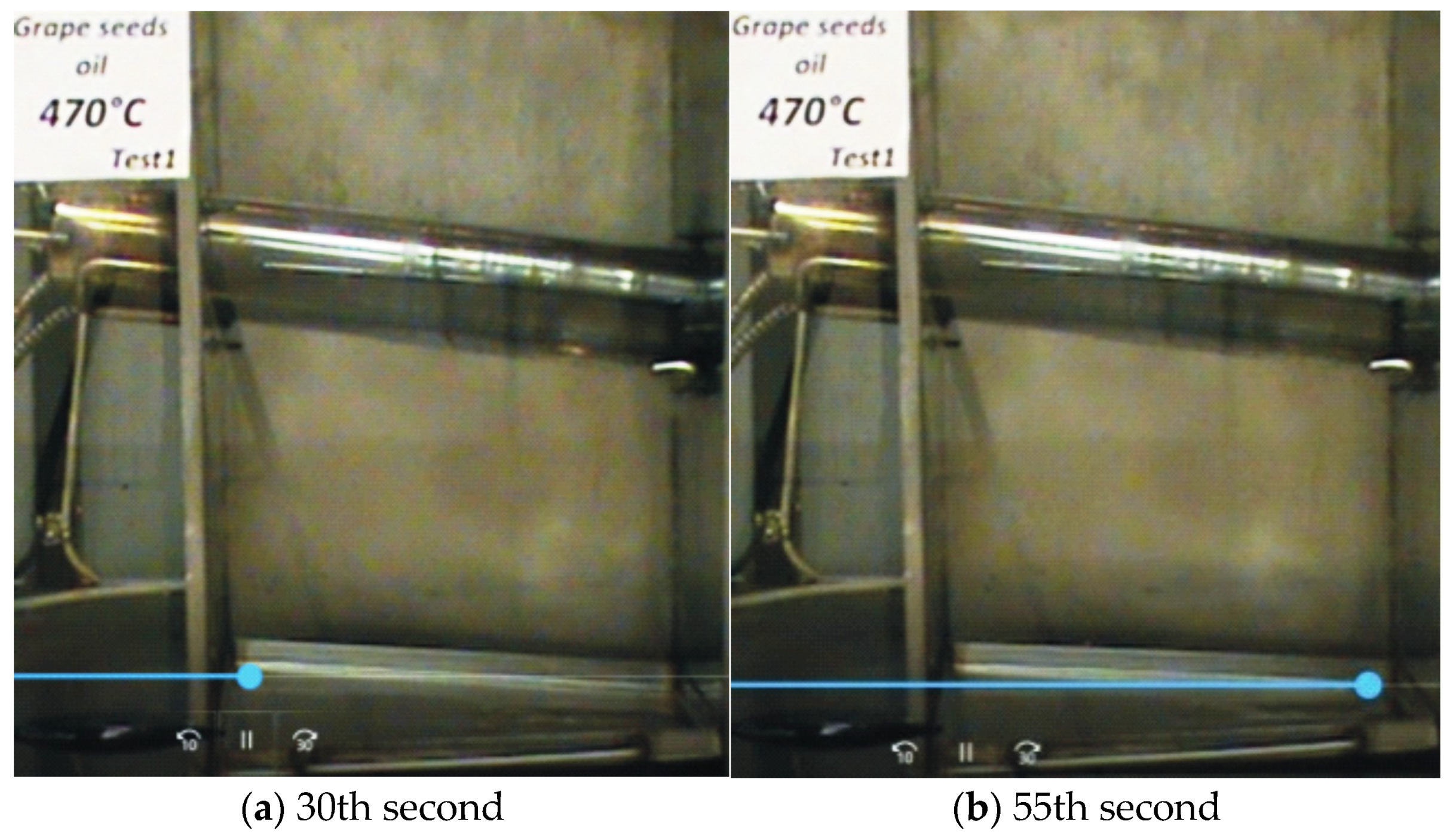
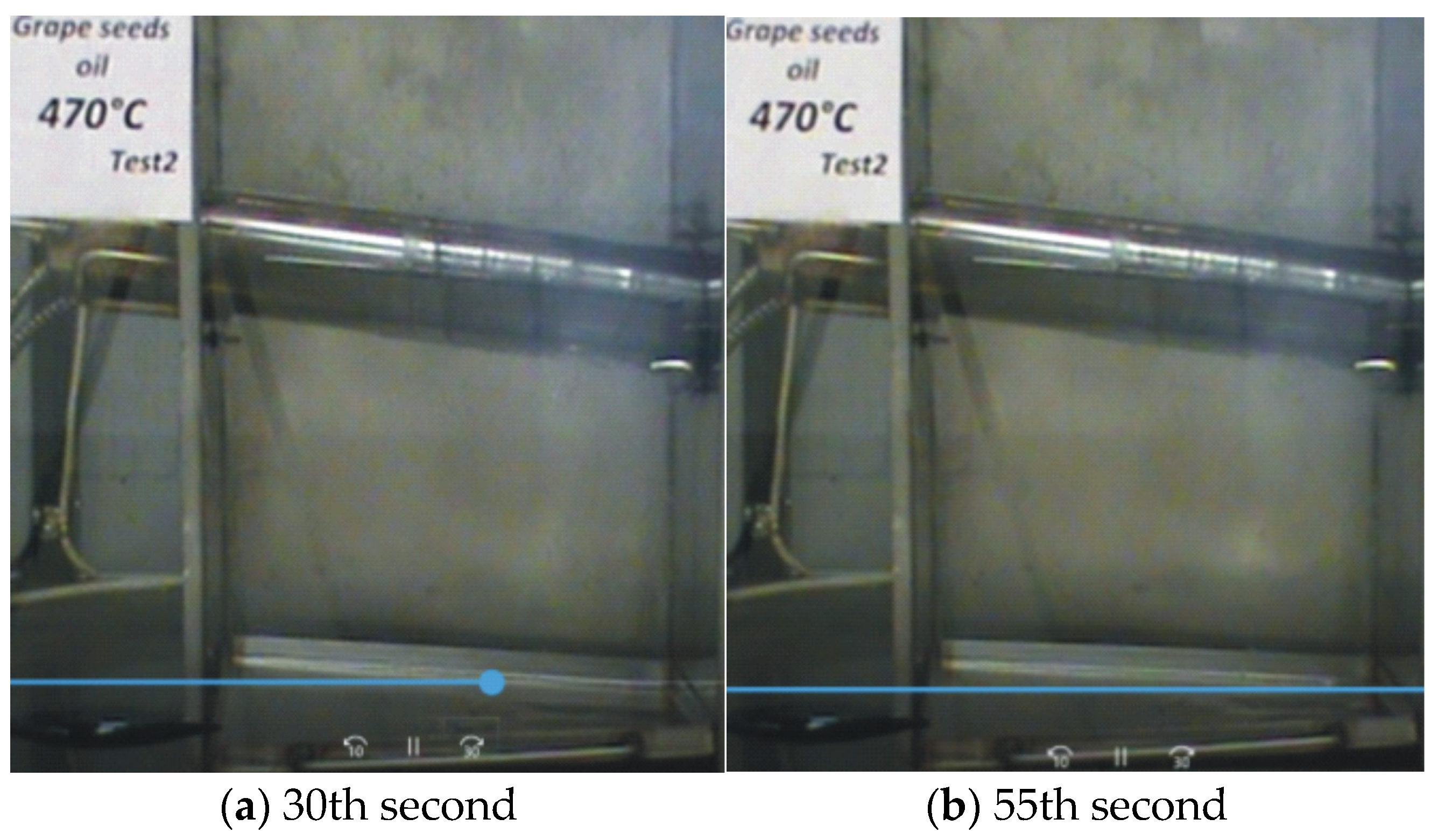

| Grape Seed Oils 100 °C | Non-Oxidized | 4 h Oxidized | 8 h Oxidized | 10 h Oxidized | |
|---|---|---|---|---|---|
| Trichromatic components * | X | 76.986 ± 0.684 | 77.011 ± 0.685 | 70.581 ± 0.627 | 79.510 ± 0.707 |
| Y | 79.735 ± 0.708 | 79.775 ± 0.709 | 72.490 ± 0.644 | 82.344 ± 0.732 | |
| Z | 68.268 ± 0.607 | 66.057 ± 0.587 | 42.744 ± 0.380 | 72.817 ± 0.647 | |
| Trichromatic coordinates | x | 0.342 ± 0.003 | 0.346 ± 0.004 | 0.380 ± 0.004 | 0.339 ± 0.003 |
| y | 0.354 ± 0.003 | 0.358 ± 0.004 | 0.390 ± 0.004 | 0.351 ± 0.003 | |
| z | 0.303 ± 0.003 | 0.296 ± 0.003 | 0.230 ± 0.002 | 0.310 ± 0.003 | |
| Grape Seed Oils 100 °C | Non-Oxidized | 4 h Oxidized | 8 h Oxidized | 10 h Oxidized | |
|---|---|---|---|---|---|
| Chromatic coordinates | L* | 91.57 ± 0.814 | 91.58 ± 0.814 | 88.20 ± 0.784 | 92.73 ± 0.824 |
| a* | 2.82 ± 0.025 | 2.79 ± 0.025 | 4.00 ± 0.036 | 2.86 ± 0.025 | |
| b* | 13.44 ± 0.119 | 15.35 ± 0.136 | 32.50 ± 0.289 | 11.71 ± 0.104 | |
| a*/b* | 0.21 | 0.18 | 0.12 | 0.24 | |
| (a*/b*)2 | 0.04 | 0.03 | 0.02 | 0.06 | |
| 13.74 ± 0.122 | 15.60 ± 0.139 | 32.75 ± 0.291 | 12.05 ± 0.107 | ||
| hab | 78.15 ± 0.001 | 79.70 ± 0.002 | 82.98 ± 0.003 | 76.26 ± 0.001 | |
| Grape Seed Oils 100 °C | ∆L* | ∆a* | ∆b* | ∆hab | |
|---|---|---|---|---|---|
| 4 h oxidized | 0.02 | −0.03 | 1.91 | 1.87 | 1.54 |
| 8 h oxidized | −3.36 | 1.18 | 19.06 | 19.01 | 4.84 |
