Abstract
The requirement to improve energy efficiency is constantly driving the development of high-performance and eco-friendly friction modifiers (FMs). Herein, two innovative sulfur- and phosphorus-free melamine long-chain alcohol esters (Dodec-EG-CC and Dodec-CC) are reported as novel organic friction modifiers (OFMs). Over a wide temperature range of 100 °C to 200 °C, the synthesized melamine long-chain alcohol esters, which have exceptional thermal stability, dramatically lessen wear and friction of PAO4 base oil. Dodec-EG-CC particularly reduces friction by up to 50% and wear rate by approximately 92% within this temperature range. Detailed studies of the tribological properties at elevated temperatures demonstrate that the synergistic effect of the melamine structural unit coupled with ester groups significantly enhances adsorption properties of additives on metal surfaces, improving adsorption strength and lubricating film stability. The adsorption of additives on the metal surfaces is further confirmed by surface analysis and adsorption energy calculation, which serve as a key parameter for characterizing the binding strength between molecules and surfaces. These findings demonstrate the potential of the designed triazine-based derivatives, especially Dodec-EG-CC, as OFMs in effectively reducing friction losses in motor vehicle engines. This highlights their significant potential for industrial applications in improving energy efficiency and extending engine lifespan. These in-depth studies not only provide valuable insights for the molecular structure design of OFMs, but also advances the development of sustainable lubrication technologies.
1. Introduction
In recent decades, the protection of the environment and the improvement of energy efficiency have emerged as significant global challenges. The automotive and industrial sectors are confronted with increasingly rigorous environmental regulations while striving to enhance fuel economy and energy efficiency [1,2]. To meet these requirements, low-viscosity lubricants have received much attention for their ability to minimize viscous drag and enhance fuel efficiency [3]. In mechanical devices operating at low speeds, high loads, or elevated temperatures, as well as under various other conditions, low-viscosity lubricants frequently fail to establish an adequate oil film thickness. This inadequacy leads to direct contact between surfaces, resulting in a transition to a boundary lubrication state [4,5]. In the situation of boundary lubrication, severe friction and wear occur, which significantly reduces the service life of the mechanism [6]. At the same time, due to increasingly stringent environmental regulations, the use of some lubricant additives containing harmful elements such as S and P is restricted [7], resulting in an additional decrease in the tribological characteristics of the lubricant. To address lubrication challenges arising from reduced oil viscosity and restricted use of sulfur- and phosphorus-containing additives, friction modifiers (FMs) are emerging as widely adopted additives in engine oils for effective wear and friction reduction [8].
There are four main types of FMs: oil-soluble organic molybdenum compounds, nanoparticle additives, functional polymers, and organic friction modifiers [9]. The most often utilized friction modifiers in engine oil compositions are organic molybdenum compounds and organic friction modifiers (OFMs). Organomolybdenum compounds form MoS2 nanocrystals on the friction surface during friction, thus reducing frictional wear [10]. However, organic molybdenum compounds produce ash during friction and contain harmful elements such as S and P, which can adversely affect engines and emission control systems [6]. Therefore, in order to effectively address pressing environmental issues, there is a tendency to transition from the current sulfur- and phosphorus-based additives to ash-free OFMs [11]. These OFMs are composed entirely of C, H, O, and N atoms, eliminating the presence of hazardous elements [12]. This sulfur- and phosphorus-free design can avoid the formation of toxic by-products and protect the three-way catalytic converters from contamination, thereby ensuring efficient exhaust purification and reducing harmful emissions. Meanwhile, it minimizes ash and sediment, thus enhancing fuel economy and lowering energy consumption. OFMs consist of a polar head group and an alkane tail chain, whose polar end group forms a stable adsorption model at the friction surface interface by physical or chemical adsorption, while the tail hydrocarbon chain forms a vertically oriented, close-packed adsorption film by van der Waals forces [13,14]. These films prevent direct metal-to-metal contact, thereby effectively reducing friction and wear [5]. Reports on OFMs in recent years have mostly concentrated on comparing the properties, developing formulations, and researching mechanisms of existing molecular structures [6,13,15].
Rapid development of modern industrial and automotive technology continuously drives the improvement of engine performance, while also leading to stringent challenges for the performance of OFMs [16,17]. The lubrication performance of most traditional OFMs decreases significantly at elevated temperatures [18]. This severely limits their application in high-temperature environments such as modern high-performance engines. To solve this issue, researchers have begun to focus on nitrogen-containing heterocyclic compounds with higher thermal stability. Due to their special molecular structure, these compounds not only possess positive tribological properties, but also exhibit excellent high-temperature performance [19,20,21,22]. Triazine derivatives are among them that have garnered a lot of interest because of their exceptional qualities and distinctive molecular architectures [23,24]. Triazine derivatives are a class of six-membered heterocyclic compounds containing three nitrogen atoms, with a high degree of symmetry and stability in their molecular structures [25]. Chen et al. showed that the incorporation of triazine derivatives improved the tribological properties of ethylene glycol, confirming that triazine derivatives are suitable candidates for further improving the tribological properties of friction systems [19]. Desanker et al. further showed that the triazine ring can form a strong adsorption with the metal surface and can maintain good lubrication at high temperatures [20]. In addition, the compounds synthesized by Jin et al. with a triazine ring as the core showed excellent antioxidant properties, and the antioxidant property is also one of the important factors influencing the lubrication performance of the compounds in high-temperature environments [26]. However, the reported explanations on the friction mechanism of triazine derivatives at high temperatures have always been limited, so it is necessary to further explain the mechanism of action of triazine derivatives at high temperatures. Moreover, how to further improve the thermal stability and lubrication properties of triazine derivatives is one of the key directions of current research.
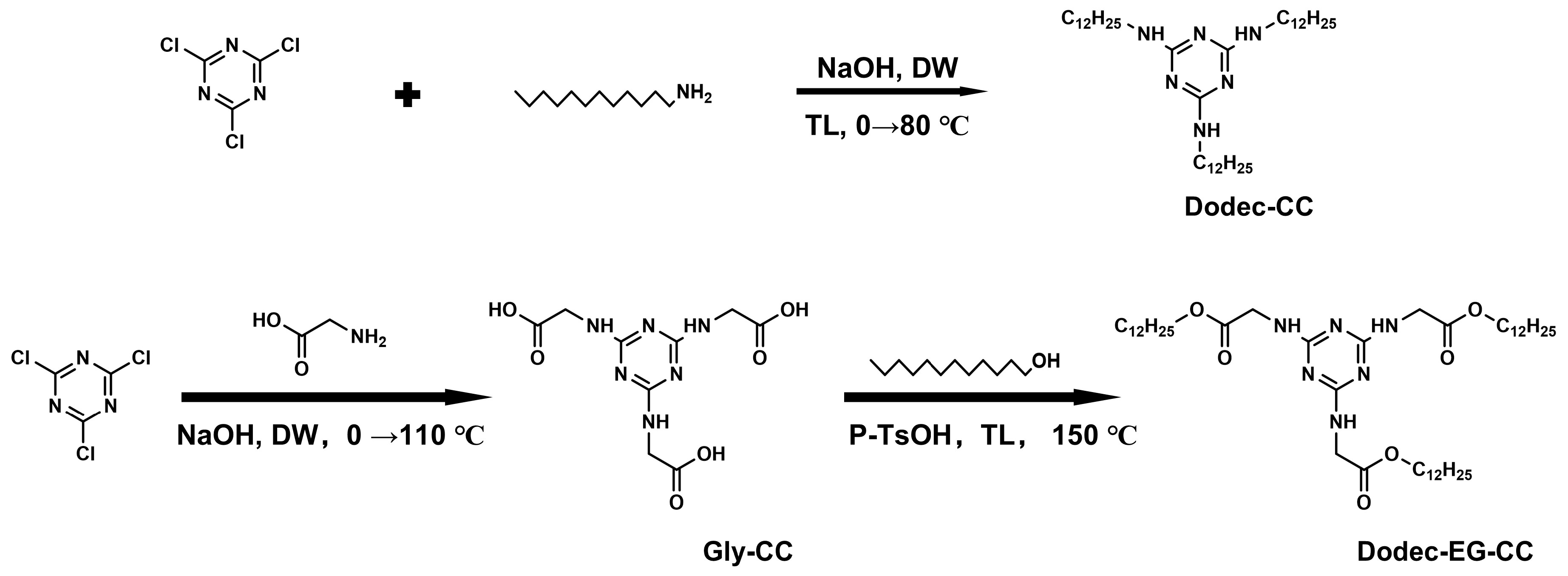
In this study, two novel sulfur- and phosphorus-free triazine-based OFMs were designed, with their molecular structures shown in Scheme 1. Based on the core structure of melamine, long-chain alkyl groups were introduced to synthesize two organic compounds with excellent thermal stability. Subsequently, their tribological performance and mechanisms under high-temperature conditions were systematically investigated. Among them, Dodec-EG-CC introduces a long chain structure containing ester groups compared to Dodec-CC. To elucidate the lubrication mechanism, we employed techniques such as scanning electron microscopy, Raman spectroscopy, XPS, and molecular dynamics simulations to calculate the adsorption energy of molecules. More research was done on how the molecular structure affects the additives’ lubricating qualities.
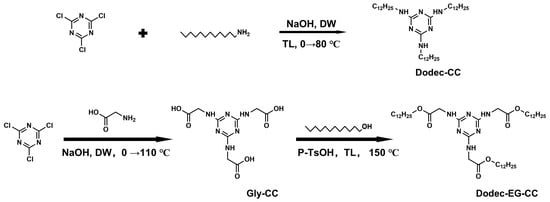
Scheme 1.
Synthetic scheme of triazine derivatives (DW: distilled water; TL: toluene).
2. Experimental Section
2.1. Materials
The compounds 2,4,6-Trichloro-1,3,5-Triazine (98.0%), Glycine (99.0%), 1-Dodecanol (99.0%), NaOH (98.0%), Ethyl Acetate (99.9%), p-Toluenesulfonic Acid (98.0%), and Petroleum Ether were obtained from Shanghai Titan Scientific Co., Ltd., Shanghai, China. Dodecylamine (98.0%) and Toluene (99.5%) were obtained from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. The reagents used are commercial products and do not require further purification.
2.2. Synthesis
2.2.1. Synthesis of Dodec-CC
In a 500 mL round-bottom flask, 2,4,6-trichloro-1,2,3-triazine (6.1 g, 33 mmol), dodecylamine (19.2 g, 103 mmol), and 180 mL toluene were added, and an ice bath was used to chill the mixture. After dissolving 4.4 g (110 mmol) of sodium hydroxide in 50 mL of water, the mixture was gradually poured into the round-bottom flask. The reaction mixture was heated to reflux and stirred for 8 h. After the reaction, the aqueous phase was separated, and the organic phase was poured into methanol to precipitate the solid. With an 89% yield, the target product Dodec-CC was produced by recrystallizing the crude product. 1H NMR (500 MHz, Chloroform- d) δ 4.82 (s, 3H), 3.32 (s, 6H), 1.52 (p, J = 7.4, 7.0 Hz, 6H), 1.31–1.17 (m, 54H), 0.87 (t, J = 6.9 Hz, 9H) ppm. 13C NMR (126 MHz, Chloroform-d) δ 58.47, 40.81, 32.07, 29.79, 27.10, 22.83, 18.58, 14.26 ppmv. MS (ESI): calcd C39H79N6 [M + H]+ 631.63; found, 631.51.
