The Synovial Lining and Synovial Fluid Properties after Joint Arthroplasty
Abstract
:1. Introduction
1.1. Synovial Tissue
1.2. Synovial Fluid
2. Methods
2.1. Synovial Lining Papers

| Author | Bearing Type 1 | Patients | Characterization of Synovial-Like Membrane | Main Synovium Findings | ||
|---|---|---|---|---|---|---|
| Layer Thickness | Synovial Lining Cells | Presence of Particulate Debris in Synovial Lining 2 | ||||
| Goldring et al. (1983) [17] | Metal on Polyethylene | N = 20 | cells |
| 0 | Membrane at cement-bone interface with histological and histochemical characteristics of normal synovium found in patients with implant loosening. |
| ||||||
| ||||||
| Goldring et al. (1986) [16] | Metal on Polyethylene | N = 41 | 1–2 cells |
| 1 | Study confirmed formation of synovial-like lining at bone-cement interface taken from patients with loosened THA components |
| ||||||
| Lennox et al. (1987) [19] | Metal on Polyethylene; Ceramic on Polyethylene | N = 61 | 1–3 cells |
| 1 | Cemented, press-fit, and biologic ingrowth prostheses showed similar formation of pseudosynovial lining at the implant-bone interface membrane. |
| ||||||
| Lalor and Revell (1993) [18] | Metal on Polyethylene and Polyethylene on Delrin THA and TKA | N = 29, 23 hip; 6 knee | 1–10 cells |
| 1 | The newly formed bone-implant interface membrane closely resembled true synovium and contained macrophage-like type A cells and fibroblast type B cells, but not necessarily always in distinct layers |
| ||||||
| Burkandt et al. (2011) [15] | Metal on Polyethylene THA; Metal on Metal HRA | N = 22, 10 with synovitis; 12 with arthroplasty. | 1–5 cells |
| 1 | Tissues from patients revised due to suggested metal hypersensitivity showed increased proliferation of synovial lining cell layer similar to cases with rheumatoid arthritis and high-grade synovitis, with 2 patients showing paucicellular synovial membrane covered by a fibrinous exudate. |
| ||||||
| Author | Arthroplasty | Experiment | Key Findings |
|---|---|---|---|
| Costa et al. (2001) [36] | 10 UHMWPE hip implants | Mass spectrophotometry and FTIR were performed after cyclohexane extraction for adsorbed products on the liners. | Methyl esters of hexadecanoic acid, octadecanoic acid, squalene, and of cholesterol were found in the extracts as well as a protein-like material at the surface. |
| Mazzucco et al. (2002) [37] | 58 index TKA; 19 revision TKA; 2 effused previous TKA | Sufficient SF samples were obtained from 36 index TKA, 14 revised TKA, and 2 effused previous TKA for flow property examination. | SF from revision TKA tended to have lower viscosity than that from index TKA. The difference was found to not be statistically significant. |
| Mazzucco et al. (2004) [38] | 77 index TKA; 20 revised TKA; 3 effused previous TKA | SF from 24 index TKA and 7 revised TKA had their composition of protein, phospholipids and HA determined and correlated. | Protein and phospholipids were found to have a positive correlation in regards to each other. Protein and phospholipids were found to have a negative correlation with HA. |
| Gale et al. (2007) [39] | 38 Metal on Polyethylene THA; 2 Metal on Polyethylene TKA | The bearing surfaces of the implants were rinsed and analyzed by HPLC for phospholipids. | 8 species of phosphatidylcholine were identified. 3 species of unsaturated phosphatidylcholine predominated; PLPC, POPC, and SLPC. |
| Bergmann et al. Part 1 (2001) [40] | Type 1 telemeterized cemented PE cup, 1 temperature measurement at neck; Type 2 telemeterized non-cemented, AC head, PE or AC cup, titanium shaft | Patients were monitored doing various physical activities and the temperatures inside their telemeterized implant were recorded. | The highest peak temperature were observed in the head of the implant and reached as high as 43.1 °C, greater than what is believed to affect the synovial fluids lubrication ability. |
| Bergmann et al. Part 2 (2001) [41] | (See above) | Data from Bergmann et al. 2001 part 1 was used to generate a finite element model to calculate the steady- state within the implant during walking. | The model shows that if the cup of an implant is made of a material with good conductivity, heat will be transferred away from the synovial fluid, capsule and stem towards the acetabular bone. |
2.2. Synovial Lubrication Papers

