Revealing the Molecular Interaction between CTL Base Oil and Additives and Its Application in the Development of Gasoline Engine Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Base Oil and Viscosity Index Improver Blends
2.3. The Characterization of the Viscosity Index Improver Blends
2.3.1. The Solubility Test
2.3.2. The Physico-Chemical Property Measurements
2.3.3. The Synchrotron Radiation Micro-Infrared Test
2.4. Preparation of the Gasoline Engine Oil
2.5. The Physico-Chemical Property Measurements of the Developed Gasoline Engine Oil
2.6. The Performance Evaluation of the Developed Gasoline Engine Oil
2.6.1. The Thermal Oxidation Stability Test
2.6.2. The Detergency Test
3. Results and Discussion
3.1. The Interaction between the Viscosity Index Improver and the Base Oil
3.1.1. The Solubility Analysis
3.1.2. The Influence of VII Concentration on the Viscosity of the Blends
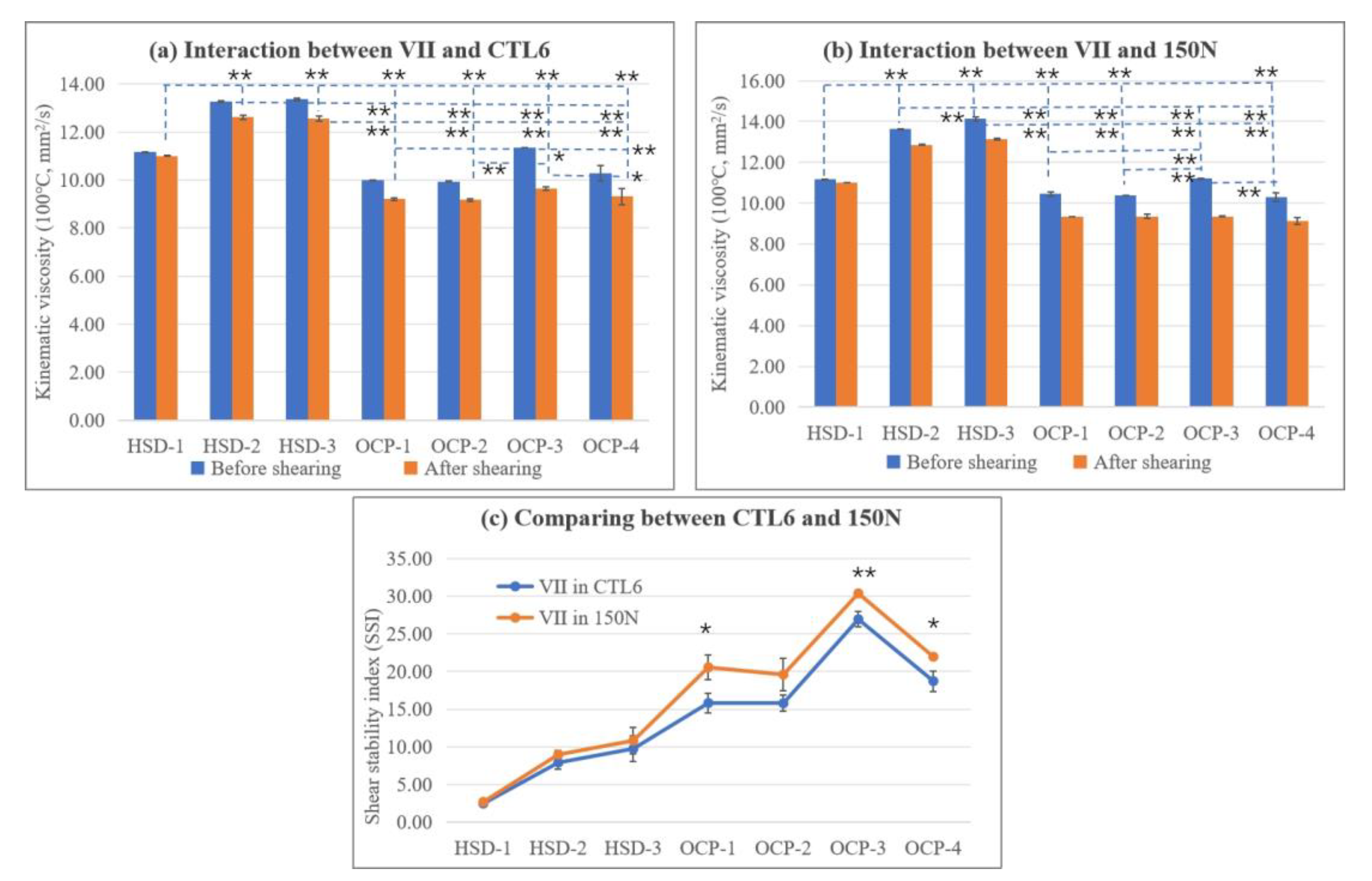
3.1.3. The Influence of Type of VII on the Shear Stability of the Blends
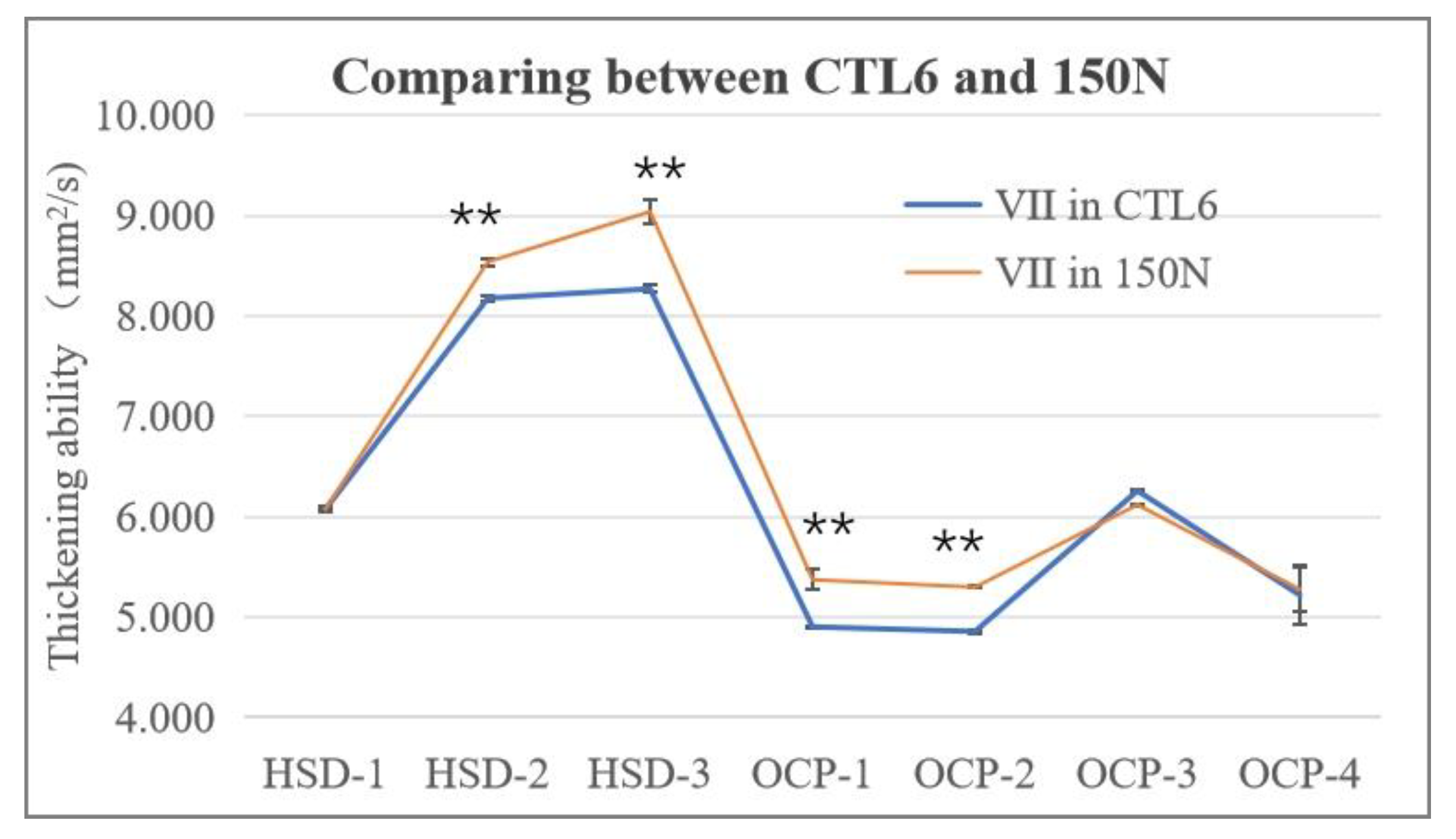
3.1.4. The Influence of Type of VII on the Thickening Ability of the Blends
3.1.5. The Influence of Type of VII on the Low-Temperature Performance of the Blends
3.2. The Characterization of the Molecular Interaction between the Viscosity Index Improver and the Base Oil
3.3. The Influence of the Concentration of the Viscosity Index Improver on the Physico-Chemical Properties of the Gasoline Engine Oil
3.4. The Performance of the Developed Gasoline Engine Oil
3.4.1. The Thermal Oxidation Stability
3.4.2. The Detergency Performance
4. Conclusions
- The interaction between three kinds of HSD-type VIIs, four kinds of OCP-type VIIs, and CTL and mineral base oils were systematically evaluated. It was found that firstly, all samples showed good solubility after storing at different temperatures. Secondly, no matter which VII and base oils were used, the kinematic viscosity and the viscosity index all increased with the increase in VII concentration. Also, the HSD-type VII illustrated a higher VI, smaller SSI, better thickening ability, and lower CCS viscosity than that of the OCP-type VII, and the HSD-type VII and CTL base oil combination exhibited the best shearing stability and low-temperature performance, which is promising for the development of gasoline engine oil.