2.2.2. Synthesis of Gly-CC
In a 1000 mL round-bottom flask, 2,4,6-trichloro-1,2,3-triazine (10 g, 54.2 mmol), glycine (4.3 g, 56.9 mmol), and 100 mL distilled water were added and stirred in an ice bath for 10 min. Subsequently, a 25 wt.% NaOH aqueous solution was gradually introduced to keep the solution’s pH within the range of 10 to 11. The solution was further stirred in an ice bath for 1.5 h, after which 8.3 g (111 mmol) of glycine and 200 mL of distilled water were introduced. After removing the ice bath, the solution was left to warm to room temperature while being agitated for five hours to keep the pH between 10 and 11. Subsequently, the solution was heated to 110 °C and refluxed for 3–6 h. Following the reaction, the solid product was filtered out and cleaned using 100 mL of a water/acetic acid (30/1 v/v) mixture and 500 mL of pure water. Finally, the product was dried in a vacuum oven at 60 °C to yield the target product with a yield of 93%. 1H NMR (500 MHz, DMSO-d6) δ 12.39 (s, 3H), 7.17–6.62 (m, 3H), 3.83 (d, J = 7.9 Hz, 6H) ppm. 13C NMR (126 MHz, DMSO-d6) δ 172.23, 165.79, 41.92 ppm. MS (ESI): calcd C9H13N6O6 [M + H]+ 301.24; found, 301.90.
2.2.3. Synthesis of Dodec-EG-CC
In a 500 mL round-bottom flask, Gly-CC (3.5 g, 11 mmol), dodecanol (6.7 g, 36 mmol), p-toluenesulfonic acid (6.29 g, 36 mmol), and 200 mL toluene were added. A Dean-Stark apparatus was used to collect the water produced during the reaction after the mixture was heated to reflux at 150 °C for 12 h in a nitrogen environment. Following the reaction, 200 mL of water and 100 mL of ethyl acetate were added to the mixture when it had cooled. The organic layer was separated and dried with anhydrous sodium sulfate. The crude product was then obtained by rotary evaporation, which eliminated the solvent. The product was purified by column chromatography (PE/EA = 3:1) to yield a white solid, with a yield of 65%. 1H NMR (500 MHz, Chloroform-d) δ 5.54 (m, 3H), 4.21–4.01 (m, 12H), 1.63 (p, J = 6.8 Hz, 6H), 1.40–1.18 (m, 54H), 0.87 (t, J = 6.8 Hz, 9H) ppm. 13C NMR (126 MHz, Chloroform-d) δ 170.84, 165.94, 65.53, 42.90, 32.06, 29.78, 29.74, 29.67, 29.50, 29.39, 28.72, 25.98, 22.83, 14.26 ppm. MS (ESI): calcd C45H85N6O6 [M + H]+ 805.65; found, 805.53.
2.3. Tribological Tests
Tribological experiments were done utilizing a ball-on-flat reciprocating tribometer (Bruker, UMT-tribolab, Billerica, MA, USA) in linear reciprocating mode. In this setup, an 8 mm diameter AISI 52100 steel ball moved back and forth against an AISI 304 steel bottom plate over a 10 mm stroke at a frequency of 1 Hz. The ball was subjected to a normal force of 5 N (900 MPa), and the bottom plate was heated to temperatures between 100 and 200 °C. The schematic diagram of the preparation process for the lubricating oil and the friction experiment setup is shown in Scheme 2. Various additives were incorporated into the PAO4 lubricant at concentrations of 0.25, 0.5, 1, 1.5, and 2 wt.%. The physicochemical properties of PAO4 were detailed in our previous article [27]. Tribology tests were conducted at elevated temperatures of 100, 150, and 200 °C. To remove any organic residues from their surfaces, both the substrate and counter-body were sonicated in petroleum ether and ethanol prior to testing. Each friction test was performed at least three times at every temperature to ensure the reliability and consistency of the average friction coefficient.
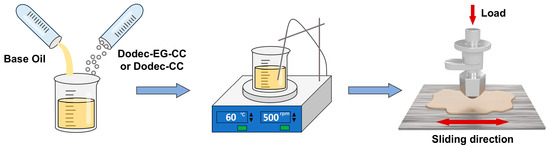
Scheme 2.
Schematic diagram of the friction experiment process.
Accurate determination of the lubrication regime is crucial in tribological research and engineering applications, as it directly affects friction, wear rates, and equipment lifespan. By figuring out the lubricating film thickness to surface roughness ratio (), the system’s lubrication state—whether boundary, mixed, or fluid lubrication—can be identified. This determination provides a basis for selecting lubricants and optimizing operating conditions, thereby enhancing system efficiency and reliability. To determine the lubrication state of a tribological system, the ratio can be calculated. The Hamrock–Dowson equation provides an estimation of the central film thickness , which can be used to identify whether the lubrication regime is in boundary, mixed, or fluid lubrication [28]. The formula for calculating the central lubrication film thickness is shown in Equation (1).
where is the dynamic viscosity of the lubricant (Pa·s); is the relative sliding speed (m/s); is the effective elastic modulus (Pa); is the pressure–viscosity coefficient of the lubricant (Pa−1); is the normal load (N); is the equivalent radius (m); and is the ellipticity parameter. The parameters of the upper and lower friction pairs used in this study can be found in our previous research [29]. The lubrication regime is given by , defined by Equation (2).
where is the composite surface roughness, which is defined as , where and are the roughnesses of the friction surfaces. Specifically, < 1, the system is in the boundary lubrication regime, where the lubrication film thickness is insufficient, and direct contact between surfaces may occur; 1 ≤ λ ≤ 3, the system is in the mixed lubrication regime, where the lubrication film partially separates the contact surfaces, with some areas of direct contact; > 3, the system is in the fluid lubrication regime, where the lubrication film fully separates the contact surfaces, minimizing friction and wear. The at 100 °C is calculated to be 18 nm, with the value of 0.283, which is less than 1. Under these experimental conditions, the system operates in a boundary lubrication regime. This indicates that the oil film is too thin to fully separate the contact surfaces, potentially leading to direct contact between the surfaces, which could increase friction and wear.
According to Aachard’s wear law, the wear rate is calculated using the following equation [30]:
where is the wear volume (μm3), F is the normal load (N), and is the sliding distance (mm). This equation allows us to estimate the wear volume under specific experimental conditions, thereby helping to assess the wear performance of the material.
2.4. Simulation Method and Conditions
Using the free and open-source molecular simulation software LAMMPS (v.2Aug2023), molecular dynamics (MD) simulations were performed to study the adsorption behavior of organic friction modifiers (OFMs) on metal surfaces [31]. A molecular dynamics model for the single-molecule adsorption process was constructed. In this single-molecule simulation system, a five-layer iron (Fe 110) structure was used as the adsorption surface to investigate the adsorption energy differences of triazine-based OFMs on its surface. The simulation model of the adsorption process consists of a single molecule at the metal interface, which is then structurally optimized to calculate its adsorption energy. Intermolecular interactions are described using the Consistent Valence Force Field (cvff), while metal iron atoms are modeled using the Embedded Atom Method (EAM) force field. The simulation used a time step of 0.001 ps, and the system was simulated for 50,000 steps (0.05 ns) in a typical NVT ensemble at 398K. The absorbed energy was calculated as follows:
where is the potential energy of the simulated system, representing the energy of the entire assembly, and and represent the potential energy of the iron surface and molecules, which are the energies of the Fe layer and the lubricant, respectively. Visualisation was performed using the OVITO software (v3.8.4).
2.5. Characterization
We employed various analytical techniques to comprehensively characterize the structure and tribological properties of the compounds. The specific methods used are as follows:
Fourier Transform Infrared Spectroscopy (FT-IR): using the PerkinElmer Frontier instrument in the 500–4000 cm−1 range to analyze the compounds and identify their functional groups. Nuclear Magnetic Resonance Spectroscopy (NMR): using a Bruker ADVANCE III HD spectrometer, we obtained hydrogen (1H) spectra at 500 MHz and carbon (13C) spectra at 126 MHz to determine the structural characteristics of the compounds. Dodec-CC and Dodec-EG-CC were dissolved in deuterated chloroform (CDCl3) as a solvent, while tetramethylsilane (TMS) served as the internal standard; CC-Gly was dissolved in deuterated dimethyl sulfoxide (DMSO-d6). Mass Spectrometry (MS): relative molecular mass information was obtained using an Agilent 6230-TOF mass spectrometer (Agilent, Santa Clara, CA, USA). Contact angle (CA): the adsorption behavior of additive molecules on the metal surface was analyzed using the DSA100 contact angle meter from Kruss, Hamburg, Germany. Thermogravimetric analysis (TGA): TGA was performed using a TGA 55 unit (TA Instruments, New Castle, DE, USA) under a nitrogen atmosphere. The temperature was increased at a rate of 10 °C/min up to 600 °C, followed by an isothermal hold for 30 min. Non-Contact Optical 3D Surface Profilometry: using the Bruker ContourGT-K profilometer, we measured the width and depth of wear scars after friction testing. Scanning Electron Microscopy with Energy Dispersive Spectroscopy (SEM-EDS): a ZEISS Sigma (Oberkochen, Germany) 300 SEM-EDS system provided images of the wear surface morphology and elemental distribution. Raman Spectroscopy: a Thermo Fisher (Waltham, MA, USA) DXR2xi Raman spectrometer was used to scan the wear scar surfaces in the 50–3400 cm−1 range with a 532 nm laser, allowing for identification of the chemical composition present on the wear surfaces after friction testing. X-ray Photoelectron Spectroscopy (XPS): using the Thermo Scientific Escalab 250xi instrument, we analyzed the chemical states of elements present on the wear surfaces.
3. Results and Discussion
3.1. Characterization of the Synthesized Triazine Derivatives
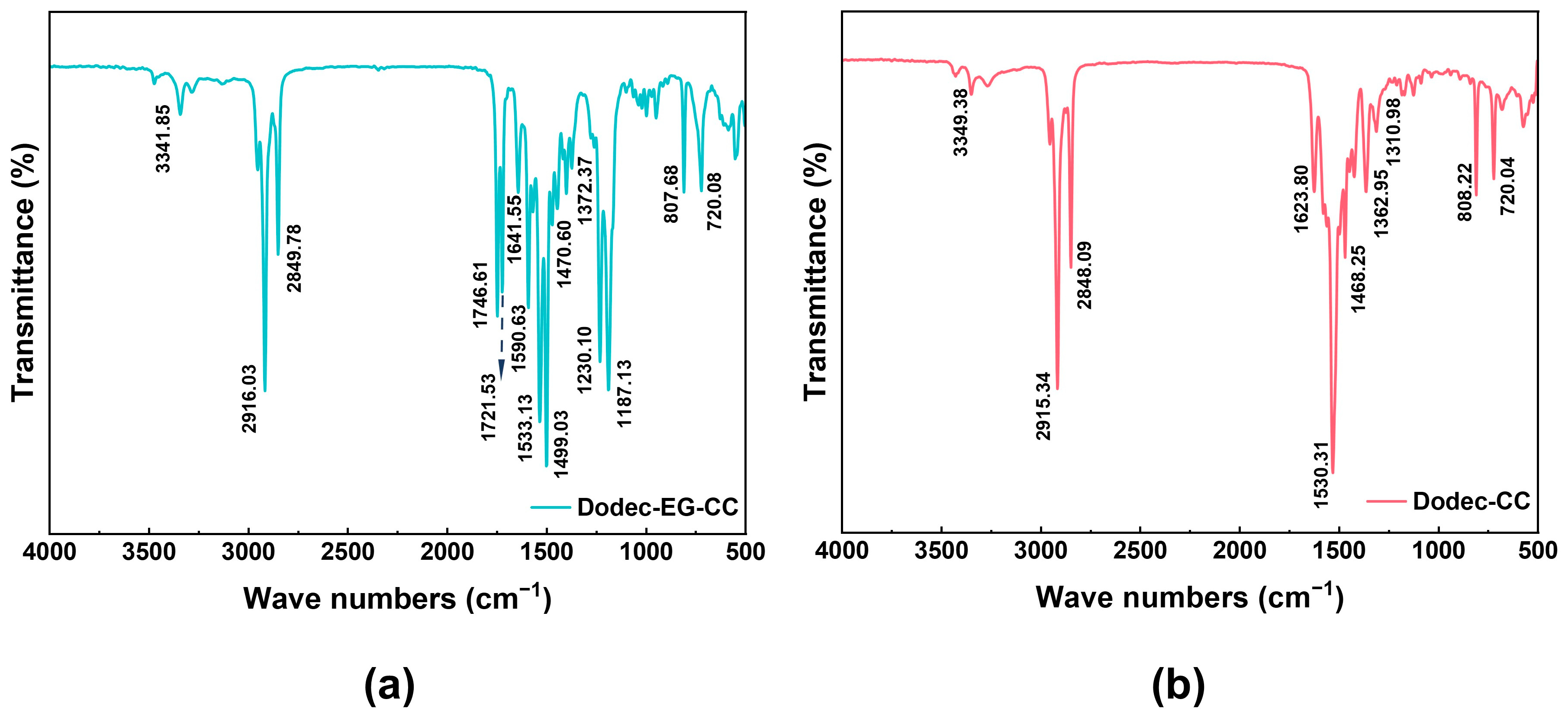
Figure 1 and Figure S3 show the FT-IR of Dodec-EG-CC, Dodec-CC, and intermediate compound CC-Gly. Table 1 lists the infrared absorption peaks of Dodec-EG-CC and Dodec-CC and their corresponding functional group information. In the infrared spectrum of Figure 1a, the peak near 3341.85 cm−1 is the N-H stretching vibration of the -NH group. The peaks at 2916.03 cm−1 and 2849.78 cm−1 correspond to the C-H stretching vibration of the -CH3 and -CH2 groups, respectively. The absorption peaks near 1746.61 cm−1 and 1721.53 cm−1 are the stretching vibrations of the ester group C=O. The absorption peak at 1590.63 cm−1 is due to the bending vibration of N-H. The characteristic peaks at 1533.13 cm−1, 1499.03 cm−1, and 807.68 cm−1 indicate the presence of triazine rings, while the absorption peaks at 1470.60 cm−1 and 1372.37 cm−1 represent the bending vibrations of -CH2 and -CH3 groups. The stretching vibration peaks of the ester group C-O and O-C=O appear at 1230.10 cm−1 and 1187.13 cm−1, respectively. The absorption peak of 720.08 cm−1 corresponds to the in-plane swing vibration of the long-chain methylene group, which supports the introduction of the long-chain structure. In the infrared spectrum of CC-Gly (Figure S3), a broad peak appeared near 3300 cm−1, representing the O-H stretching vibration of the -COOH group. However, in the spectrum of Dodec-EG-CC (Figure 1a), this peak disappeared, indicating that the -COOH group was converted during the reaction. This change, along with the appearance of other characteristic absorption peaks and the NMR and mass spectrometry data shown in Figures S1 and S2, indicates that the synthesis of Dodec-EG-CC was successful. Comparing the molecular structures of Dodec-CC and Dodec-EG-CC, they only differ in the ester group. Figure 1b and Table 1 show that the infrared absorption peaks of Dodec-CC and Dodec-EG-CC differ only in the absorption peak of the ester group. At the same time, combined with the data from Figures S1 and S2, it indicates that Dodec-CC was also successfully synthesized.