| 10 h oxidized | 1.16 | 0.04 | −1.74 | −1.69 | −1.88 |
| Grape Seed Oils 120 °C | Non-Oxidized | 4 h Oxidized | 8 h Oxidized | 10 h Oxidized | |
|---|---|---|---|---|---|
| Trichromatic components | X | 76.986 ± 0.684 | 81.526 ± 0.725 | 83.661 ± 0.744 | 82.597 ± 0.734 |
| Y | 79.735 ± 0.709 | 84.163 ± 0.748 | 86.153 ± 0.766 | 85.126 ± 0.757 | |
| Z | 68.268 ± 0.607 | 81.483 ± 0.724 | 87.065 ± 0.774 | 84.205 ± 0.748 | |
| Trichromatic coordinates | x | 0.342 ± 0.003 | 0.330 ± 0.003 | 0.326 ± 0.003 | 0.328 ± 0.003 |
| y | 0.354 ± 0.003 | 0.341 ± 0.003 | 0.335 ± 0.003 | 0.338 ± 0.003 | |
| z | 0.303 ± 0.003 | 0.330 ± 0.003 | 0.339 ± 0.003 | 0.334 ± 0.003 | |
| Grape Seed Oils 120 °C | Non-Oxidized | 4 h Oxidized | 8 H Oxidized | 10 h Oxidized | |
|---|---|---|---|---|---|
| Chromatic coordinates | L* | 91.57 ± 0.557 | 93.52 ± 0.569 | 94.38 ± 0.574 | 93.94 ± 0.571 |
| a* | 2.82 ± 0.017 | 3.39 ± 0.021 | 3.81 ± 0.023 | 3.67 ± 0.022 | |
| b* | 13.44 ± 0.082 | 6.36 ± 0.039 | 3.77 ± 0.023 | 5.07 ± 0.031 | |
| a*/b* | 0.21 | 0.53 | 1.01 | 0.72 | |
| (a*/b*)2 | 0.04 | 0.28 | 1.02 | 0.52 | |
| 13.74 ± 0.084 | 7.21 ± 0.044 | 5.36 ± 0.033 | 6.26 ± 0.038 | ||
| hab | 78.15 ± 0.475 | 61.98 ± 0.377 | 44.66 ± 0.272 | 54.14 ± 0.329 | |
| Grape Seed Oils 120 °C | ∆L* | ∆a* | ∆b* | ∆hab | |
|---|---|---|---|---|---|
| 4 h oxidized | 1.96 | 0.57 | −7.08 | −6.53 | −16.18 |
| 8 h oxidized | 2.81 | 0.99 | −9.68 | −8.38 | −33.49 |
| 10 h oxidized | 2.37 | 0.85 | −8.37 | −7.48 | −24.02 |
