Biomass-Derived Carbons as Friction Reducing Additives for Lubricants: Tribological Properties of Biochars and Activated Carbons Obtained from Sugar Cane Bagasse
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Tribological Tests
2.3. Physico-Chemical Investigations
3. Results and Discussion
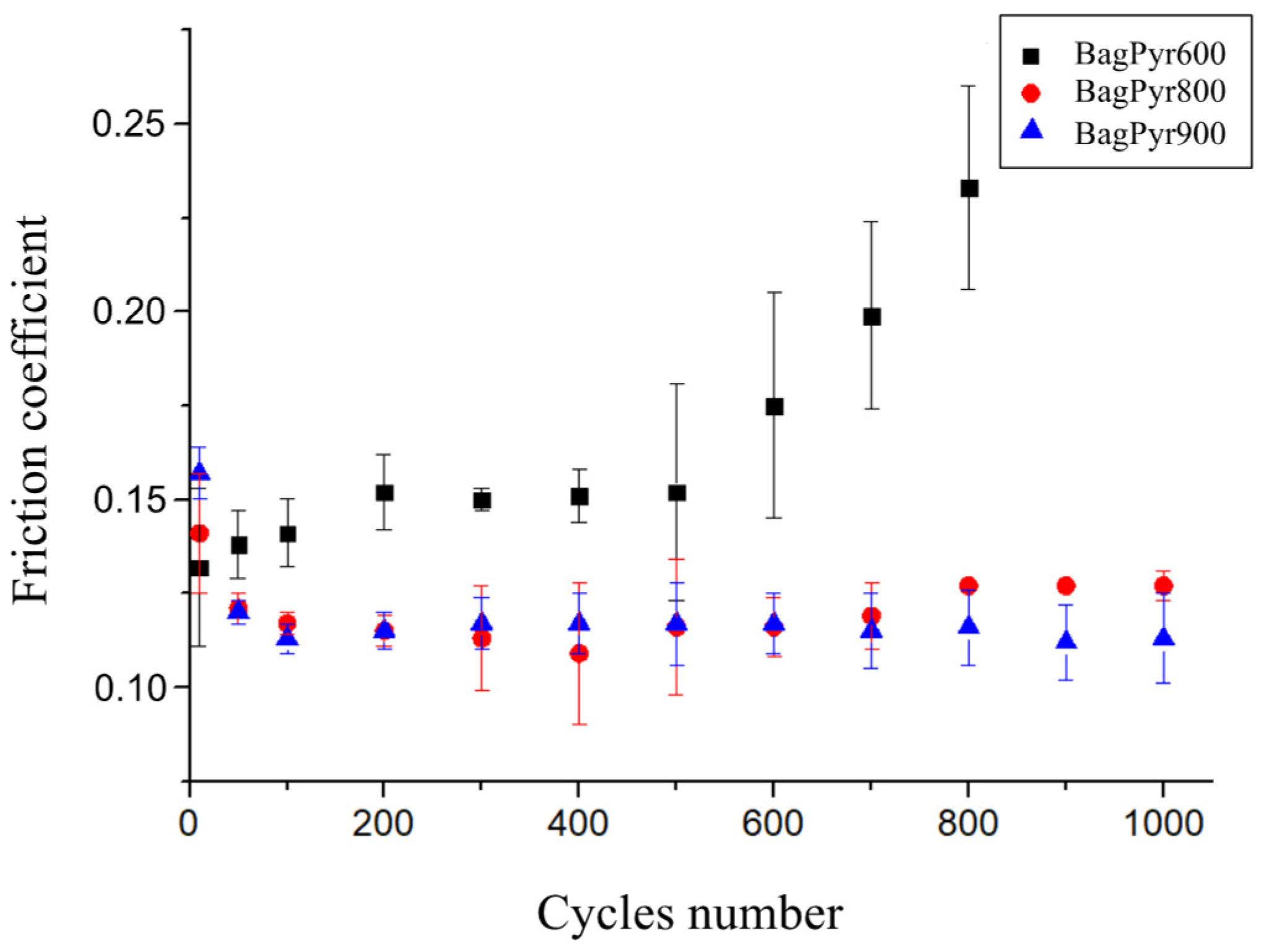
3.1. Influence of the Pyrolysis Temperature on the Friction Properties of Biochars Derived from Sugar Cane Bagasse
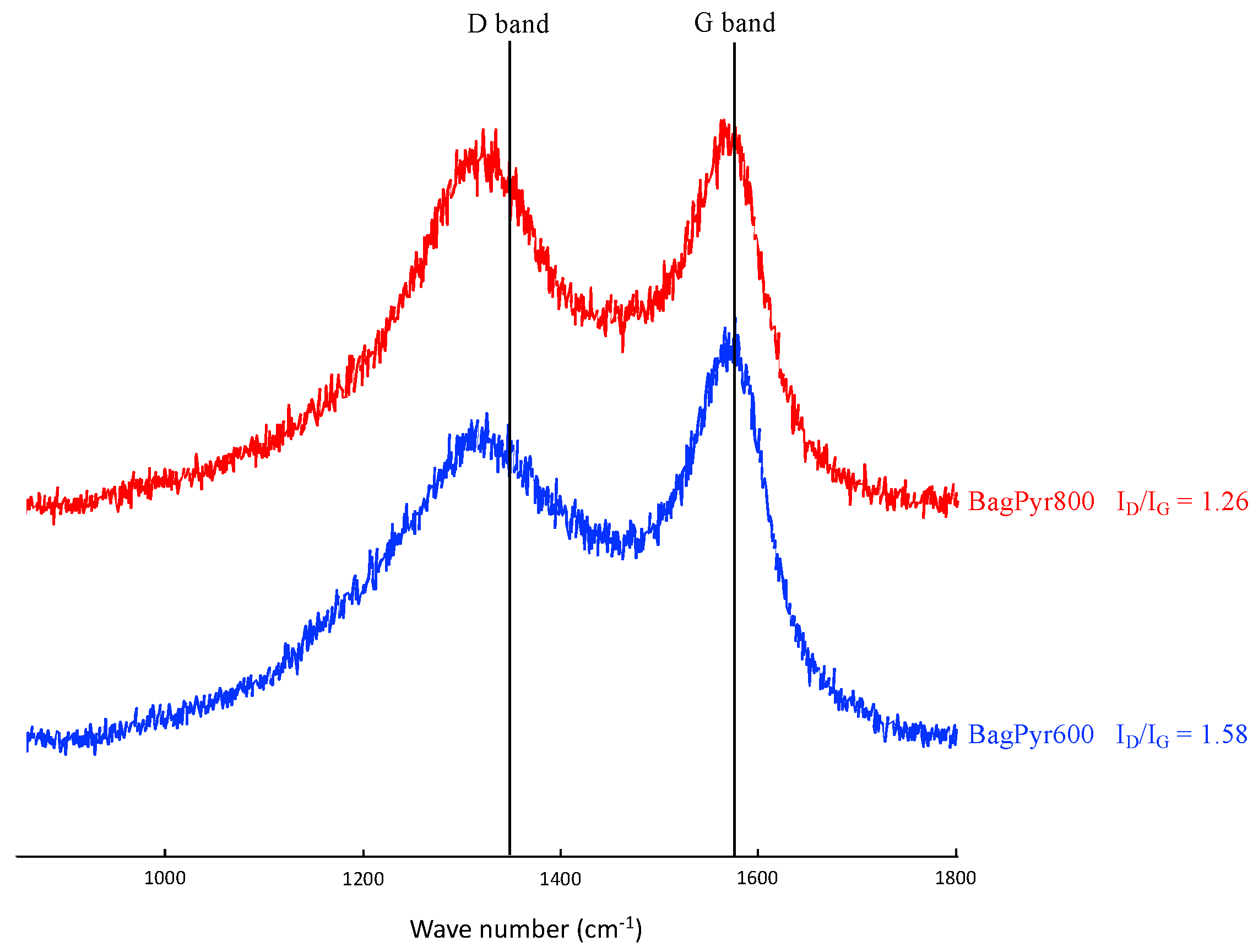
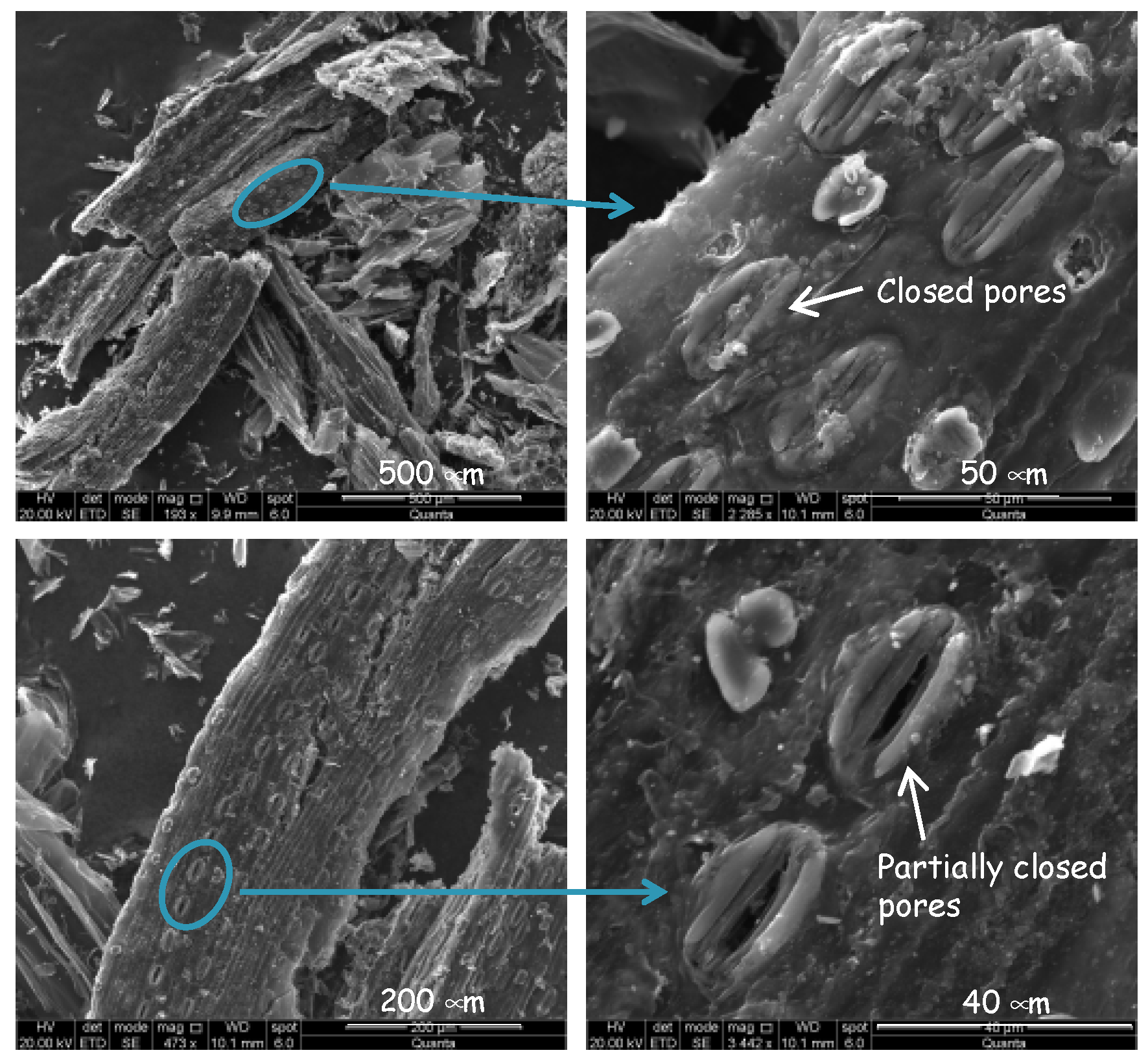
3.2. Structural and Textural Characterization of Activated Carbons Prepared from Sugar Cane Bagasse
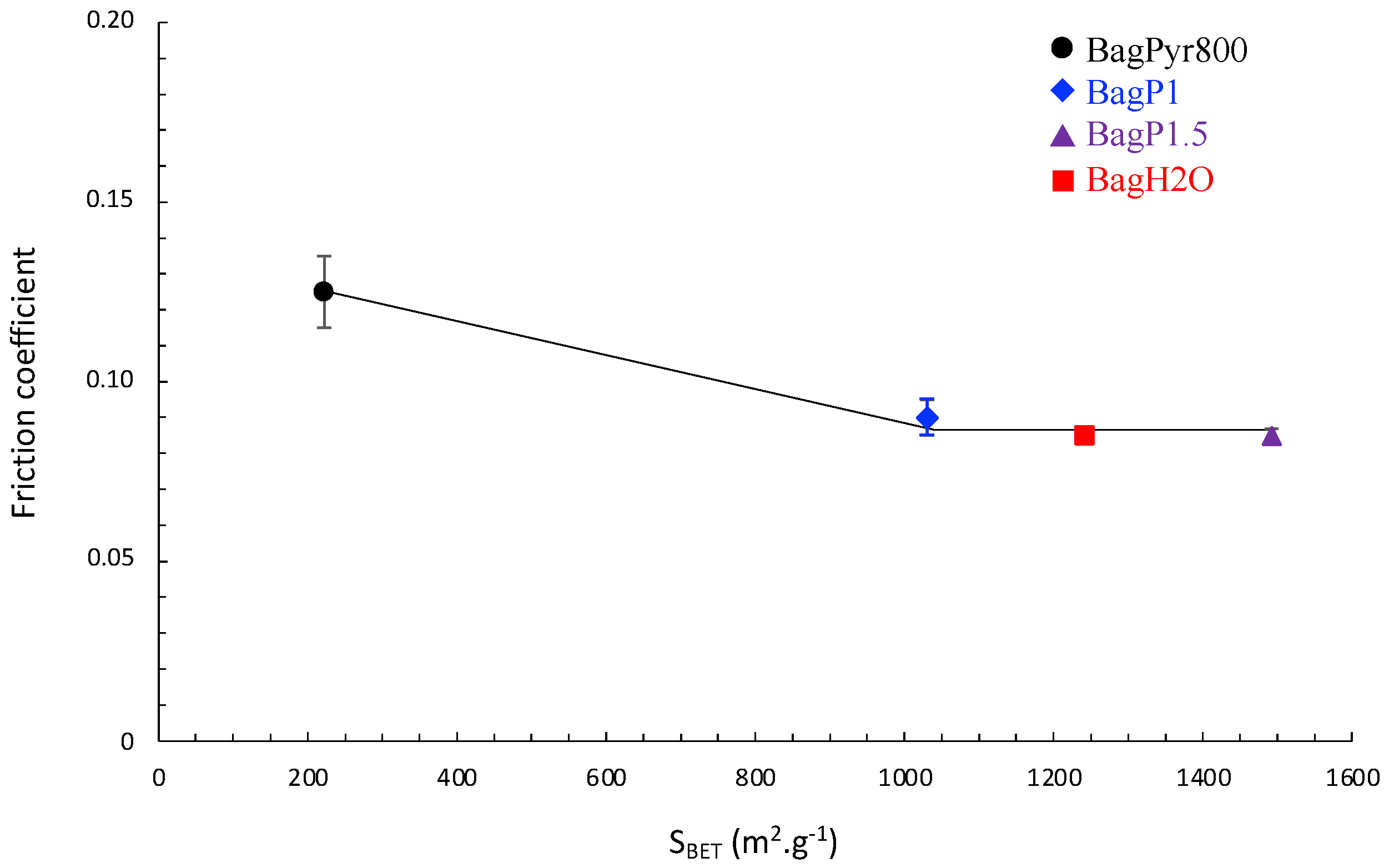
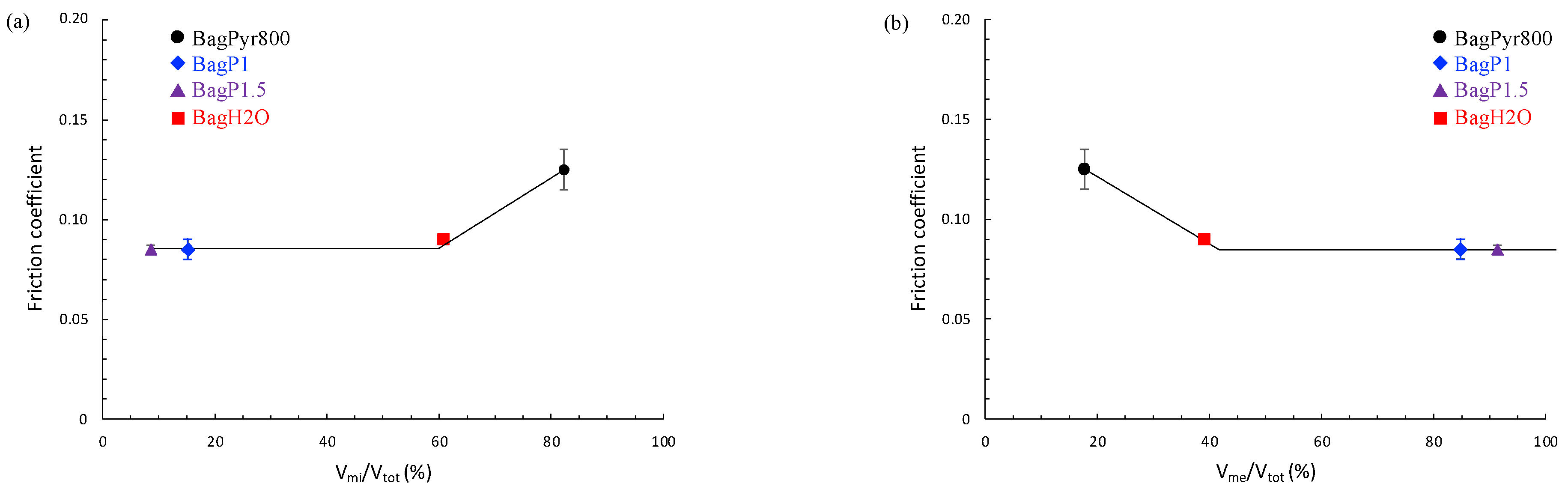
3.3. Tribological Properties of Activated Carbons
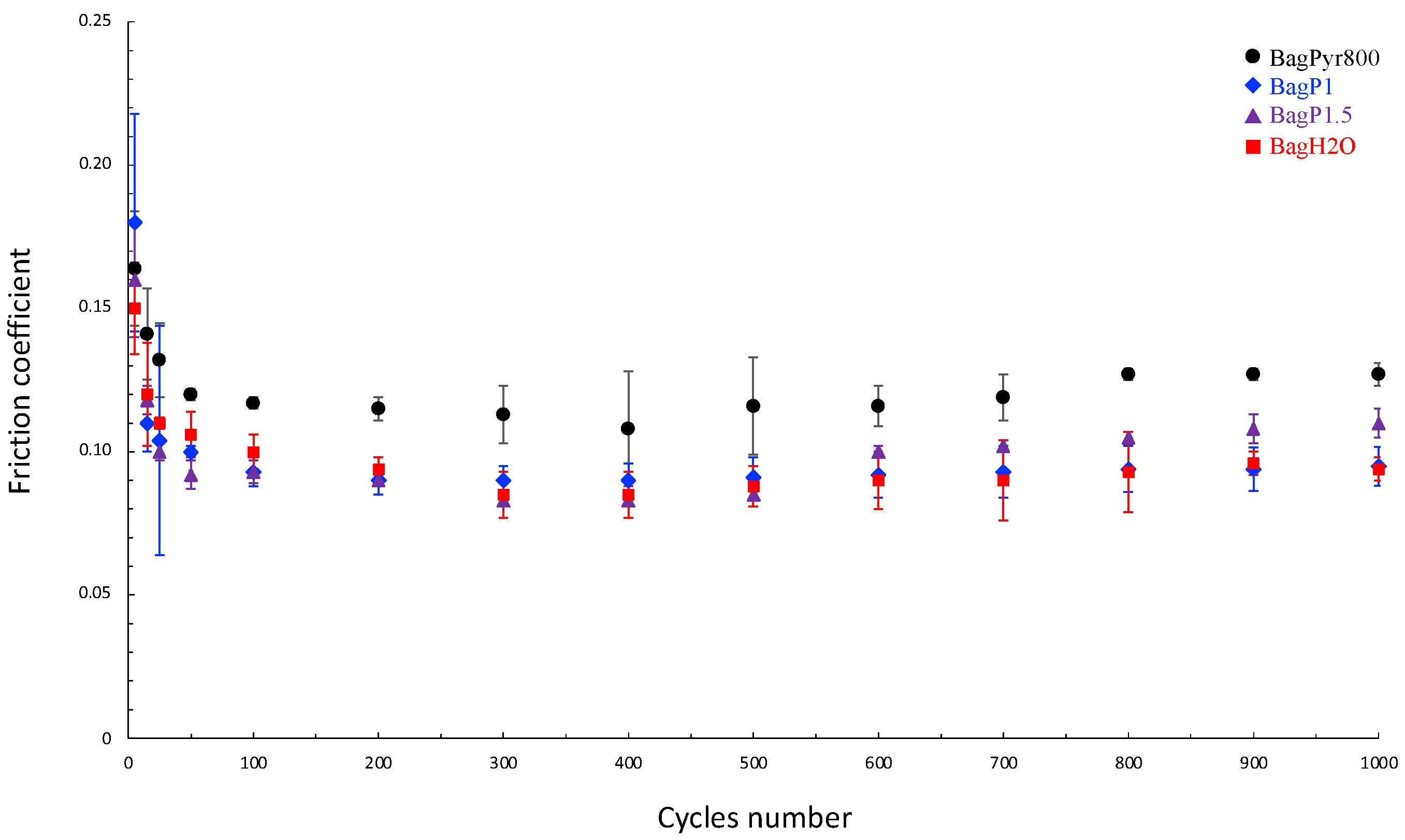
3.3.1. Friction Properties
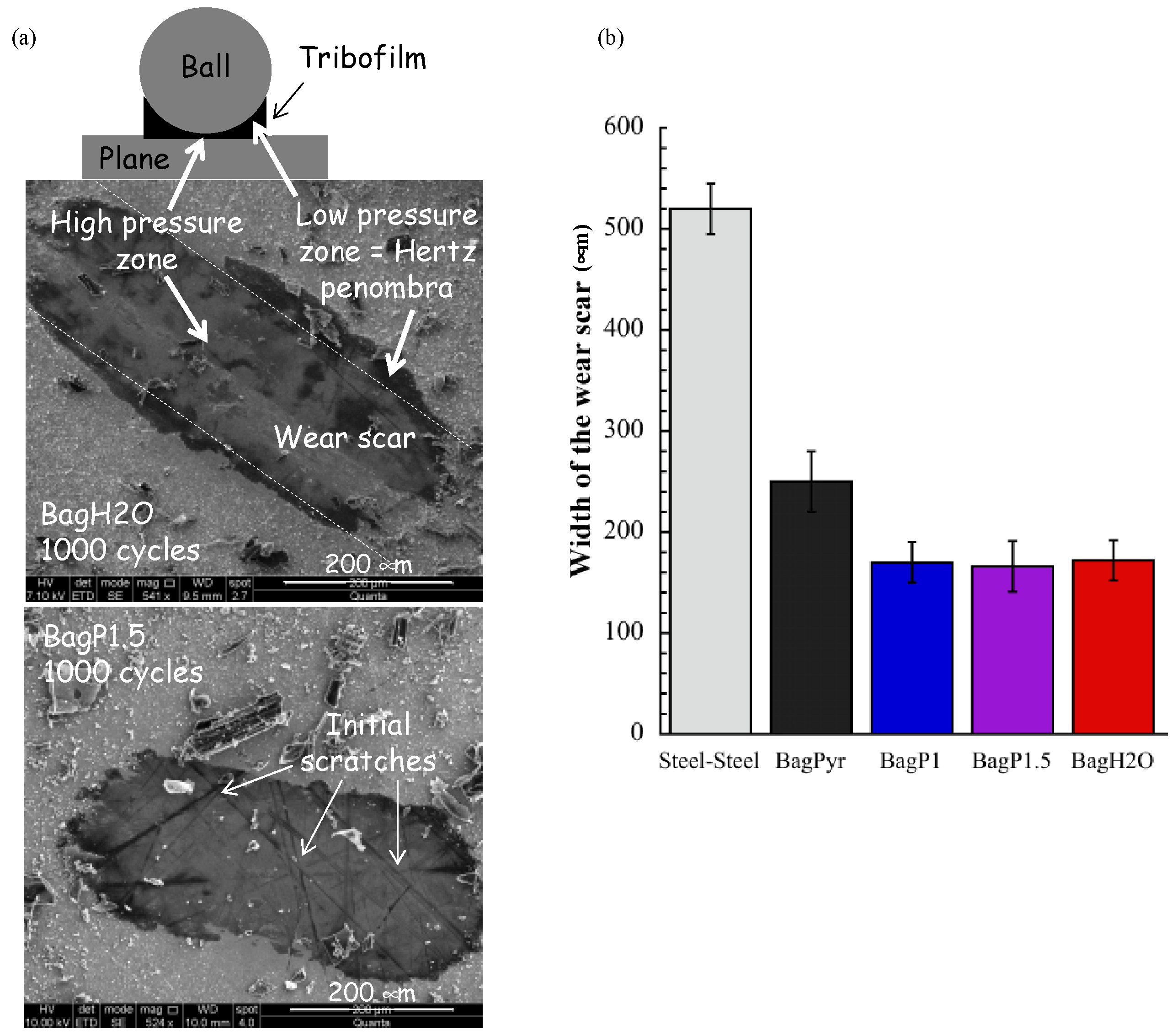
3.3.2. Estimation of the Anti-Wear Properties of Activated Carbons
4. Conclusions
5. Patents
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Booser, E.R. Tribology Data Handbook; CRC Press: New York, NY, USA, 1997. [Google Scholar]
- Georges, J.M. Frottement, Usure et Lubrification: La Tribologie ou Science des Surfaces; CNRS Editions: Paris, France, 2000. [Google Scholar]