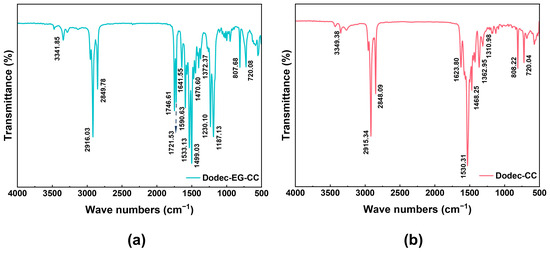
Figure 1.
The FT-IR spectra of (a) Dodec-EG-CC and (b) Dodec-CC.

Table 1.
The infrared data of Dodec-EG-CC and Dodec-CC, along with their corresponding functional group.
3.2. Analysis of Thermal Stability
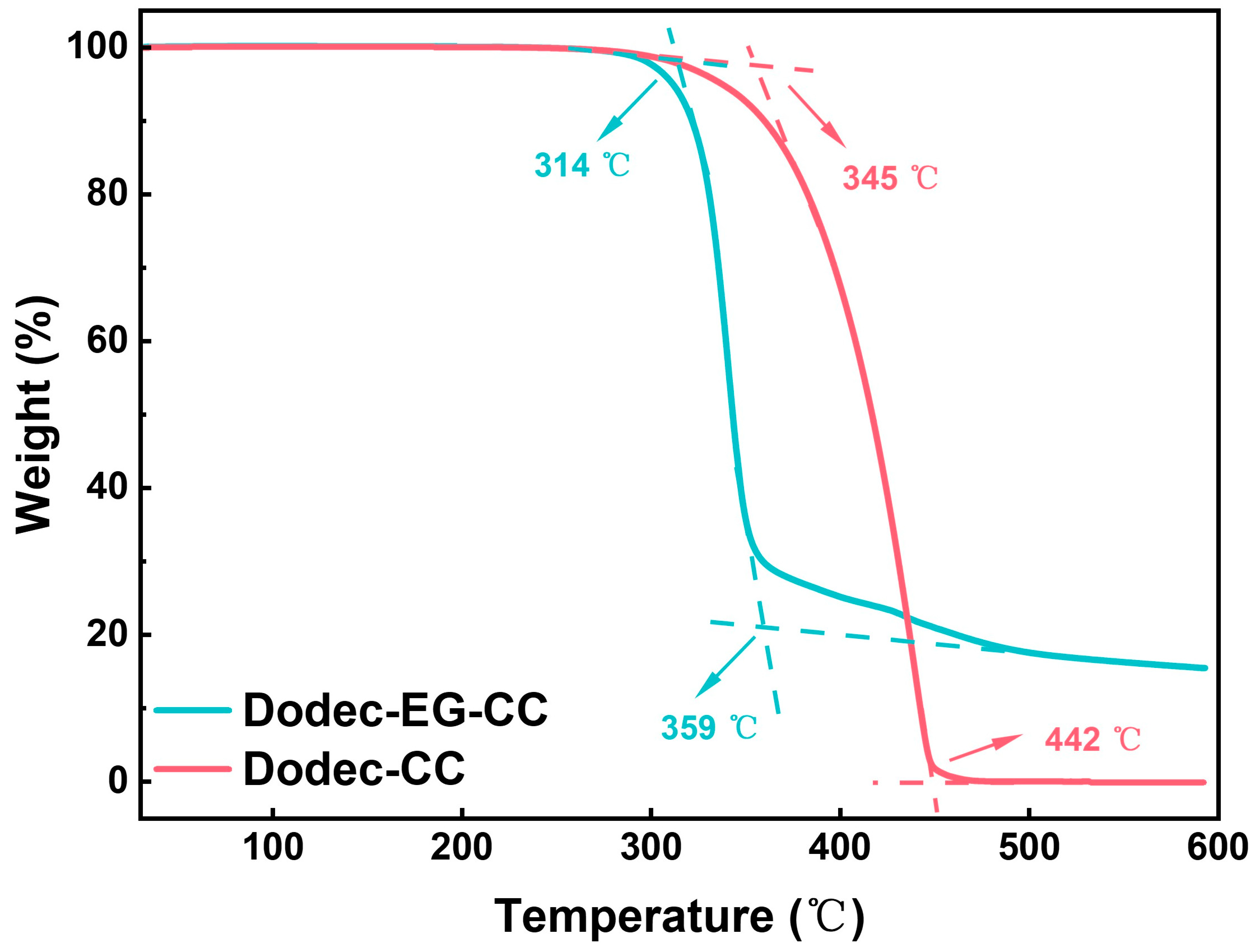
In the high-temperature and high-load operating environments of modern engines, the thermal stability of lubricating additives is critical to ensuring their effectiveness under elevated temperatures. TGA testing provides a direct insight into the thermal decomposition characteristics of additives, which is essential for evaluating their ability to perform under extreme working conditions. As shown in Figure 2, the initial decomposition temperature of Dodec-EG-CC is approximately 314 °C, with rapid decomposition occurring between 314 °C and 359 °C. Additionally, it leaves a considerable amount of residue at 600 °C. In contrast, Dodec-CC starts decomposing at around 345 °C, with rapid decomposition observed between 345 °C and 442 °C. Both synthesized additives exhibit initial decomposition temperatures exceeding 310 °C, demonstrating excellent thermal stability to meet the stringent high-temperature requirements of most modern automotive engines.
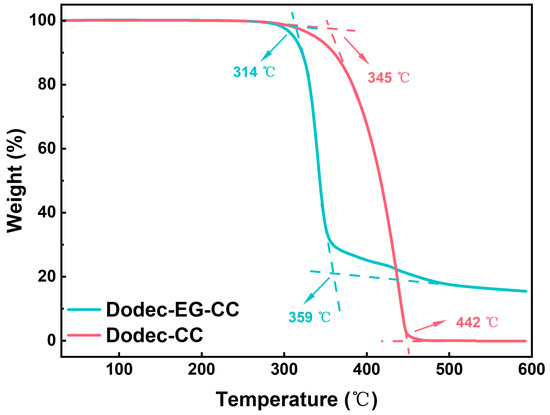
Figure 2.
TGA data of Dodec-EG-CC and Dodec-CC.
3.3. Analysis of Adsorption Behavior
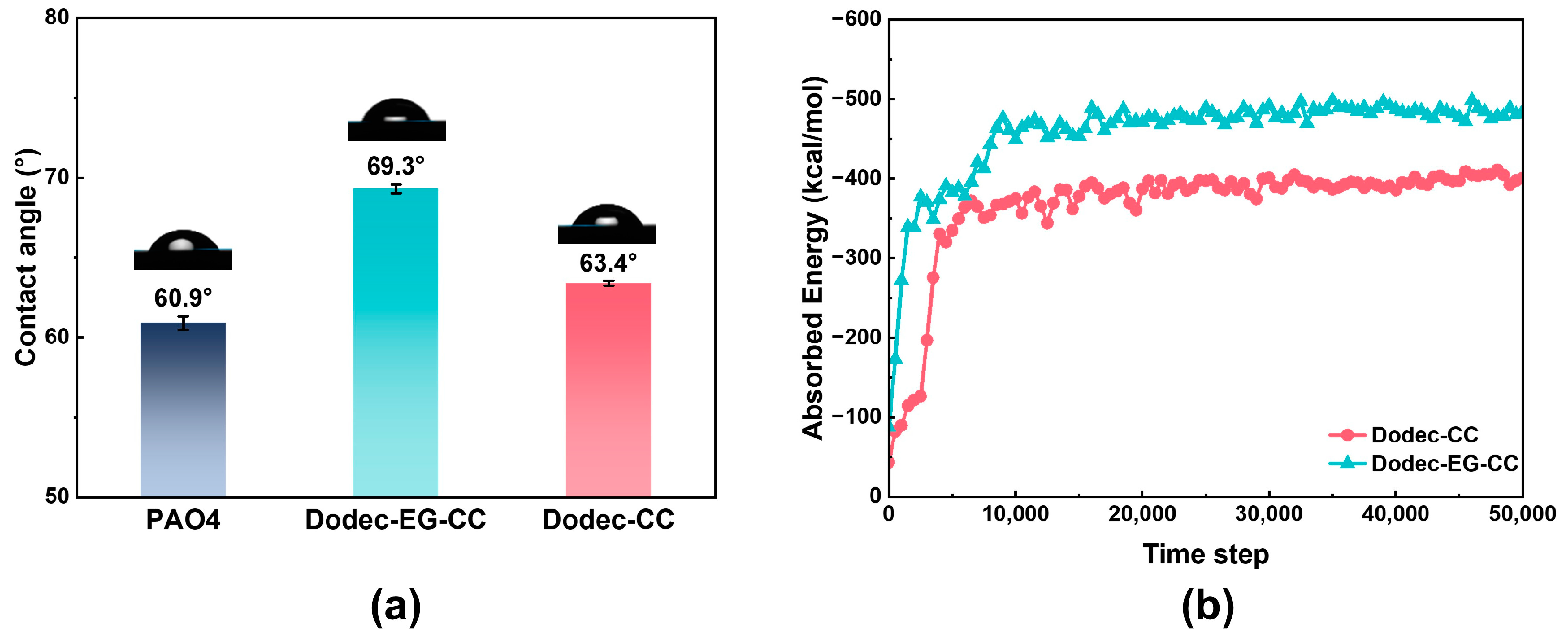
One important element influencing how well OFMs work is the additive’s capacity to adsorb onto metal surfaces. The adsorption behavior of additive molecules on metal surfaces was investigated through both experimental and computational analyses. As shown in Figure 3a, the water contact angle was measured to assess the adsorption properties of the additive molecules on the metal surface. To prepare the samples for contact angle measurement, AISI 304 stainless steel was immersed for 30 min in base oil at 100 °C, either with or without triazine-based OFMs. The substrate surface was then rinsed with toluene and dried with nitrogen. The experimental results showed that the addition of triazine-based OFMs altered the wettability of the metal surface, indicating that these additives can adsorb onto the metal surface and modify its chemical environment. Analysis of the water contact angle data revealed that Dodec-EG-CC caused a significantly greater change in the wettability of the metal surface compared to Dodec-CC. This suggests that Dodec-EG-CC has a more pronounced effect on the metal surface and can more effectively alter its physicochemical properties.