| L* | a* | b* | hab | t | ||
|---|---|---|---|---|---|---|
| a Grape seed oils, 100 °C | ||||||
| L* | 1.000 | |||||
| a* | −0.947 | 1.000 | ||||
| b* | −0.985 | 0.979 | 1.000 | |||
| −0.985 | 0.980 | 0.998 | 1.000 | |||
| hab | −0.985 | 0.981 | 0.999 | 0.998 | 1.000 | |
| t | −0.124 | 0.405 | 0.627 | 0.629 | 0.291 | 1.000 |
| b Grape seed oils, 120 °C | ||||||
| L* | 1.000 | |||||
| a* | 0.990 | 1.000 | ||||
| b* | −0.999 | −0.985 | 1.000 | |||
| −0.996 | −0.977 | 0.999 | 1.000 | |||
| hab | −0.972 | −0.988 | 0.962 | 0.948 | 1.000 | |
| t | 0.896 | 0.934 | −0.892 | −0.885 | −0.888 | 1.000 |
| Grape Seed Oils | Untested Oil | 470 °C (Test 1) (It Does Not Ignite) | 475 °C (Test 1) (It Ignites) | |
|---|---|---|---|---|
| Trichromatic components | X | 79.002 ± 0.702 | 72.930 ± 0.648 | 76.997 ± 0.684 |
| Y | 81.857 ± 0.728 | 75.355 ± 0.670 | 79.661 ± 0.708 | |
| Z | 71.418 ± 0.635 | 64.971 ± 0.577 | 69.259 ± 0.616 | |
| Trichromatic coordinates | x | 0.340 ± 0.003 | 0.342 ± 0.003 | 0.341 ± 0.003 |
| y | 0.352 ± 0.003 | 0.353 ± 0.003 | 0.353 ± 0.003 | |
| z | 0.307 ± 0.003 | 0.305 ± 0.003 | 0.307 ± 0.003 | |
| Grape Seed Oils | Untested Oil | 470 °C (Test 1) (It Does Not Ignite) | 475 °C (Test 1) (It Ignites) | |
|---|---|---|---|---|
| Chromatic coordinates | L* | 92.51 ± 0.557 | 89.56 ± 0.545 | 91.53 ± 0.563 |
| a* | 2.78 ± 0.017 | 3.13 ± 0.019 | 2.99 ± 0.018 | |
| b* | 12.47 ± 0.076 | 12.80 ± 0.078 | 12.56 ± 0.076 | |
| a*/b* | 0.22 | 0.24 | 0.24 | |