- Zhai, W.; Srikanth, N.; Kong, L.B.; Zhou, K. Carbon nanomaterials in tribology. Carbon 2017, 119, 150–171. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, Z.; Han, S.; Bian, H. Effect of Adding Pyrolysis Carbon Black (CBp) on Soft Friction and Metal Wear during Mixing. Polymers 2022, 14, 1319. [Google Scholar] [CrossRef] [PubMed]
- Joly-Pottuz, L.; Ohmae, N. Carbon-based Nanolubricants. In Nanolubricants; Martin, J.M., Ohmae, N., Eds.; John Wiley & Sons: Chichester, UK, 2008; Chapter 3; pp. 93–147. [Google Scholar]
- Lee, J.; Cho, S.; Hwang, Y.; Lee, C.; Kim, S.H. Enhancement of lubrication properties of nano-oil by controlling the amount of fullerene nanoparticle additives. Tribol. Lett. 2007, 28, 203–208. [Google Scholar] [CrossRef]
- Baik, S.; Lee, G.S.; Yoon, D.K.; Lee, Y.Z. Tribological Performance of Multi-Walled Carbon Nanotubes in Mineral Oils under Boundary Lubricated Sliding. Key Eng. Mater. 2006, 321, 694–698. [Google Scholar]
- Chen, W.X.; Tu, J.P.; Wang, L.Y.; Gan, H.Y.; Xu, Z.D.; Zhang, X.B. Tribological Application of Carbon Nanotubes in a Metal-Based Composite Coating and Composites. Carbon 2003, 41, 215–222. [Google Scholar] [CrossRef]
- Hwang, Y.; Lee, C.; Choi, Y.; Cheong, S.; Kim, D.; Lee, K.; Lee, J.; Kim, S.H. Effect of the size and morphology of particles dispersed in nano-oil friction performance between rotating discs. J. Mech. Sci. Technol. 2011, 25, 2853–2857. [Google Scholar] [CrossRef]