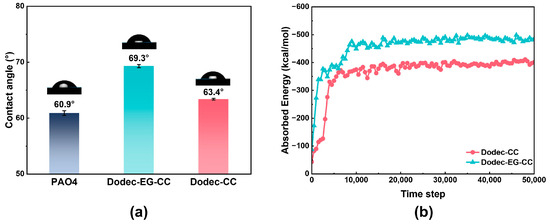
Figure 3.
(a) Comparison of contact angle of steel plate surfaces after oil impregnation treatment at 100 °C; (b) adsorption energy of Dodec-EG-CC and Dodec-CC obtained from molecular dynamics (MD) simulations at 100 °C.
Furthermore, molecular dynamics simulations were utilized to assess the adsorption energies of the two additive compounds at 100 °C, as illustrated in Figure 3b. The results indicate that after the ester group is added, the adsorption energy of Dodec-EG-CC is significantly higher than that of Dodec-CC.
From the perspective of the additive molecule adsorption behavior, Dodec-EG-CC forms a more stable adsorption layer on the metal surface, which enhances its effectiveness and stability in the lubrication process.
3.4. Tribological Properties Analysis
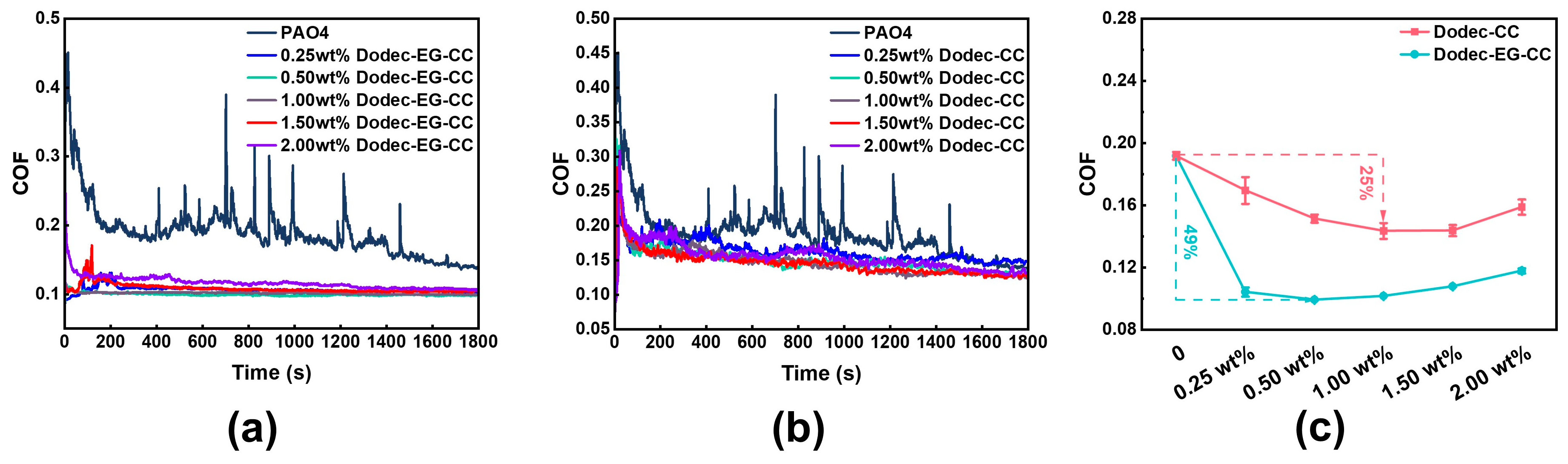
Using UMT reciprocating friction tests, the impact of additive concentration on tribological performance was investigated. The experimental results indicate that both ester-functionalized Dodec-EG-CC and non-ester Dodec-CC significantly improved the friction-reducing performance of the base oil, with the effect closely related to concentration, as shown in Figure 4. Overall, with the increase in additive concentration, the tribological performance of the lubricant exhibited a “first increase, then decrease” trend. Specifically, Dodec-EG-CC achieved the best friction reduction performance at a concentration of 0.5 wt.%, with the average friction coefficient reduced by approximately 49% compared to the pure base oil. In contrast, Dodec-CC performed best at a concentration of 1 wt.%, with the friction coefficient reduced by about 25%. These results suggest that the introduction of the ester group allows Dodec-EG-CC to form a complete and dense lubricating film at lower concentrations through polar adsorption, while Dodec-CC requires a higher concentration to achieve a similar effect. Further analysis revealed that, in the tribological tests, the friction coefficient curve of Dodec-EG-CC was more stable, reflecting better repeatability and stability. This indicates that the lubricating film formed by Dodec-EG-CC is not only denser, but also exhibits excellent dynamic self-repairing ability. When the lubricating film experiences local damage or detachment, Dodec-EG-CC molecules can quickly repair the film through physical or chemical adsorption, thereby maintaining the stability of the friction interface. The key advantage of Dodec-EG-CC over Dodec-CC lies in the ester groups within its structure, which demonstrate superior chemical reactivity [32]. This functional group can strongly physically adsorb and chemically bond with the metal surface, forming a robust lubricating layer [4,33,34]. This interaction further enhances the lubricating film’s shear resistance and structural stability, thereby improving its tribological performance.
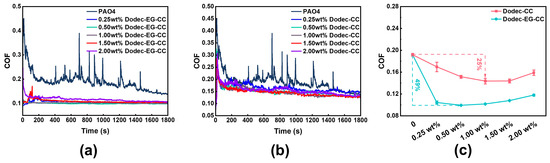
Figure 4.
Friction coefficient of (a) Dodec-EG-CC and (b) Dodec-CC at different mass fractions at 100 °C, and (c) average friction coefficient of the triazine-based OFMs.
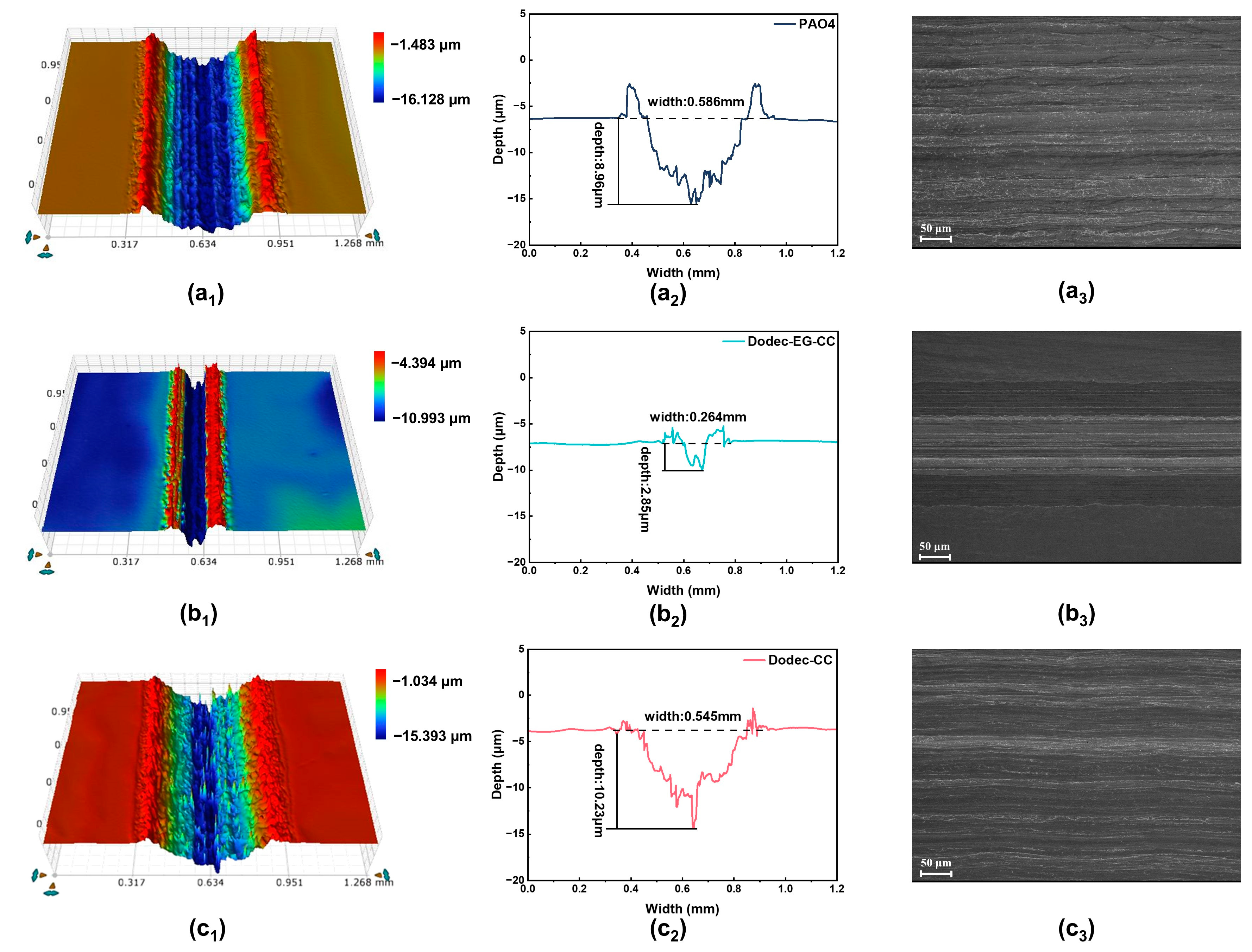
To further verify the lubrication performance and mechanisms of Dodec-EG-CC and Dodec-CC, white light interferometry and SEM were used to quantitatively characterize the surface morphology of the friction surfaces (Figure 5 and Table 2). The results showed that, under base oil lubrication, the wear scar depth reached 8.96 μm with a width of 0.586 mm, while the wear rate (.03 × 105 μm3/(N·mm)) was the highest. Surface roughness data further indicated a significant increase in surface roughness after friction, suggesting that base oil, without additives, has limited lubrication effectiveness and fails to effectively reduce surface wear. In contrast, Dodec-EG-CC exhibited significantly better performance under the same conditions, with a wear scar depth of only 2.85 μm and a width of 0.264 mm. The wear rate decreased markedly to 4.93 × 104 μm3/(N·mm). Additionally, surface roughness data revealed a noticeable reduction in surface damage and improved smoothness of the metal surface after lubrication with Dodec-EG-CC. This indicates that Dodec-EG-CC can effectively reduce wear and form a compact lubrication film, thereby greatly enhancing its friction-reducing performance. These properties are closely associated with the presence of ester groups and triazine rings in its molecular structure, which enhance adsorption onto the metal surface and facilitate the formation of a stable and dense lubrication film, reducing wear. However, the performance of Dodec-CC was inferior to that of Dodec-EG-CC, with a wear scar depth of 10.23 μm, a width of 0.545 mm, and a wear rate of 5.94 × 105 μm3/(N·mm). Although Dodec-CC outperformed the base oil to some extent, surface roughness data indicated that the friction surface remained relatively rough, with significant wear still present. The insufficient stability of its lubrication film may be attributed to the lack of ester groups in its molecular structure. Although Dodec-CC contains a triazine ring with polar functional groups, it fails to form a sufficiently stable and dense lubrication film, resulting in limited friction-reducing and anti-wear performance. While Dodec-CC demonstrates better performance than the base oil, its properties remain significantly inferior to those of Dodec-EG-CC. In summary, the results of white light interferometry are consistent with previous tribological performance tests, further confirming the excellent friction-reducing and anti-wear properties of Dodec-EG-CC.