- The SR Micro-IR analysis revealed that HSD-1 had a better molecular interaction with CTL6 than 150N because of better uniformity of the C=C group distribution.
- By adjusting the base oil ratio and VII concentration, a gasoline engine oil F#1 was developed, with comparable viscosity characteristics and detergency performance to that of the reference oil SP 0W-20, and with better low-temperature fluidity and cold start property, lower oil consumption characteristic and better thermal stability than that of the reference oil SP 0W-20.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tóth, Á.D.; Szabó, Á.I.; Leskó, M.Z.; Rohde-Brandenburger, J.; Kuti, R. Tribological properties of the nanoscale spherical Y2O3 particles as lubricant additives in automotive application. Lubricants 2022, 10, 28. [Google Scholar] [CrossRef]
- Arumugam, S.; Ellappan, R.; Sriram, G. Degradation of engine components upon exposure to chemically modified vegetable oil-based automotive lubricant. J. Indian Chem. Soc. 2021, 98, 100227. [Google Scholar] [CrossRef]
- GF-6B:API 1509; Engine Oil Licensing and Certification System. API Publishing Services: Washington, DC, USA, 2019.
- Srivastava, G. Framework for brand positioning of automotive lubricants by using structural equation modelling. Int. J. Manag. Pract. 2023, 16, 89–103. [Google Scholar] [CrossRef]
- Tóth1, Á.D.; Knaup, J. Investigation of the tribological properties of nano-scaled ZrO2 and CuO additive in automotive lubricants. IOP Conf. Ser. Mater. Sci. Eng. 2020, 903, 012015. [Google Scholar] [CrossRef]
- Kang, G.; Zhang, F.; Liu, H.; Wei, B.; Jin, Z.; Lv, H. Rapid separation and API grades identification of base oil in low viscosity gasoline engine oil SN 0W-16. ACS Omega 2024, 9, 21270–21275. [Google Scholar] [CrossRef]
- Kallas, M.M.; Al Sabek, M.S.; Saoud, Y. Experimental comparison of the effect of using synthetic, semi-synthetic, and mineral engine oil on gasoline engine parts wear. Adv. Tribol. 2024, 2024, 5997292. [Google Scholar] [CrossRef]
- Tong, R.; Zhang, B.; Yang, X.; Wang, Y.; Zhang, L. A life cycle analysis comparing coal liquefaction techniques: A health-based assessment in China. Sustain. Energy Technol. Assess. 2021, 44, 101000. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Yang, X. Comprehensive competitiveness assessment of four coal-to-liquid routes and conventional oil refining route in China. Energy 2021, 235, 121442. [Google Scholar] [CrossRef]
- Song, M.; Wang, Z.; Wang, W. An empirical study on technological innovation and corporate competitiveness of listed coal-to-liquids companies in China. Front. Environ. Sci. 2022, 10, 1043094. [Google Scholar] [CrossRef]
- Kong, Z.; Dong, X.; Jiang, Q. Forecasting the development of China’s coal-to-liquid industry under security, economic and environmental constraints. Energy Econ. 2019, 80, 253–266. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, C.; Wang, H.; Wang, W.; Jiang, C.; Peng, C.; Yang, K. Oxidation degradation analysis of antioxidant added to CTL base oils: Experiments and simulations. J. Therm. Anal. Calorim. 2023, 148, 7033–7046. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, C.; Cai, P.; Jing, Z.; Wen, J.; Li, Y.; Wang, H.; An, L.; Zhang, J. The potential of coal-to-liquid as an alternative fuel for diesel engines: A review. J. Energy Inst. 2023, 109, 101306. [Google Scholar] [CrossRef]