- Thomas, P.; Bilas, P.; Molza, A.; Legras, L.; Mansot, J.L.; Guerin, K.; Dubois, M. Fluorinated nanocarbons for lubrication. In New Fluorinated Carbons: Fundamentals and Applications; Progress in Fluorine Science Series; Botalina, O., Nakajima, T., Tressaud, A., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 325–360. [Google Scholar]
- Gaspard, S.; Passé-Coutrin, N.; Durimel, A.; Cesaire, T.; Jeanne-Rose, V.R. Activated carbon from biomass for water treatment. RSC Green Chem. 2014, 46–105. [Google Scholar] [CrossRef]
- Yacou, C.; Altenor, S.; Carene, B.; Gaspard, S. Chemical structure investigation of tropical Turbinaria turbinata seaweeds and its derived carbon sorbents applied to the removal of hexavalent chromium in water. Algal Res. 2018, 34, 25–36. [Google Scholar] [CrossRef]
- Ranguin, R.; Delannoy, M.; Yacou, C.; Jean-Marius, C.; Feidt, C.; Rychen, G.; Gaspard, S. Biochar and activated carbons preparation from invasive algae Sargassum spp. for Chlordecone availability reduction in contaminated soils. J. Environ. Chem. Eng. 2021, 9, 105280. [Google Scholar] [CrossRef]
- Abuelnoor, N.; AlHajaj, A.; Khaleel, M.; Vega, L.F.; Abuzahra, M.R.M. Activated carbons from biomass-based sources for CO2 capture applications. Chemosphere 2021, 282, 131111. [Google Scholar] [CrossRef]
- Khuong, D.A.; Nguyen, H.N.; Tsubota, T. Activated carbon produced from bamboo and solid residue by CO2 activation utilized as CO2 adsorbents. Biomass Bioenergy 2021, 148, 106039. [Google Scholar] [CrossRef]