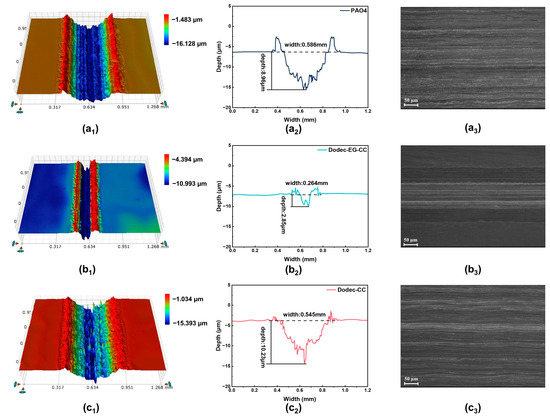
Figure 5.
Surface morphology of steel wear scars after friction tests at 100 °C: (a1,a2): PAO4; (b1,b2): Dodec-EG-CC; (c1,c2): Dodec-CC; SEM images: (a3): PAO4; (b3): Dodec-EG-CC; (c3): Dodec-CC.

Table 2.
Wear rate and surface roughness results for different additives at 100 °C.
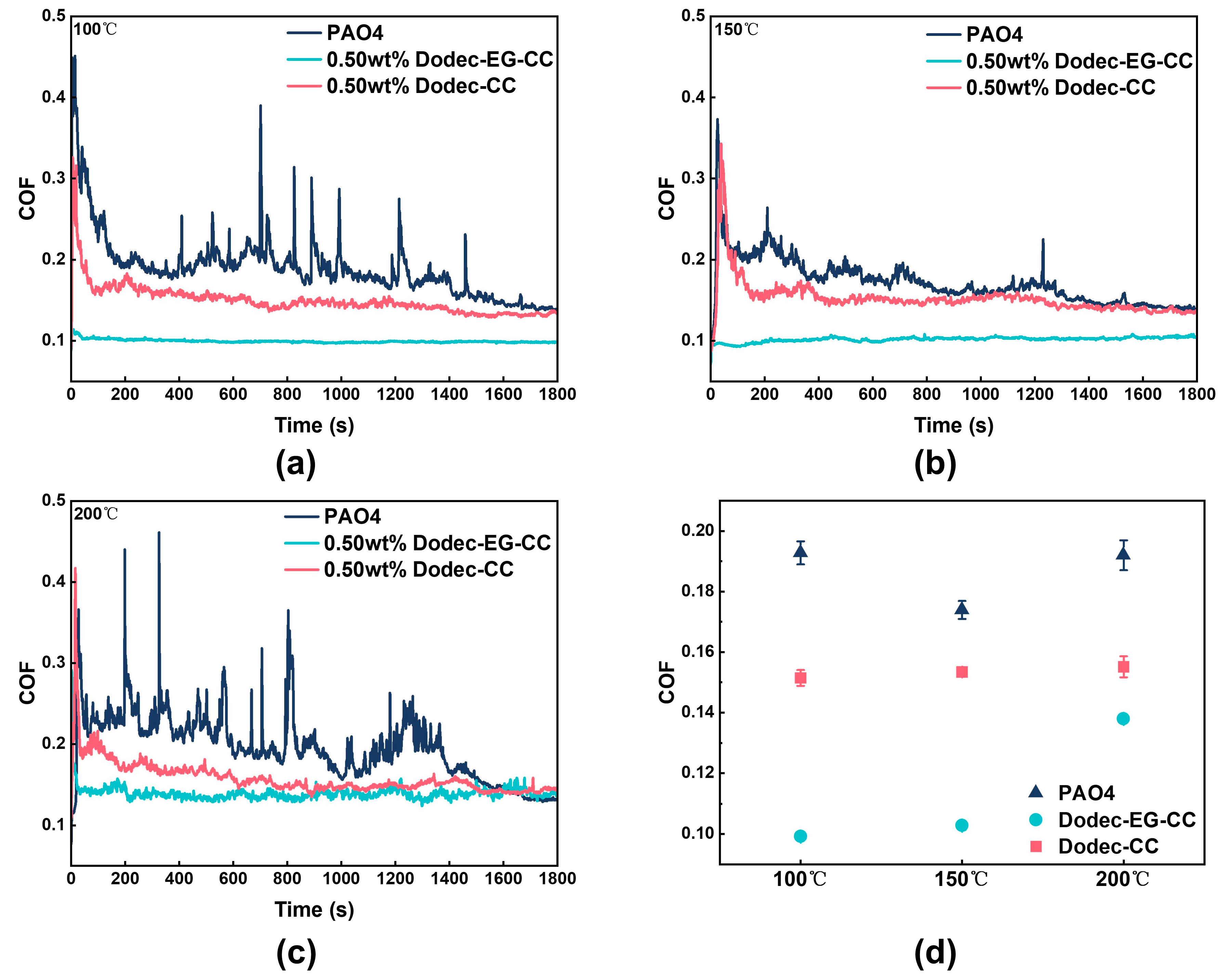
Friction tests were conducted at an additional concentration of 0.5 wt.% at temperatures ranging from 100 to 200 °C in order to better examine the tribological performance of triazine-based OFMs at elevated temperatures. The results showed that the synthesized triazine-based OFMs could still significantly improve the friction-reducing performance of the base oil under high-temperature conditions, as shown in Figure 6. In detail, the friction coefficient of the PAO4 base oil fluctuated sharply, indicating that it is difficult to form a stable lubricating film at elevated temperatures.
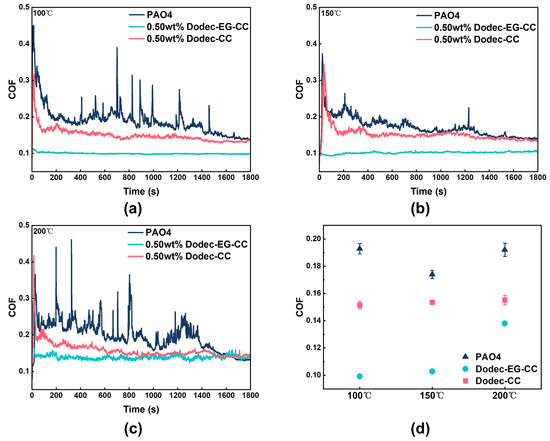
Figure 6.
The real-time friction coefficient of melamine long-chain alcohol ester at different temperatures: (a) 100 °C, (b) 150 °C, (c) 200 °C; (d) average friction coefficients.
At 200 °C, the average friction coefficient was reduced by up to 28%. Dodec-EG-CC particularly demonstrated excellent tribological performance, with the friction coefficient significantly lower than that of the base oil PAO4 and Dodec-CC, exhibiting superior stability and friction reduction performance. When a certain amount of Dodec-CC was added to the base oil, both the fluctuation of the friction coefficient and the friction coefficient itself were improved to a certain extent. However, they were still inferior to those of Dodec-EG-CC. As can be seen from the comparison of the friction coefficients of Figure 6a,b, the friction coefficient curve of Dodec-EG-CC remained steady and at a lower level. Even if the temperature continues to rise to 200 °C, the coefficient of friction remained low relative to the base oil, indicating its ability to effectively form a stable lubricating protective film at elevated temperatures (Figure 6c). The excellent friction-reducing performance can be attributed to the long-chain ester-functionalized structure introduced into the molecular design, which enhanced the interaction between the molecules and the metal surface, further accelerating the formation of the lubricating film and improving its durability. The synthesized compound Dodec-CC in this study exhibits a structure similar to the triazine derivatives reported by Desanker et al. [20]. However, comparative tribological performance results demonstrate that Dodec-EG-CC significantly outperforms Dodec-CC. This indicates that the organic friction modifiers synthesized in this study exhibit outstanding performance advantages under high-temperature boundary lubrication conditions. This result provides new theoretical support for the design of high-temperature friction modifiers and confirms the potential application of Dodec-EG-CC as an efficient friction modifier in high-temperature lubrication systems.
3.5. Analysis of Wear Surfaces
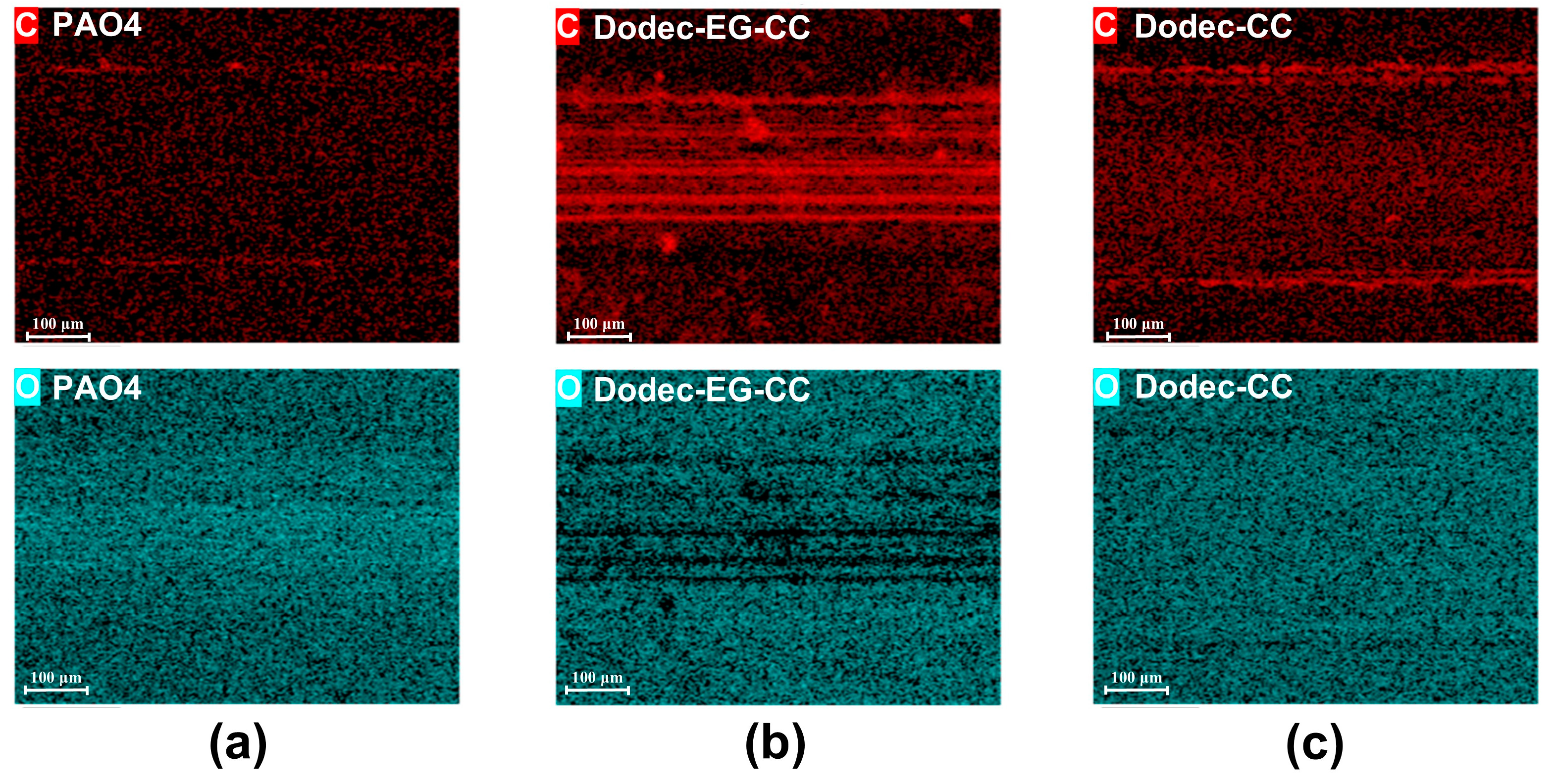
To reveal the lubrication mechanism of melamine long-chain alcohol ester derivatives, this study analyzes the friction surface using EDS mapping. As shown in Figure 7 and Figure S4, significant carbon enrichment appears on the friction interface when Dodec-EG-CC is used as an OFM. This indicates that Dodec-EG-CC molecules form a carbon-based protective film on the metal substrate during the friction process. This film effectively passivates the metal surface through a physical barrier effect, significantly reducing direct contact and adhesive wear between the friction pairs. In contrast, the carbon enrichment on the lubricated surface of Dodec-CC is notably weaker, as evidenced by the lower carbon concentration detected by EDS. This weaker enrichment fails to effectively passivate the metal surface, resulting in severe wear. Additionally, no significant carbon enrichment was observed in the base oil system, suggesting that the base oil itself does not have the ability to form such an obvious carbon-based film. This finding highlights the importance of the additive’s chemical structure in facilitating the formation of protective films and improving lubrication performance. To further analyze the chemical composition and structural characteristics of the carbon film, Raman spectroscopy and XPS analyses were performed on the wear tracks.