- Sun, W.; Sun, Y.; Guo, L.; Zhang, H.; Yan, Y.; Zeng, W.; Lin, S. Comparative assessment of n-butanol addition in CTL on performance and exhaust emissions of a CI engine. Fuel 2021, 303, 121223. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, H.; Yu, X.; Peng, C.; Zhang, A.; Liang, X.; Yan, Y. Correlation between the molecular structure and viscosity index of CTL base oils based on ridge regression. ACS Omega 2022, 7, 18887–18896. [Google Scholar] [CrossRef]
- Markandan, K.; Nagarajan, T.; Walvekar, R.; Chaudhary, V.; Khalid, M. Enhanced tribological behaviour of hybrid MoS2@Ti3C2 MXene as an effective anti-Friction additive in gasoline engine oil. Lubricants 2023, 11, 47. [Google Scholar] [CrossRef]
- Rahimi, B.; Semnani, A.; Nezamzadeh-Ejhieh, A.; Langeroodi, H.S.; Davood, M.H. Monitoring of the physical and chemical properties of a gasoline engine oil during its usage. J. Anal. Methods Chem. 2012, 2012, 819524. [Google Scholar] [CrossRef]
- Kaleli, E.H.; Demirtas, S. Experimental investigation of the effect of tribological performance of reduced graphene oxide additive added into engine oil on gasoline engine wear. Lubr. Sci. 2023, 35, 118–143. [Google Scholar] [CrossRef]
- Coutinho, F.M.B.; Teixeira, S.C.S. Polymers used as viscosity index improvers A comparative study. Polym. Test. 1993, 12, 415–422. [Google Scholar] [CrossRef]
- Boussaid, M.; Haddadine, N.; Benmounah, A.; Dahal, J.; Bouslah, N.; Benaboura, A.; El-Shall, S. Viscosity-boosting effects of polymer additives in automotive lubricants. Polym. Bull. 2023, 81, 6995–7011. [Google Scholar] [CrossRef]
- Mohamad, S.A.; Ahmed, N.S.; Hassanein, S.M.; Rashad, A.M. Investigation of polyacrylates copolymers as lube oil viscosity index improvers. J. Pet. Sci. Eng. 2012, 100, 173–177. [Google Scholar] [CrossRef]
- Ghosh, P.; Das, M. Study of the influence of some polymeric additives as viscosity index improvers and pour point depressants-synthesis and characterization. J. Pet. Sci. Eng. 2014, 119, 79–84. [Google Scholar] [CrossRef]
- Lomège, J.; Mohring, V.; Lapinte, V.; Negrel, C.; Robin, J.J.; Caillol, S. Synthesis of plant oil-based amide copolymethacrylates and their use as viscosity index improvers. Eur. Polym. J. 2018, 109, 435–446. [Google Scholar] [CrossRef]
- Rathika, S.; Raghavan, P.S. Influence of polyvinyl palmitate copolymer as viscosity index improvers For lube. Mater. Today Proc. 2019, 16, 978–986. [Google Scholar] [CrossRef]
- Esfea, M.H.; Aranib, A.A.A.; Esfandeh, S. Improving engine oil lubrication in light-duty vehicles by using of dispersing MWCNT and ZnO nanoparticles in 5W50 as viscosity index improvers (VII). Appl. Therm. Eng. 2018, 143, 493–506. [Google Scholar] [CrossRef]
- Méheust, H.; Le Meins, J.-F.; Brûlet, A.; Sandre, O.; Grau, E.; Cramail, H. Fatty-acid based comb copolyesters as viscosity Index improvers in lubricants. Eur. Polym. J. 2022, 181, 111674. [Google Scholar] [CrossRef]
- Sparnacci, K.; Frison, T.; Podda, E.; Antonioli, D.; Laus, M.; Notari, M.; Assanelli, G.; Atzeni, M.; Merlini, G.; Pó, R. Core-crosslinked star copolymers as viscosity index improvers for lubricants. ACS Appl. Polym. Mater. 2022, 4, 8722–8730. [Google Scholar] [CrossRef]