- Suarez-Guevara, J.; Ruiz, V.; Gomez-Romero, P. Hybrid energy storage: High voltage aqueous supercapacitors based on activated carbon-phosphotungstate hybrid materials. J. Mater. Chem. A 2014, 2, 1014–1021. [Google Scholar] [CrossRef]
- Sevilla, M.; Mokaya, R. Energy storage applications of activated carbons: Supercapacitors and hydrogen storage. Energy Environ. Sci. 2014, 7, 1250–1280. [Google Scholar] [CrossRef]
- Tan, X.F.; Liu, S.B.; Liu, Y.G.; Gu, Y.L.; Zeng, G.M.; Hu, X.J.; Wang, X.; Liu, S.H.; Jiang, L.H. Biochar as potential sustainable precursors for activated carbon production: Multiple applications in environmental protection and energy storage. Bioresour. Technol. 2017, 227, 359–372. [Google Scholar] [CrossRef]
- Ayinla, R.T.; Dennis, J.O.; Zaid, H.M.; Sanusi, Y.K.; Usman, F.; Adebayo, L.L. A review of technical advances of recent palm bio-waste conversion to activated carbon for energy storage. J. Clean. Prod. 2019, 229, 1427–1442. [Google Scholar] [CrossRef]
- Baig, M.M.; Gul, I.H. Conversion of wheat husk to high surface area activated carbon for energy storage in high performance supercapacitors. Biomass Bioenergy 2021, 144, 105909. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, X.; Liu, A.; Zhu, H.; Ma, T. Recent progress in biomass-derived carbon materials used for secondary batteries. Sustain. Energy Fuels 2021, 5, 3017–3038. [Google Scholar] [CrossRef]