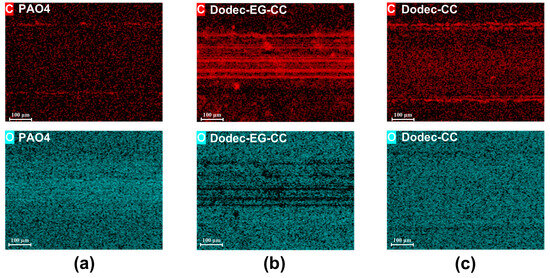
Figure 7.
EDS-mapping of steel wear scars after friction tests at 100 °C: (a): PAO4; (b): Dodec-EG-CC; (c): Dodec-CC.
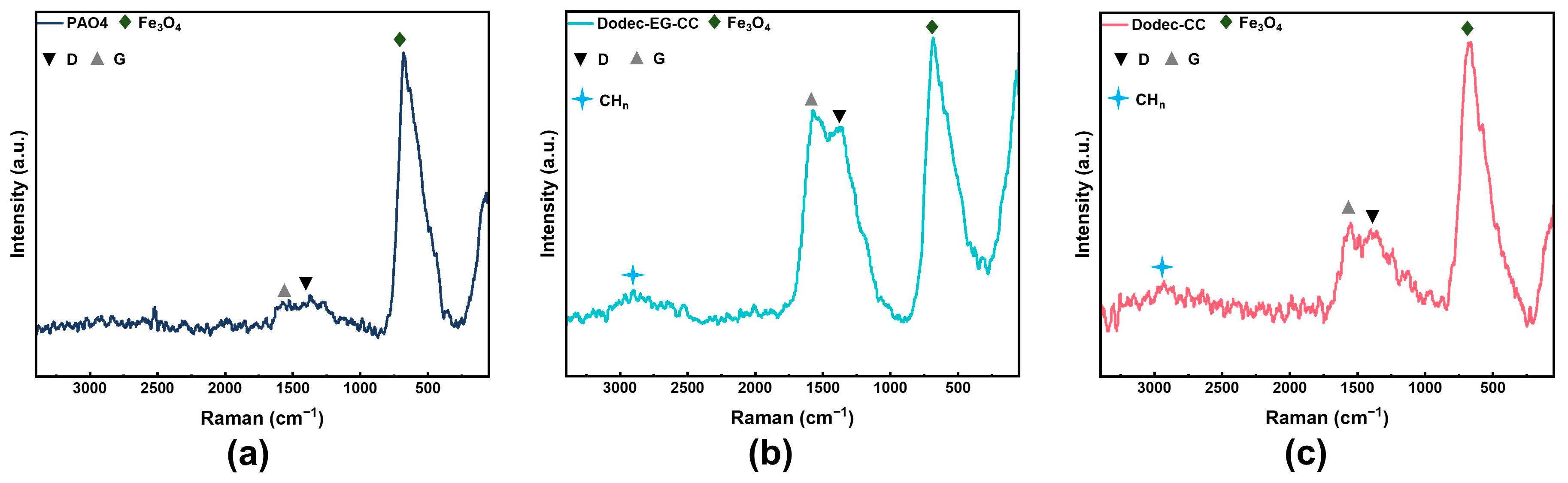
Raman spectroscopy was performed on the worn tracks following friction in order to examine the makeup of the protective layer that developed on the metal surface following friction, as illustrated in Figure 8. The magnetite characteristic peak in the spectrum, located close to 680 cm−1, shows that metal oxides have formed on the metal surface [3]. Notably, the D peak at 1350 cm−1 reflects the amorphous carbon structure in carbon-based materials, while the G peak at 1580 cm−1 corresponds to the stretching vibration of all sp2 hybridized carbon atoms [35,36]. The presence of these peaks suggests the existence of carbon-based materials on the metal surface. The peak around 2900 cm−1 is associated with the vibrations of -CHx (x = 1/2/3) groups in alkyl chains [37]. From the comparison of Figure 8a–c, it can be observed that the addition of triazine-based OFMs results in the appearance of significant alkyl vibration peaks on the frictional surface, indicating that the triazine derivative molecules can effectively adsorb onto the metal surface to form an adsorption protective film. Additionally, after adding OFMs, the relative intensity of the D or G peak increases significantly. This suggests that under high-temperature and high-pressure conditions during friction, the additives may decompose and further undergo tribochemical reaction to form carbon-based materials, which is consistent with the EDS-mapping analysis. The intensity ratio of the D and G peaks (ID/IG) further reflects the structural characteristics of the carbon film on the metal surface [38]. For the metal surface lubricated with Dodec-EG-CC, the ID/IG ratio is about 0.92, while for the metal surface lubricated with Dodec-CC, the ID/IG ratio is about 0.94 [39]. This suggests that the carbon film formed on the metal surface is amorphous carbon material with a higher level of sp2 bonding carbon [36,40].
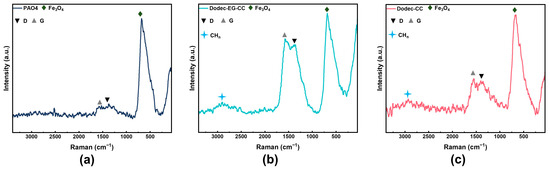
Figure 8.
Raman spectra of wear surface lubricated by (a) PAO4, (b) 0.5 wt.% Dodec-EG-CC in PAO4, and (c) 0.5 wt.% Dodec-CC in PAO4.
Further comparison indicates that the relative intensity of the D or G peak is significantly higher on the surface lubricated with Dodec-EG-CC compared to the surface lubricated with Dodec-CC. This indicates that a greater amount of carbon-based materials is formed on the surface lubricated with Dodec-EG-CC. The enhanced formation of carbon-based materials can be attributed to the presence of the ester group in its molecular structure. The ester group provides additional adsorption sites, allowing Dodec-EG-CC molecules to form stronger physical and chemical bonds with the metal surface. During the friction process, these bonds facilitate the formation of a robust carbon-film. Furthermore, the ester group in Dodec-EG-CC is more prone to decomposition under high-temperature and high-pressure conditions, leading to the generation of carbon-based materials. This process not only enhances the film’s ability to passivate the metal surface, but also improves its tribological performance by providing a protective barrier that reduces direct metal-to-metal contact. In contrast, Dodec-CC, which lacks the ester group, exhibits weaker adsorption and film-forming ability. As a result, it forms fewer carbon-based materials and a less effective protective film, leading to inferior lubrication performance compared to Dodec-EG-CC.
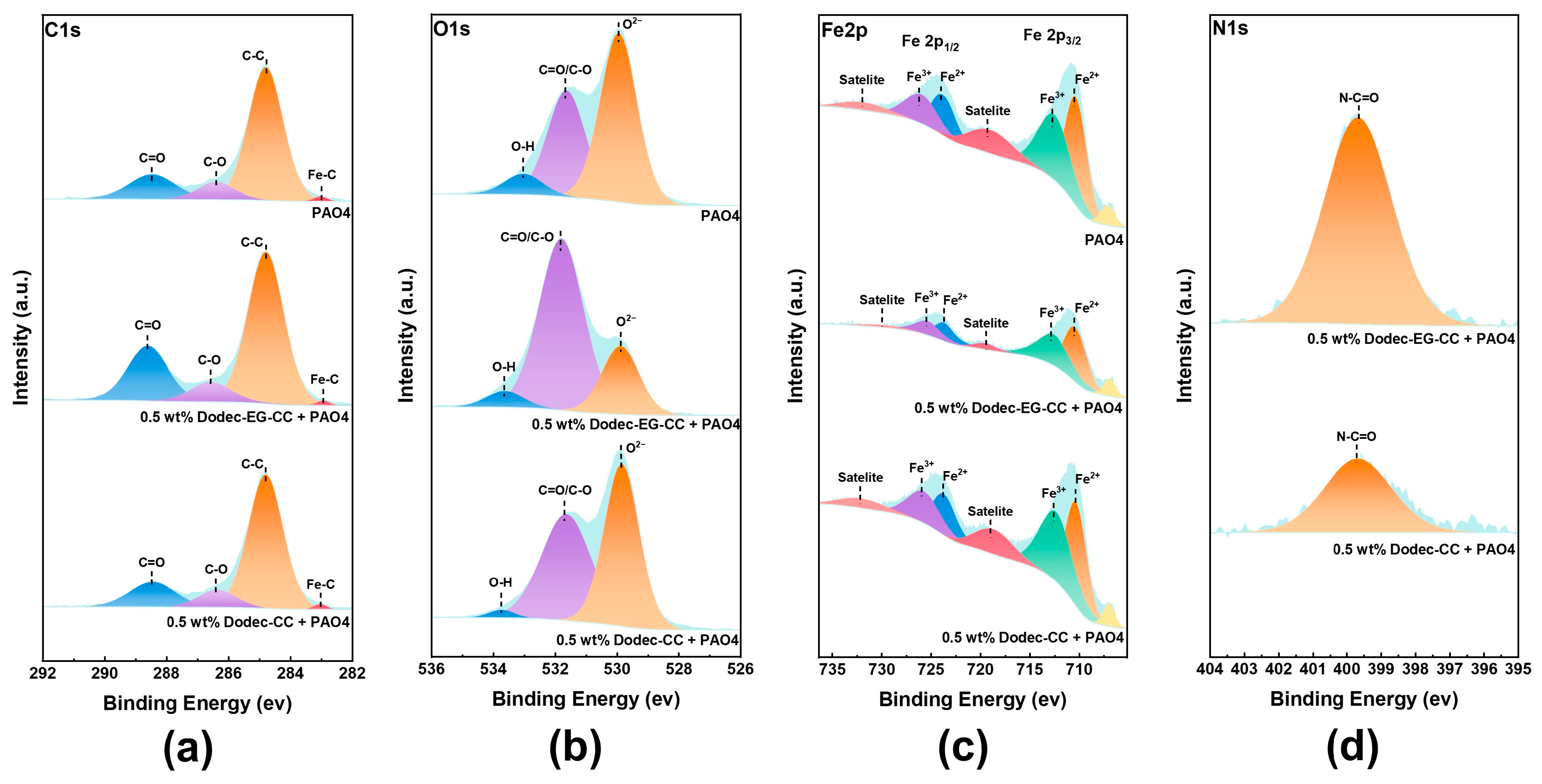
To further investigate the tribological mechanism of triazine-based OFMs, XPS was utilized to study the chemical composition of the friction surface. Based on the XPS results shown in Figure 9 and Figure S5, the lubricating films are composed of similar chemical substances, primarily carbon, oxygen, and metal oxides. This suggests that the base oil’s lubricating mechanism was not significantly changed by the addition of the additives. The differences in peak intensity among the samples reveal the strength of the lubricating films they form and their varying ability to protect the metal surface. As shown in Figure 9a, the C1s spectrum mainly consists of three peaks at 284.8 eV (C-C), 286 eV (C-O), and 288.4 eV (C=O) [3]. However, a more pronounced C=O peak was observed on the friction surface lubricated with Dodec-EG-CC. Similarly, the O1s spectrum also showed that the peak intensity of C-O/C=O (531.6 eV) on the Dodec-EG-CC lubricated friction surface was significantly higher than that of PAO4 and Dodec-CC, while the peak intensity of O2− (531.6 eV) was markedly lower [41]. C1s and O1s spectral analysis shows that Dodec-EG-CC is more likely to form a stable lubricating protective film on the metal surface, which is consistent with the Raman test results. The ester group in Dodec-EG-CC may have facilitated this phenomenon. Furthermore, the Fe2p spectrum showed that the peaks representing metal oxides (Fe2p1/2 and Fe2p3/2) were notably lower on the surface lubricated with Dodec-EG-CC. This is consistent with the relative weakening of the iron oxide characteristic peaks on the metal surface in the Raman spectrum, also lubricated with Dodec-EG-CC. These observations indicate that Dodec-EG-CC provides better protection to the metal surface and inhibits the formation of metal oxides compared to Dodec-CC. Additionally, the peak intensity representing C-N=O [5] in the N1s spectrum was significantly higher on the friction surface lubricated with Dodec-EG-CC. This suggests that the triazine ring structure in Dodec-EG-CC interacts more readily with the metal surface during lubrication, promoting the formation of the lubricating film and further passivating the metal surface. Compared to Dodec-CC, the ester group in Dodec-EG-CC significantly affects the lubrication mechanism of the triazine-based OFMs.