| (a*/b*)2 | 0.05 | 0.06 | 0.057 | |
| 12.78 ± 0.078 | 13.18 ± 0.080 | 12.91 ± 0.079 | ||
| hab | 77.43 ± 0.471 | 76.26 ± 0.464 | 76.63 ± 0.466 | |
| Grape Seed Oils | ∆L* | ∆a* | ∆b* | ∆hab | ||
|---|---|---|---|---|---|---|
| 470 °C (Test 1) (it does not ignite) | −2.95 | 0.35 | 0.33 | 0.40 | −1.17 | 2.9 |
| 475 °C (Test 1) (it ignites) | −0.98 | 0.21 | 0.09 | 0.13 | −0.80 | 1.01 |
| L* | a* | b* | hab | FT | ||
|---|---|---|---|---|---|---|
| L* | 1.000 | |||||
| a* | −0.998 | 1.000 | ||||
| b* | −0.992 | 0.991 | 1.000 | |||
| C*ab | −0.993 | 0.992 | 0.998 | 1.000 | ||
| hab | 0.207 | −0.213 | −0.083 | −0.089 | 1.000 | |
| FT | −0.749 | 0.914 | 0.704 | 0.743 | −0.948 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Șolea, L.C.; Crețu, R. Oxidation and Flammability Tests for Grape (Vitis vinifera L.) Seed Oil. Lubricants 2024, 12, 263. https://doi.org/10.3390/lubricants12080263
Șolea LC, Crețu R. Oxidation and Flammability Tests for Grape (Vitis vinifera L.) Seed Oil. Lubricants. 2024; 12(8):263. https://doi.org/10.3390/lubricants12080263
Chicago/Turabian StyleȘolea, Liviu Cătălin, and Romică Crețu. 2024. "Oxidation and Flammability Tests for Grape (Vitis vinifera L.) Seed Oil" Lubricants 12, no. 8: 263. https://doi.org/10.3390/lubricants12080263
APA StyleȘolea, L. C., & Crețu, R. (2024). Oxidation and Flammability Tests for Grape (Vitis vinifera L.) Seed Oil. Lubricants, 12(8), 263. https://doi.org/10.3390/lubricants12080263