- Jayachandran, M.; Kishore Babu, S.; Maiyalagan, T.; Rajadurai, N.; Vijayakumar, T. Activated carbon derived from bamboo-leaf with effect of various aqueous electrolytes as electrode material for supercapacitor applications. Mater. Lett. 2021, 301, 130335. [Google Scholar] [CrossRef]
- Baik, S.; Kim, J.M.; Lee, K.S.; Yoon, D.K. Tribological Performance of Ordered Mesoporous Carbons in Mineral Oils Under Boundary Lubricated Sliding. Key Eng. Mater. 2006, 326, 353–356. [Google Scholar]
- Om Prakash, M.; Raghavendra, G.; Ojha, S.; Panchal, M.; Kumae, D. Investigation of tribological properties of biomass developed porous nano activated carbon composites. Wear 2021, 466–467, 203523. [Google Scholar] [CrossRef]
- Om Prakash, M.; Gujjala, R.; Ojha, S.; Panchal, M. Mechanical characterization of arhar biomass based porous nano activated carbon polymer composites. Polym. Compos. 2020, 1–11. [Google Scholar] [CrossRef]
- Noor Ayuma, M.T.; Mohd Fadzli, B.A.; Radifah, H.; Hilmi, A. The effect of temperature on the tribological properties of palm kernel activated carbon-epoxy composite. Tribol. Online 2015, 10, 428–433. [Google Scholar]
- Talib, N.; Jamaluddin, N.A.; Sheng, T.K.; Kiow, L.W.; Abdullah, H.; Ahmad, S.; Saleh, A. Tribological study of activated carbon nanoparticle in nonedible nanofluid for machining application. Evergr. Jt. J. Nov. Carbon Resour. Sci. Green Asia Strategy 2021, 8, 454–460. [Google Scholar] [CrossRef]