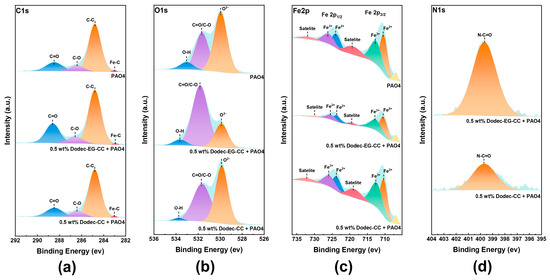
Figure 9.
XPS spectral comparison of lubricated wear surfaces: (a) C1s, (b) O1s, (c) Fe2p, (d) N1s.
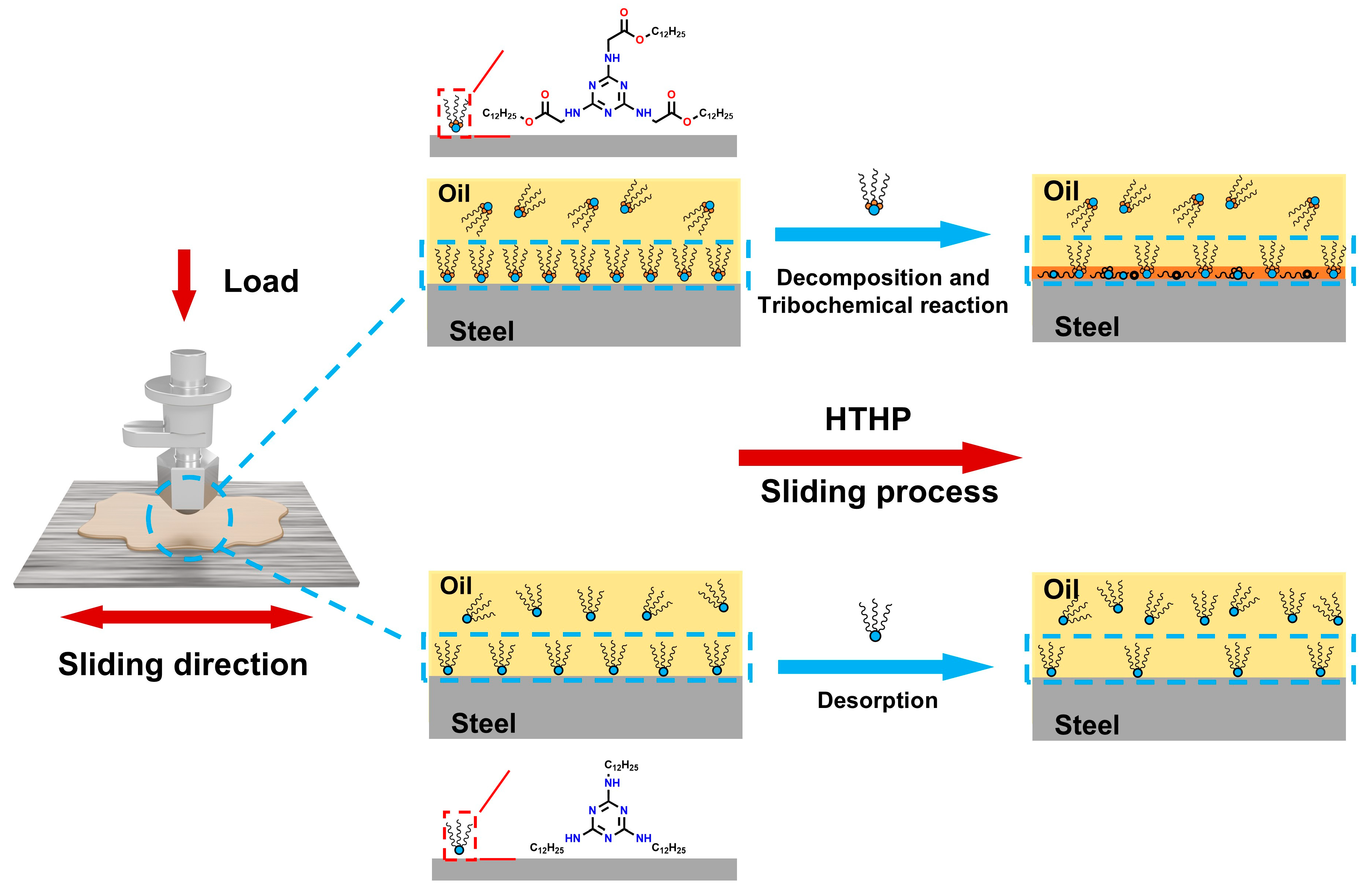
3.6. Mechanism Hypothesis
Based on the experimental and characterization results mentioned above, we hypothesize the lubrication mechanism of melamine long-chain alcohol ester as an organic friction modifier, as shown in Figure 10. First, due to the adsorption sites of the triazine ring and ester group, the additive molecules can form hydrogen bonds, coordinate bonds, or van der Waals forces with the metal surface, promoting their adsorption and replacement of the weakly adsorbed base oil film [15,42]. This adsorption film acts as a barrier, passivating the metal surface and effectively preventing direct contact between the metal surfaces. During the formation of this adsorption film, the additional ester group in Dodec-EG-CC may provide an extra adsorption site, which enhances its interaction with the metal surface through additional van der Waals forces or electrostatic forces, leading to a denser and more stable adsorption film compared to Dodec-CC. During further friction processes, harsh environmental factors such as high temperature and pressure may cause the additive molecules to desorb, decompose, and undergo tribochemical reactions, leading to the formation of carbon-based materials [20,35]. Dodec-CC, with its weaker adsorption ability, tends to desorb at elevated temperatures, leading to a more chaotic and disordered adsorption film. Consequently, Dodec-CC detaches from the friction surface and does not participate in tribochemical reactions. In contrast, Dodec-EG-CC, with its excellent adsorption performance on the metal surface, firmly adheres to the surface. Under high temperature and pressure, Dodec-EG-CC may decompose near the ester group under high temperature and further undergo tribochemical reactions, forming a highly disordered carbon film [33,34]. This carbon film fills the metal surface, further passivating it, inhibiting the formation of metal oxides, and displaying superior lubrication performance.
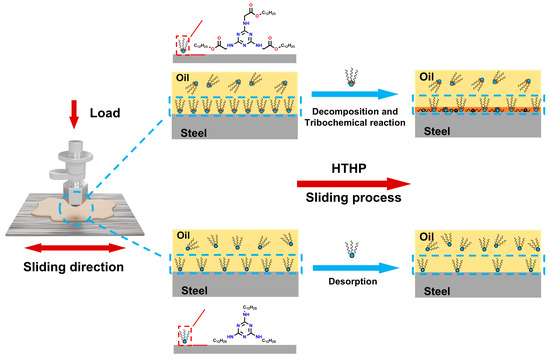
Figure 10.
Schematic illustration of the lubrication mechanism of Dodec-EG-CC and Dodec-CC.
4. Conclusions
Based on the above, we synthesized two novel ash-free OFMs with a triazine ring as the polar core, which serve as environmentally friendly additives to reduce boundary lubrication under high-temperature conditions. This study also investigated the structural effects on the tribological performance of the additive molecules, revealing the mechanisms of action of the synthesized additives at elevated temperatures. The main conclusions are as follows:
- Two triazine derivatives were synthesized using a simple and efficient synthetic method, and their structures were confirmed by NMR, IR, and MS characterization techniques. Thermogravimetric analysis indicated that the synthesized compounds exhibit excellent thermal stability, with initial decomposition temperatures exceeding 310 °C. Such outstanding thermal stability is a key performance characteristic of high-temperature lubricant additives.
- Adsorption behavior analysis showed that both triazine derivatives could effectively adsorb onto metal surfaces. Further analysis revealed that Dodec-EG-CC exhibited superior adsorption on metal substrates compared to Dodec-CC. The difference in adsorption behavior is likely a key factor contributing to the variation in tribological performance.
- Tribological test results demonstrated that both additive molecules significantly improved the friction-reducing performance of base oil PAO4 over a wide temperature range of 100–200 °C. Notably, Dodec-EG-CC achieved a 28% reduction in the average friction coefficient even at 200 °C. The friction-reducing and anti-wear performance of Dodec-EG-CC was comprehensively superior to that of Dodec-CC, indicating that the incorporation of ester groups into the alkyl chain effectively enhances the tribological properties of the additive molecules.
- Friction surface analysis revealed that additive molecules form an adsorption film on the metal surface, effectively preventing direct contact between the friction pairs. During the friction process, harsh environmental factors such as high temperature and pressure promote the desorption, decomposition, and further tribochemical reactions of the additive molecules, leading to the formation of carbon-based materials. Dodec-EG-CC, with its excellent adsorption performance on the metal surface, adheres firmly and is more likely than Dodec-CC to generate carbon-based materials. This process forms a protective friction film that prevents direct contact between the friction pairs and inhibits the formation of metal oxides.
- This study provides a feasible solution for equipment lubrication under harsh working conditions, especially in high-temperature environments. It enhances the stability and service life of machinery by effectively reducing wear and preventing surface degradation. Furthermore, this research offers new theoretical insights into the structure design and development of environmentally friendly additives. By highlighting the role of functional groups, such as the ester moiety, in enhancing lubrication performance, this work contributes to the advancement of green innovation in the lubricants field. Future work will focus on further optimizing the molecular structure of these additives to improve their thermal stability and film-forming capabilities.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/lubricants13030114/s1, Figure S1: 1H NMR spectra of (a1) Dodec-CC, (b1) Gly-CC, (c1) Dodec-EG-CC; 13C NMR spectra of (a2) Dodec-CC, (b2) Gly-CC, (c2) Dodec-EG-CC; Figure S2: MS spectral of (1) Dodec-CC, (b) Dodec-CC, (c) Dodec-EG-CC; Figure S3: The FT-IR spectra of CC-Gly; Figure S4: EDS-mapping of steel wear scars after friction tests at 100 °C: a: PAO4; b: Dodec—EG-CC; c: Dodec-CC; Figure S5: XPS survey spectrum.
Author Contributions
Conceptualization, W.H. and J.L.; methodology, W.H.; validation, J.Z.; formal analysis, J.Z.; investigation, J.Z.; resources, J.L.; data curation, J.Z.; writing—original draft preparation, J.Z.; writing—review and editing, W.H.; supervision, W.H. and J.L.; project administration, J.L. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to thank the financial support for this work by “The Key Project of the Henan Province Science and Technology Research and Development Joint Funds, grant number 235200810015”, “International Partnership Program of Chinese Academy of Sciences grant number 307GJHZ2022034GC”.