- Khalafvandi, S.A.; Pazokian, M.A.; Fathollahi, E. The investigation of viscometric properties of the most reputable types of viscosity index improvers in different lubricant base oils: API groups I, II, and III. Lubricants 2022, 10, 6. [Google Scholar] [CrossRef]
- Tseregounis, S.I.; McMillan, M.L.; Olree, R.M. Engine oil effects on fuel economy in GM vehicles-separation of viscosity and friction modifier effects. In SAE Technical Paper Series-Fuel Economy and Wear Performance on Engine Oils; Society of Automotive Engineers, Inc.: Warrendale, PA, USA, 1998; p. 982502. [Google Scholar]
- Zhang, D.; Li, Z.; Wei, X.; Wang, L.; Xu, J.; Liu, Y. Study tribological properties of MoDTC and its interactions with metal detergents. J. Tribol. 2020, 142, 122201. [Google Scholar] [CrossRef]
- Pirouz, S.; Duhamel, J. Using pyrene excimer fluorescence to probe the interactions between viscosity index improvers and waxes present in automotive oil. Macromolecules 2017, 50, 2467–2476. [Google Scholar] [CrossRef]
- Gholami, K.; Frasca, F.; Duhamel, J. Probing the interactions between pour point depressants (PPDs), viscosity index improvers (VIIs), and wax in octane using fluorescently labeled PPDs Can. J. Chem. 2022, 100, 688–696. [Google Scholar] [CrossRef]
- Tan, L.; Huang, T.; Ma, J.; Li, J.; Song, Z.; Zhou, X.; Tang, Y.; Yang, L.; Zeng, X. Study on the effect of end group on the anti-corrosion behaviour of polyether derivatives. J. Mol. Liq. 2022, 347, 117991. [Google Scholar] [CrossRef]
- Guo, Y.; Li, J.; Zhou, X.; Tang, Y.; Zeng, X. Formulation of lyotropic liquid crystal emulsion based on natural sucrose ester and its tribological behaviour as novel lubricant. Friction 2022, 10, 1879–1892. [Google Scholar] [CrossRef]
- GB/T 265; Petroleum Products-Determination of Kinematic Viscosity and Calculation of Dynamic Viscosity. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 1998.
- GB/T 1995; Petroleum Products-Calculation of Viscosity Index. The State Bureau of Quality and Technical Supervision: Beijing, China, 1998.
- GB/T 3535; Petroleum Products-Determination of Pour Point. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2006.
- GB/T 3536; Petroleum Products-Determination of Flash and Fire Points-Cleveland Open Cup Method. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2008.
- GB/T 6538; Determination of Apparent Viscosity of Engine Oils-Using the Cold-Cranking Simulator. The State Bureau of Quality and Technical Supervision: Beijing, China, 2022.
- SH/T 0562; Standard Test Method for Determination of Yield Stress and Apparent Viscosity of Engine Oils at Low Temperature. National Energy Administration: Beijing, China, 2013.
- SH/T 0703; Standard Test Method for Measuring Apparent Viscosity at High-Temperature and High-Shear Rate by Multicell Capillary Viscometer. National Energy Administration: Beijing, China, 2021.
- SH/T 0059; Standard Test Method for Evaporation Loss of Lubricating Oils by the Noack Method. National Energy Administration: Beijing, China, 2011.
- GB/T 12579; Determination of Foaming Characteristics of Lubricating Oils. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2002.