- Jumali, S.N.Q.A.; Embong, Z.; Rahim, E.A.; Mohid, Z.; Samion, S. Surface Morphology and Tribological Studies of Activated Carbon Additive Blended in RBD Palm Olein as Sustainable Machining Lubricant. In Recent Trends in Manufacturing and Materials towards Industry 4.0; Springer: Berlin/Heidelberg, Germany, 2021; pp. 257–266. [Google Scholar] [CrossRef]
- Baik, S.; Yoon, D.; Lee, H.I.; Kim, J.M.; Lee, G.S.; Lee, Y.Z. Frictional Performances of Activated Carbon and Carbon Blacks as Lubricant Additives. Tribol. Trans. 2008, 52, 133–137. [Google Scholar] [CrossRef]
- Nassef, M.G.A.; Hassan, H.S.; Nassef, G.A.; Nassef, B.G.; Soliman, M.; Elkady, M.F. Activated Carbon Nanoparticles from Recycled Polymers Waste as Novel Nano-Additive to Grease Lubrication. Lubricants 2022, 10, 214. [Google Scholar] [CrossRef]
- Abouelkasem, Z.A.; Nassef, G.A.; Abdelnaeem, M.; Nassef, M.G.A. Enhancing the Elastohydrodynamic Lubrication and Vibration Behavior of Rolling Bearings Using a Hybrid Bio-Grease Blended with Activated Carbon Nanoparticles. Tribol. Lett. 2024, 72, 46. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Pimenta, M.A.; Ecklund, P.C.; Dresselhaus, G. Raman Scattering in Fullerenes and Related Carbon-Based Materials; Weber, W.H., Merlin, R., Eds.; Springer: New York, NY, USA, 2000; pp. 315–364. [Google Scholar]