Data Availability Statement
The data presented in this study are available in the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tung, S.C.; McMillan, M.L. Automotive tribology overview of current advances and challenges for the future. Tribol. Int. 2004, 37, 517–536. [Google Scholar] [CrossRef]
- Ji, H.; Zhang, X.; Tan, T. Preparation of a Water-Based Lubricant from Lignocellulosic Biomass and Its Tribological Properties. Ind. Eng. Chem. Res. 2017, 56, 7858–7864. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Wen, P.; Ma, L.; Fan, M.; Dong, R.; Zhang, C. Low-viscosity oligoether esters (OEEs) as high-efficiency lubricating oils: Insight on their structure–lubricity relationship. Friction 2023, 12, 1133–1153. [Google Scholar] [CrossRef]
- Zachariah, Z.; Nalam, P.C.; Ravindra, A.; Raju, A.; Mohanlal, A.; Wang, K.; Castillo, R.V.; Espinosa-Marzal, R.M. Correlation Between the Adsorption and the Nanotribological Performance of Fatty Acid-Based Organic Friction Modifiers on Stainless Steel. Tribol. Lett. 2019, 68, 11. [Google Scholar] [CrossRef]
- Hu, M.; Ma, R.; Zhang, S.; Han, Y.; Zhao, J.; Zhang, M.; Li, W.; Liu, H. Preparation and tribological study of novel amide-based organic friction modifiers. Tribol. Int. 2024, 194, 109465. [Google Scholar] [CrossRef]
- Cyriac, F.; Tee, X.Y.; Poornachary, S.K.; Chow, P.S. Influence of structural factors on the tribological performance of organic friction modifiers. Friction 2020, 9, 380–400. [Google Scholar] [CrossRef]
- Xu, X.; Yang, F.; Yang, H.; Zhao, Y.; Sun, X.; Tang, Y. Preparation and Tribological Behaviors of Sulfur- and Phosphorus-Free Organic Friction Modifier of Amide–Ester Type. Lubricants 2024, 12, 196. [Google Scholar] [CrossRef]
- Song, W.; Campen, S.; Shiel, H.; Gattinoni, C.; Zhang, J.; Wong, J.S.S. Position of Carbonyl Group Affects Tribological Performance of Ester Friction Modifiers. ACS Appl. Mater. Interfaces 2024, 16, 14252–14262. [Google Scholar] [CrossRef]
- Eickworth, J.; Wagner, J.; Daum, P.; Dienwiebel, M.; Rühle, T. Gas phase lubrication study with an organic friction modifier. Lubr. Sci. 2022, 35, 40–55. [Google Scholar] [CrossRef]
- Vaitkunaite, G.; Espejo, C.; Thiebaut, B.; Neville, A.; Morina, A. Low friction tribofilm formation and distribution on an engine cylinder tested with MoDTC-containing low viscosity engine lubricants. Tribol. Int. 2022, 171, 107551. [Google Scholar] [CrossRef]
- Davidson, J.E.; Hinchley, S.L.; Harris, S.G.; Parkin, A.; Parsons, S.; Tasker, P.A. Molecular dynamics simulations to aid the rational design of organic friction modifiers. J. Mol. Graph. Model. 2006, 25, 495–506. [Google Scholar] [CrossRef]
- Wei, P.; Gao, P.; Pu, W. Nonequilibrium molecular dynamics investigation on friction behavior of organic friction modifiers under dynamic load. J. Mol. Liq. 2024, 407, 125195. [Google Scholar] [CrossRef]
- Gu, H.; Hirayama, T.; Yamashita, N.; Xu, J.; Yamada, M. Relationship between friction reduction effect and solubility in base oil of organic friction modifiers. Tribol. Int. 2025, 202, 110304. [Google Scholar] [CrossRef]
- Fry, B.M.; Moody, G.; Spikes, H.A.; Wong, J.S.S. Adsorption of Organic Friction Modifier Additives. Langmuir 2020, 36, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Cyriac, F.; Yamashita, N.; Hirayama, T.; Yi, T.X.; Poornachary, S.K.; Chow, P.S. Mechanistic insights into the effect of structural factors on film formation and tribological performance of organic friction modifiers. Tribol. Int. 2021, 164, 107243. [Google Scholar] [CrossRef]
- Meng, Y.; Wen, X.; Cheng, J.; Bai, P.; Meng, Y.; Tian, Y. High temperature lubrication performance of chlorophenyl silicone oil. Friction 2024, 12, 1716–1727. [Google Scholar] [CrossRef]
- Meng, Y.; Xu, J.; Jin, Z.; Prakash, B.; Hu, Y. A review of recent advances in tribology. Friction 2020, 8, 221–300. [Google Scholar] [CrossRef]
- Wang, W.; Shen, B.; Li, Y.; Ni, Q.; Zhou, L.; Du, F. Friction reduction mechanism of glycerol monooleate-containing lubricants at elevated temperature—Transition from physisorption to chemisorption. Sci. Prog. 2021, 104, 36850421998529. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, R.; He, Z.; Xiong, L. Tribological performance of N-containing heterocyclic triazine derivative as lubricant additive in ethylene glycol. Surf. Interface Anal. 2021, 53, 1027–1034. [Google Scholar] [CrossRef]
- Desanker, M.; He, X.; Lu, J.; Johnson, B.A.; Liu, Z.; Delferro, M.; Ren, N.; Lockwood, F.E.; Greco, A.; Erdemir, A.; et al. High-Performance Heterocyclic Friction Modifiers for Boundary Lubrication. Tribol. Lett. 2018, 66, 50. [Google Scholar] [CrossRef]
- Mao, S.; Wang, B.; Dai, S.; Lu, H. Synthesizing Benzotriazole Group-Terminated Carbon Dots as Multifunctional Additives of Poly(ethylene glycol). Langmuir 2024, 40, 1922–1930. [Google Scholar] [CrossRef] [PubMed]
- Desanker, M.; He, X.; Lu, J.; Liu, P.; Pickens, D.B.; Delferro, M.; Marks, T.J.; Chung, Y.-W.; Wang, Q.J. Alkyl-Cyclens as Effective Sulfur- and Phosphorus-Free Friction Modifiers for Boundary Lubrication. ACS Appl. Mater. Interfaces 2017, 9, 9118–9125. [Google Scholar] [CrossRef]
- Wu, Y.; He, Z.; Zeng, X.; Ren, T.; de Vries, E.; van der Heide, E. Tribological and anticorrosion behaviour of novel xanthate-containing triazine derivatives in water-glycol. Tribol. Int. 2017, 110, 113–124. [Google Scholar] [CrossRef]
- Zeng, X.; Wu, H.; Yi, H.; Ren, T. Tribological behavior of three novel triazine derivatives as additives in rapeseed oil. Wear 2007, 262, 718–726. [Google Scholar] [CrossRef]
- Capel Berdiell, I.; Farmiloe, S.E.; Kulmaczewski, R.; Halcrow, M.A. Molecular squares, coordination polymers and mononuclear complexes supported by 2,4-dipyrazolyl-6H-1,3,5-triazine and 4,6-dipyrazolylpyrimidine ligands. Dalton Trans. 2019, 48, 17310–17320. [Google Scholar] [CrossRef]
- Jin, Y.; Li, J.; Jia, D.; Tu, J.; Zhan, S.; Yang, T.; Duan, H. Online infrared spectra analysis of multi-phenol antioxidants in ester lubricant during friction under high-temperature oxidation. Tribol. Int. 2022, 176, 107877. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, Z.; Zeng, X.; Li, J. Correlation between the Degree of Alkylation and Tribological Properties of Amino-PEG2-amine-Based Organic Friction Modifiers. Ind. Eng. Chem. Res. 2019, 59, 215–225. [Google Scholar] [CrossRef]
- Lee, C.T.; Lee, M.B.; Hamdan, S.H.; Chong, W.W.F.; Chong, C.T.; Zhang, H.; Chen, A.W.L. Trimethylolpropane trioleate as eco-friendly lubricant additive. Eng. Sci. Technol. Int. J. 2022, 35, 101068. [Google Scholar] [CrossRef]
- Hu, W.; Xu, Y.; Zeng, X.; Li, J. Alkyl-Ethylene Amines as Effective Organic Friction Modifiers for the Boundary Lubrication Regime. Langmuir 2020, 36, 6716–6727. [Google Scholar] [CrossRef]
- Zhou, F.; Chen, K.; Wang, M.; Xu, X.; Meng, H.; Yu, M.; Dai, Z. Friction and wear properties of CrN coatings sliding against Si3N4 balls in water and air. Wear 2008, 265, 1029–1037. [Google Scholar] [CrossRef]
- Thompson, A.P.; Aktulga, H.M.; Berger, R.; Bolintineanu, D.S.; Brown, W.M.; Crozier, P.S.; in’t Veld, P.J.; Kohlmeyer, A.; Moore, S.G.; Nguyen, T.D.; et al. LAMMPS—A flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales. Comput. Phys. Commun. 2022, 271, 108171. [Google Scholar] [CrossRef]
- Knothe, G.; Steidley, K.R. Lubricity of Components of Biodiesel and Petrodiesel. The Origin of Biodiesel Lubricity. Energy Fuels 2005, 19, 1192–1200. [Google Scholar] [CrossRef]
- Boiko, M.V.; Sidashov, A.V.; Boiko, T.G.; Burykin, I.V.; Uflyand, I.E. The mechanism of formation of boundary lubricating films during friction in a medium of di(2-ethylhexyl) sebacate. Tribol. Int. 2022, 165, 107222. [Google Scholar] [CrossRef]
- Yu, H.; Chen, H.; Zheng, Z.; Ba, Z.; Qiao, D.; Feng, D.; Gong, Z.; Dong, G. Transformation mechanism between the frictional interface under dioctyl sebacate lubrication. Tribol. Int. 2021, 155, 106745. [Google Scholar] [CrossRef]
- Qiu, F.; Feng, W.; Song, H.; Yang, Z.; Zhang, F.; Hu, X. On the Structure–Activity Relationship of Glyceryl oleate Friction Modifiers and Its Synergistic Mechanism on Phosphate ester Antiwear Additives. Tribol. Lett. 2023, 71, 90. [Google Scholar] [CrossRef]
- Saini, V.; Seth, S.; Ramakumar, S.S.V.; Bijwe, J. Carbon Nanoparticles of Varying Shapes as Additives in Mineral Oil Assessment of Comparative Performance Potential. ACS Appl. Mater. Interfaces 2021, 13, 38844–38856. [Google Scholar] [CrossRef]
- Liu, K.; Ren, E.; Ma, J.; Cao, Y.; Du, J.; Ming, W.; Li, X.; Li, B. Controllable preparation of graphene-based film deposited on cemented carbides by chemical vapor deposition. J. Mater. Sci. 2019, 55, 4251–4264. [Google Scholar] [CrossRef]
- Johnson, B.; Wu, H.; Desanker, M.; Pickens, D.; Chung, Y.-W.; Jane Wang, Q. Direct Formation of Lubricious and Wear-Protective Carbon Films from Phosphorus- and Sulfur-Free Oil-Soluble Additives. Tribol. Lett. 2017, 66, 2. [Google Scholar] [CrossRef]
- Wu, H.; Khan, A.M.; Johnson, B.; Sasikumar, K.; Chung, Y.-W.; Wang, Q.J. Formation and Nature of Carbon-Containing Tribofilms. ACS Appl. Mater. Interfaces 2019, 11, 16139–16146. [Google Scholar] [CrossRef]
- Erdemir, A.; Ramirez, G.; Eryilmaz, O.L.; Narayanan, B.; Liao, Y.; Kamath, G.; Sankaranarayanan, S.K.R.S. Carbon-based tribofilms from lubricating oils. Nature 2016, 536, 67–71. [Google Scholar] [CrossRef]
- Niu, Y.; Pang, X.; Yue, S.; Shangguan, B.; Zhang, Y. The friction and wear behavior of laser textured surfaces in non-conformal contact under starved lubrication. Wear 2021, 476, 203723. [Google Scholar] [CrossRef]
- Sukjit, E.; Poapongsakorn, P.; Dearn, K.D.; Lapuerta, M.; Sánchez-Valdepeñas, J. Investigation of the lubrication properties and tribological mechanisms of oxygenated compounds. Wear 2017, 376–377, 836–842. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).