| Name | Kinematic Viscosity (100 °C, mm2/s) | Kinematic Viscosity (40 °C, mm2/s) | Viscosity Index | CCS Viscosity (−30 °C, mPa·s) | Pour Point (°C) | Flash Point by Open Cup (°C) | Evaporation Loss (NOACK, 250 °C, 1 h, %) |
|---|---|---|---|---|---|---|---|
| CTL4 | 4.155 | 17.80 | 141 | 922 | −33 | 210 | 11.3 |
| CTL6 | 6.181 | 31.34 | 150 | 2497 | −36 | 247 | 3.9 |
| KL15 | 5.350 | 27.00 | 138 | 4151 | −63 | 235 | 7.5 |
| 150N | 5.258 | 29.17 | 112 | 4483 | −24 | 229 | 12.4 |
| Base Oil | Temperature (°C) | HSD-1 | HSD-2 | HSD-3 | OCP-1 | OCP-2 | OCP-3 | OCP-4 |
|---|---|---|---|---|---|---|---|---|
| CTL6 | 0 |  |  |  |  |  |  |  |
| −10 |  |  |  |  |  |  |  | |
| −20 |  |  |  |  |  |  |  | |
| rt. |  |  |  |  |  |  |  | |
| 150N | 0 |  |  |  |  |  |  |  |
| −10 |  |  |  |  |  |  |  | |
| −20 |  |  |  |  |  |  |  | |
| rt. |  |  |  |  |  |  |  |
| Items | CTL4 | CTL6 | KL15 | HSD-1 Liquid | AP | PPD | DF | In Total |
|---|---|---|---|---|---|---|---|---|
| F#1 | 62.59% | 10.00% | 10.00% | 4.00% | 13.10% | 0.30% | 0.01% | 100.00% |
| F#2 | 64.59% | 10.00% | 10.00% | 2.00% | 13.10% | 0.30% | 0.01% | 100.00% |
| F#3 | 12.00% | 62.59% | 10.00% | 2.00% | 13.10% | 0.30% | 0.01% | 100.00% |
| Items | Qualification | F#1 | F#2 | F#3 | Reference SP 0W-20 | Test Method |
|---|---|---|---|---|---|---|
| Kinematic viscosity (100 °C, mm2/s) | 5.6–9.3 | 8.90 | 6.60 | 8.45 | 8.76 | GB/T 265 [35] |
| Viscosity index | report | 177 | 168 | 170 | 171 | GB/T 1995 [36] |
| Pour point, °C | ≤−40 | −48 | −45 | −45 | −51 | GB/T 3535 [37] |
| Flash point (opening), °C | ≥200 | 235 | 231 | 228 | 229 | GB/T 3536 [38] |
| Low-temperature dynamic viscosity (−35 °C, mPa·s) | ≤6200 | 4636 | 4530 | 6100 | 3381 | GB/T 6538 [39] |
| Low-temperature pumping viscosity (−40 °C, mPa·s, no yield stress) | ≤60,000 | 14,887 | 11,562 | 22,624 | 16,300 | SH/T 0562 [40] |
| High temperature and high shear viscosity (150 °C, mPa·s) | ≥2.6 | 2.800 | 2.519 | 2.817 | 2.611 | SH/T 0703 [41] |
| Evaporation loss (%) | ≤15 | 8.30 | 8.42 | 5.80 | 10.0 | SH/T 0059 [42] |
| Foam property (ml/mL) | GB/T 12579 [43] | |||||
| Procedure Ⅰ (24 °C) | ≤10/0 | 0/0 | 0/0 | 0/0 | 0/0 | |
| Procedure Ⅱ (93.5 °C) | ≤50/0 | 10/0 | 10/0 | 15/0 | 20/0 | |
| Procedure Ⅲ (Last 24 °C) | ≤10/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Items | F#1 | Reference SP 0W-20 |
|---|---|---|
| Deposit weight (mg) | 5 | 15 |
| Color scale | 1 + 1 | 3 + 0 |
| Results description | Even, light yellow lacquer, carbon deposits level 1 | Uneven bright patterns, carbon deposits level 2 |
| Images of the plate surface |  |  |
| Items | F#1 | Reference SP 0W-20 |
|---|---|---|
| Results description | Yellow lacquer | Yellow lacquer |
| Color scale | 6.5 | 6.5 |
| Images of the tube |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Zhang, X.; Yan, Q.; Wang, L.; Zeng, X. Revealing the Molecular Interaction between CTL Base Oil and Additives and Its Application in the Development of Gasoline Engine Oil. Lubricants 2024, 12, 275. https://doi.org/10.3390/lubricants12080275
Zhang C, Zhang X, Yan Q, Wang L, Zeng X. Revealing the Molecular Interaction between CTL Base Oil and Additives and Its Application in the Development of Gasoline Engine Oil. Lubricants. 2024; 12(8):275. https://doi.org/10.3390/lubricants12080275
Chicago/Turabian StyleZhang, Chunfeng, Xiaojun Zhang, Qiang Yan, Liyang Wang, and Xiangqiong Zeng. 2024. "Revealing the Molecular Interaction between CTL Base Oil and Additives and Its Application in the Development of Gasoline Engine Oil" Lubricants 12, no. 8: 275. https://doi.org/10.3390/lubricants12080275
APA StyleZhang, C., Zhang, X., Yan, Q., Wang, L., & Zeng, X. (2024). Revealing the Molecular Interaction between CTL Base Oil and Additives and Its Application in the Development of Gasoline Engine Oil. Lubricants, 12(8), 275. https://doi.org/10.3390/lubricants12080275