- Merlin, R.; Pinczuk, A.; Weber, W.H. Overview of Phonon Raman Scattering in Solids; Weber, W.H., Merlin, R., Eds.; Springer: New York, NY, USA, 2000; pp. 1–29. [Google Scholar]
- Tuinstra, F.; Koenig, J.L. Raman spectrum of graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
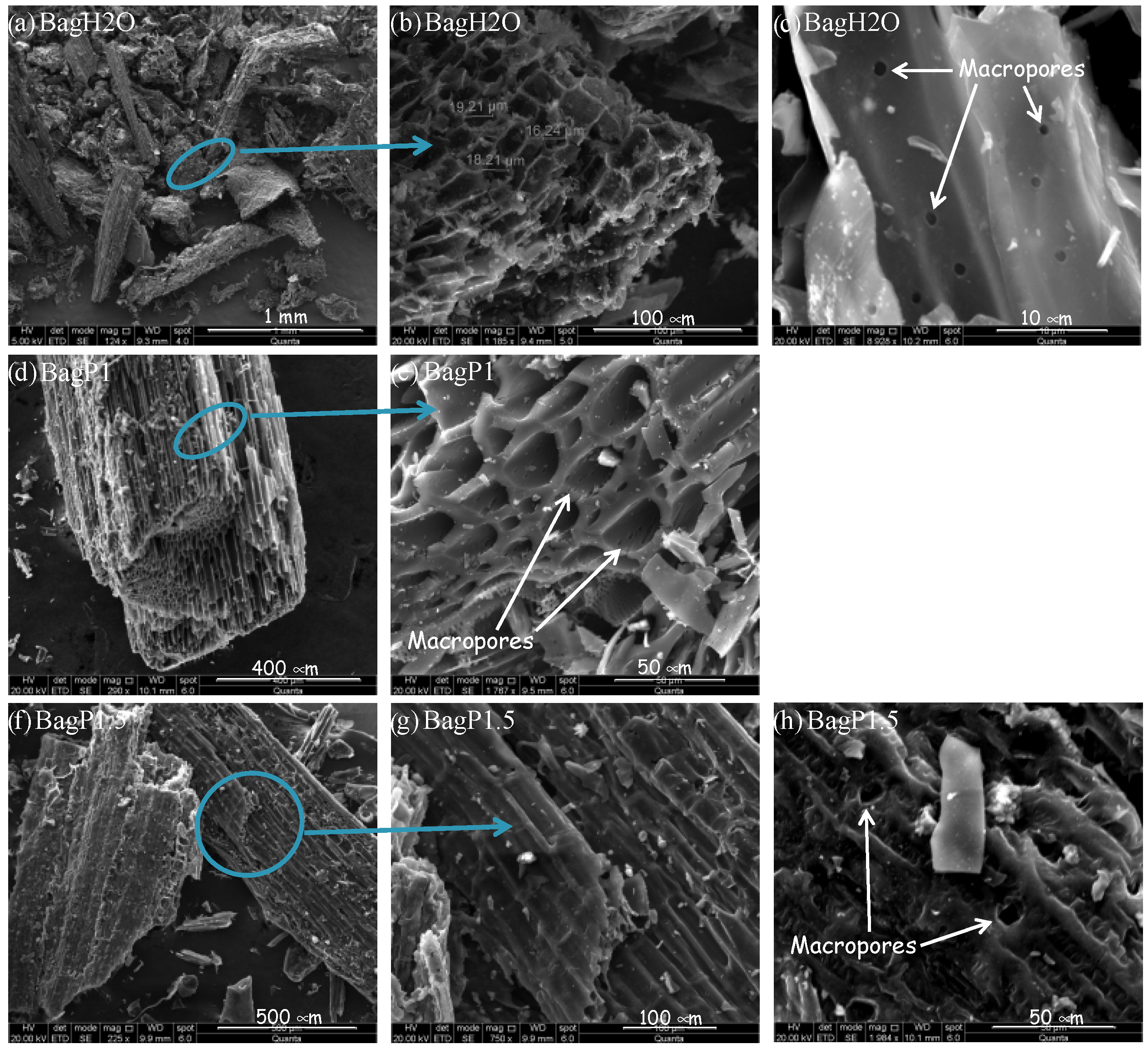
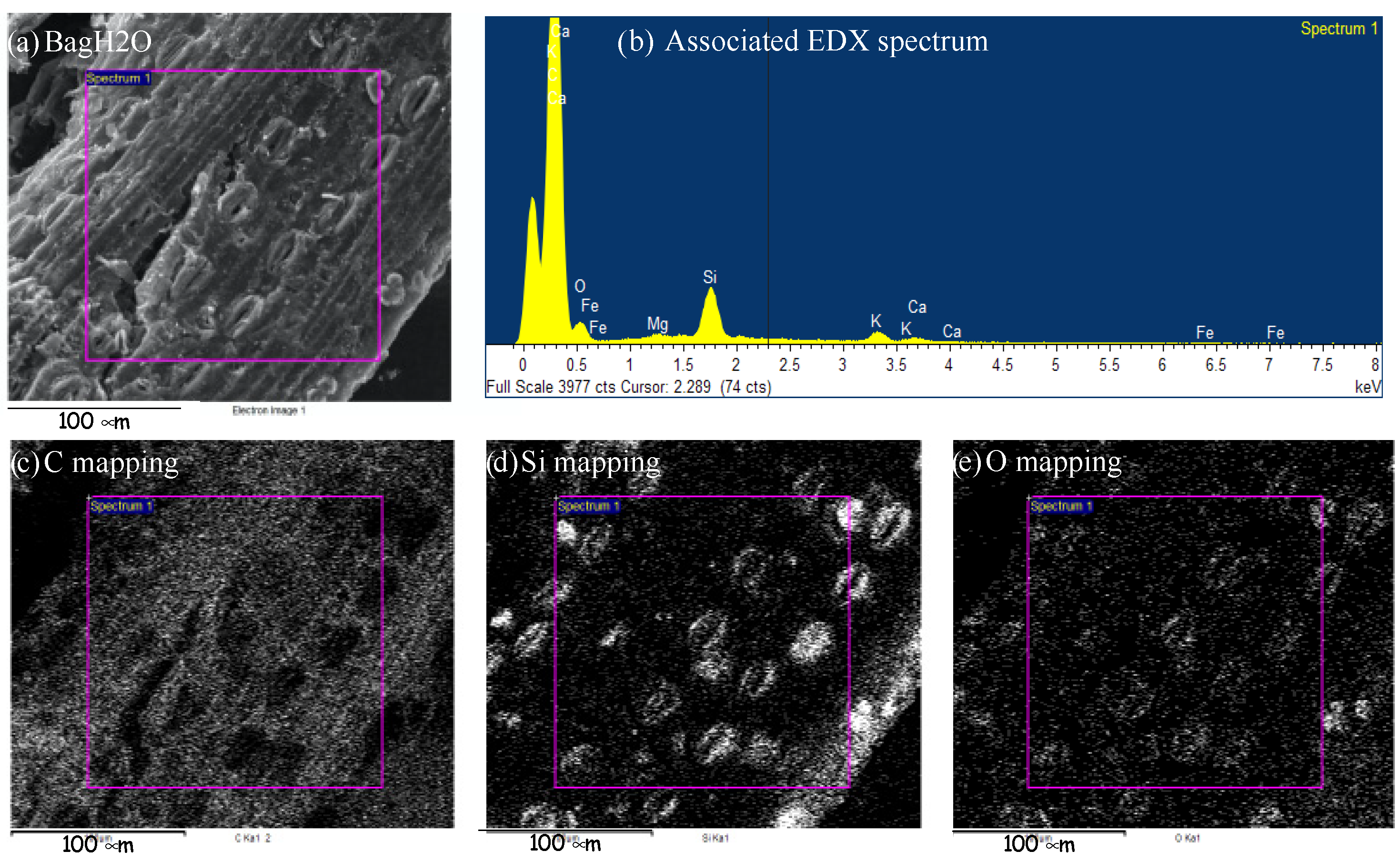


| Atomic Concentration (%) | BagPyr800 | BagH2O | BagP1 | BagP1.5 |
|---|---|---|---|---|
| C | 85.50 | 89.35 | 90.20 | 91.73 |
| O | 12.64 | 7.93 | 8.73 | 5.74 |
| Si | 1.33 | 0.70 | 0.21 | 0.31 |
| P | - | 0.06 | 0.37 | 1.10 |
| Textural Parameters | BagPyr800 | BagH2O | BagP1 | BagP1.5 |
|---|---|---|---|---|
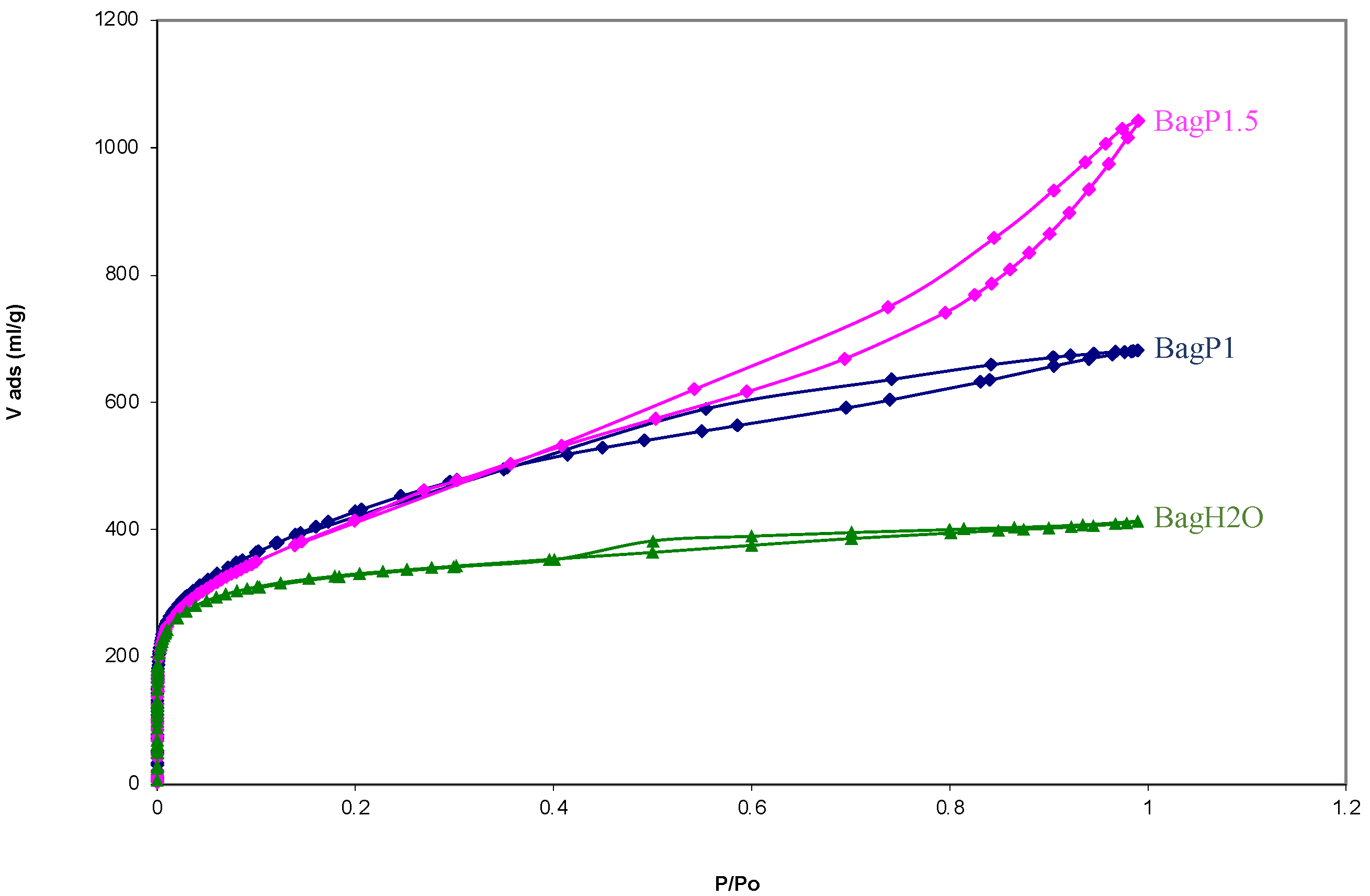
| SBET (m2·g−1) | 222 | 1242 | 1030 | 1492 |
| Vmi (cm3·g−1) | 0.078 | 0.420 | 0.094 | 0.140 |
| Vme (cm3·g−1) | 0.017 | 0.270 | 0.524 | 1.490 |
| Vtot (cm3·g−1) | 0.095 | 0.690 | 0.618 | 1.630 |
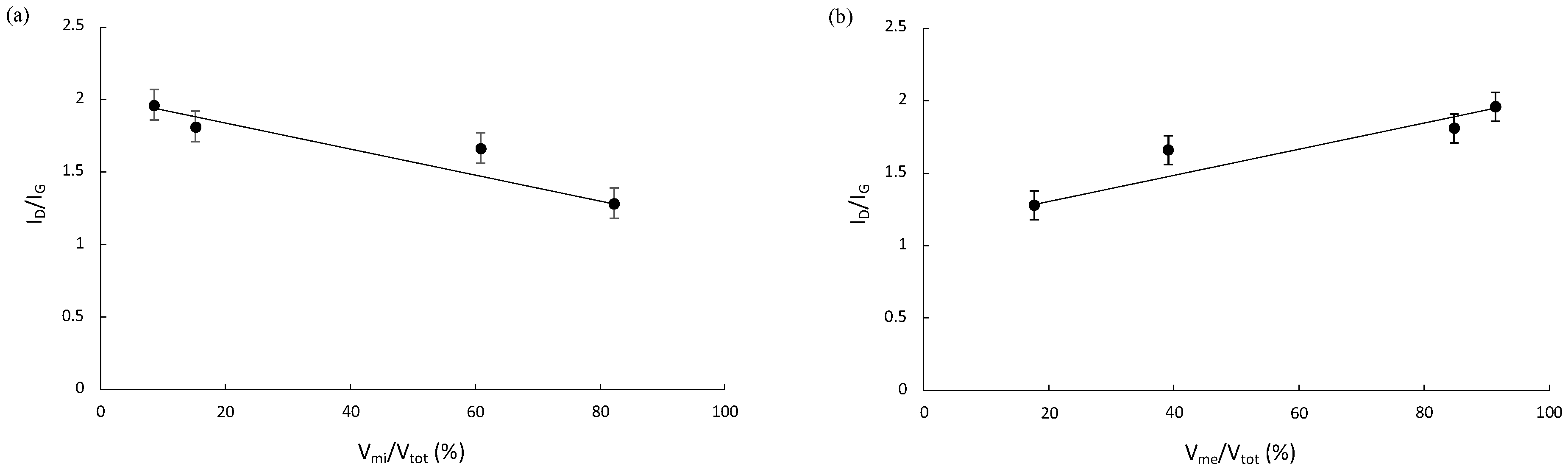
| Vmi/Vtot (%) | 82.278 | 60.869 | 15.210 | 8.589 |
| Vme/Vtot (%) | 17.722 | 39.131 | 84.790 | 91.411 |
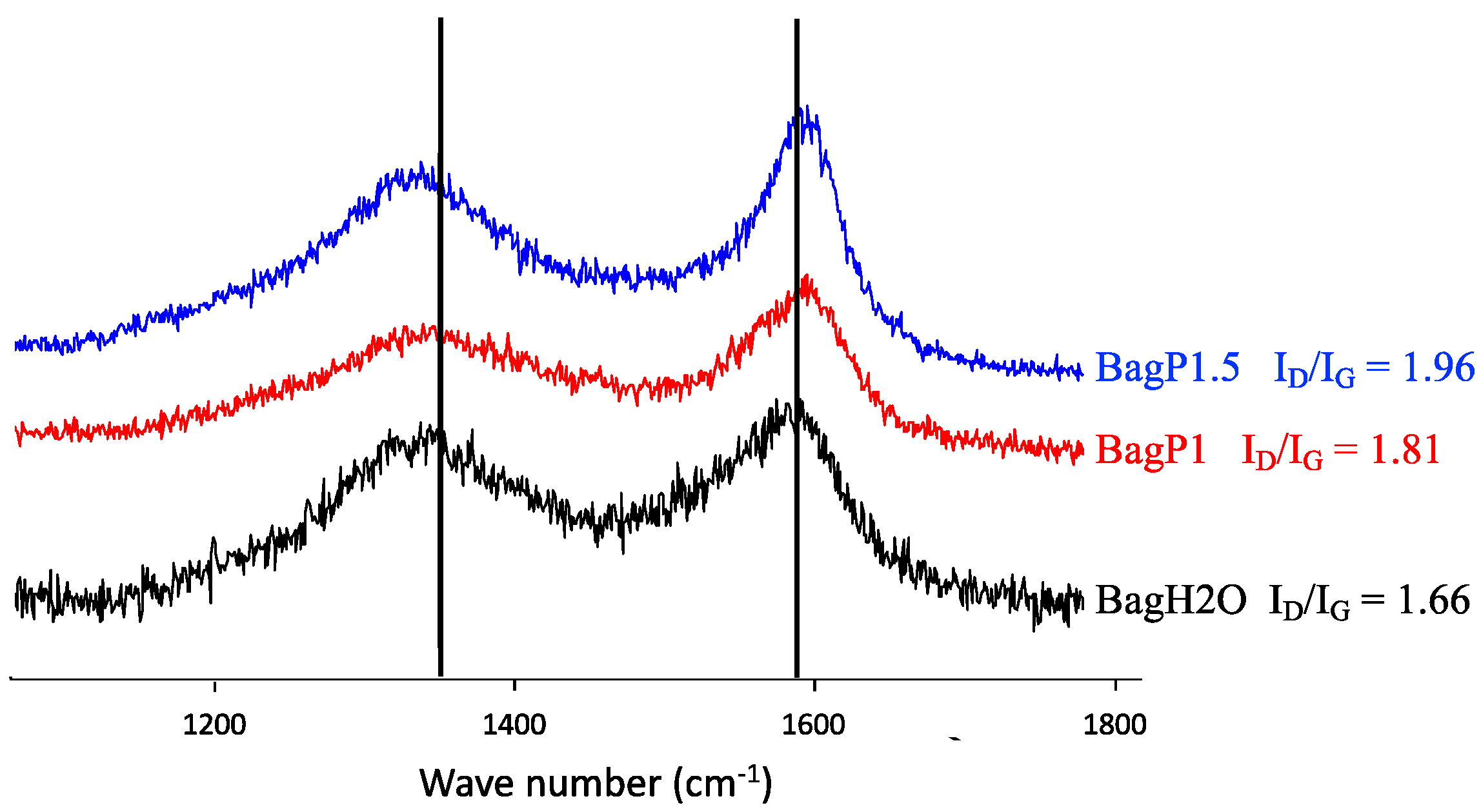
| ID/IG Ratio before Friction | ID/IG Ratio after 1000 Cycles of Friction | La before Friction (nm) | La after 1000 Cycles of Friction (nm) | |
|---|---|---|---|---|
| BagPyr800 | 1.26 | 1.54 | 15.23 | 12.47 |
| BagH2O | 1.66 | 1.92 | 11.57 | 10 |
| BagP1 | 1.81 | 1.95 | 10.60 | 9.85 |
| BagP1.5 | 1.96 | 2.17 | 9.79 | 8.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molza, A.; Bilas, P.; Nomède-Martyr, N.; Césaire, T.; Yacou, C.; Gaspard, S.; Thomas, P. Biomass-Derived Carbons as Friction Reducing Additives for Lubricants: Tribological Properties of Biochars and Activated Carbons Obtained from Sugar Cane Bagasse. Lubricants 2024, 12, 308. https://doi.org/10.3390/lubricants12090308
Molza A, Bilas P, Nomède-Martyr N, Césaire T, Yacou C, Gaspard S, Thomas P. Biomass-Derived Carbons as Friction Reducing Additives for Lubricants: Tribological Properties of Biochars and Activated Carbons Obtained from Sugar Cane Bagasse. Lubricants. 2024; 12(9):308. https://doi.org/10.3390/lubricants12090308
Chicago/Turabian StyleMolza, Audrey, Philippe Bilas, Nadiège Nomède-Martyr, Thierry Césaire, Christelle Yacou, Sarra Gaspard, and Philippe Thomas. 2024. "Biomass-Derived Carbons as Friction Reducing Additives for Lubricants: Tribological Properties of Biochars and Activated Carbons Obtained from Sugar Cane Bagasse" Lubricants 12, no. 9: 308. https://doi.org/10.3390/lubricants12090308
APA StyleMolza, A., Bilas, P., Nomède-Martyr, N., Césaire, T., Yacou, C., Gaspard, S., & Thomas, P. (2024). Biomass-Derived Carbons as Friction Reducing Additives for Lubricants: Tribological Properties of Biochars and Activated Carbons Obtained from Sugar Cane Bagasse. Lubricants, 12(9), 308. https://doi.org/10.3390/lubricants12090308