3. Results
3.1. Synovial Lining Characterization

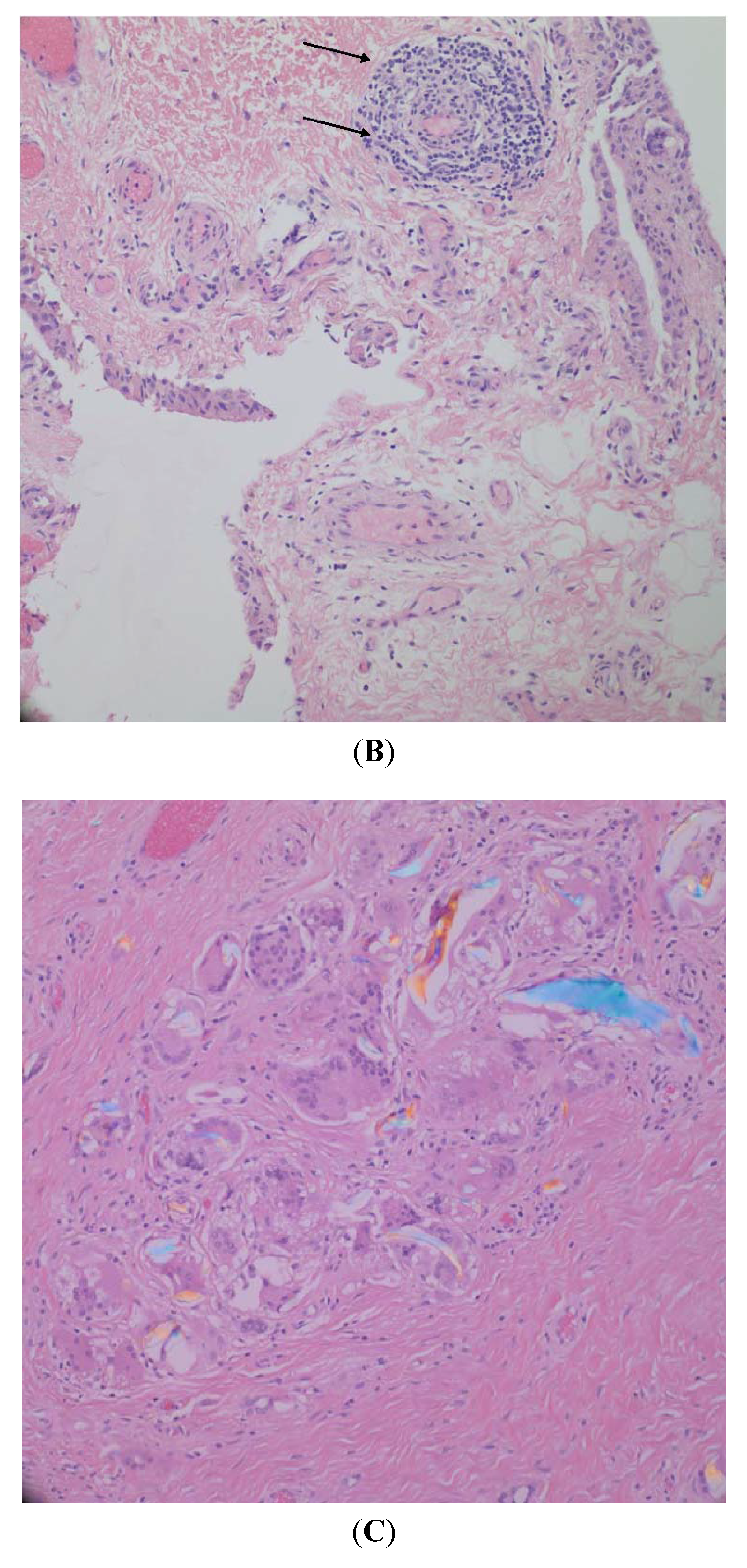
3.2. Synovial Fluid Properties
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Ghadially, F.N. Fine Structure of Synovial Joints: A Text and Atlas of the Ultrastructure of Normal and Pathological Articular Tissues; Butterworths: London, UK; Boston, MA, USA, 1983. [Google Scholar]
- Ghadially, F.N.; Roy, S. Ultrastructure of Synovial Joints in Health and Disease; Butterworths: London, UK, 1969. [Google Scholar]
- Key, J.A. The Synovial Membrane of Joints and Bursae, 2nd ed.; Paul, B., Hober: New York, NY, USA, 1932. [Google Scholar]
- Bronner, F.; Farach-Carson, M.C. Bone and Osteoarthritis; Springer-Verlag: London, UK, 2007; Volume 4. [Google Scholar]
- Pavlovich, R.I.; Lubowitz, J. Current concepts in synovial tissue of the knee joint. Orthopedics 2008, 31, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Athanasou, N.A.; Quinn, J.; Heryet, A.; Puddle, B.; Woods, C.G.; McGee, J.O. The immunohistology of synovial lining cells in normal and inflamed synovium. J. Pathol. 1988, 155, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Barland, P.; Novikoff, A.B.; Hamerman, D. Electron microscopy of the human synovial membrane. J. Cell Biol. 1962, 14, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, T.; Shikichi, M.; Kitamura, H.; Yanase, H.; Nozawa-Inoue, K. Morphology and functional roles of synoviocytes in the joint. Arch. Histol. Cytol. 2000, 63, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Mapp, P.I.; Revell, P.A. Fibronectin production by synovial intimal cells. Rheumatol. Int. 1985, 5, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Spycher, M.A.; Ruttner, J.R.; Fehr, K. The ultrastructural localization of fibronectin in the lining layer of rheumatoid arthritis synovium: The synthesis of fibronectin by type B lining cells. Rheumatol. Int. 1983, 3, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Yielding, K.L.; Tomkins, G.M.; Bunim, J.J. Synthesis of hyaluronic acid by human synovial tissue slices. Science 1957, 125, 1300. [Google Scholar] [CrossRef] [PubMed]
- Hogg, N.; Palmer, D.G.; Revell, P.A. Mononuclear phagocytes of normal and rheumatoid synovial membrane identified by monoclonal antibodies. Immunology 1985, 56, 673–681. [Google Scholar] [PubMed]
- Depalma, A.F. Diseases of the Knee: Management in Medicine and Surgery, 1st ed.; J.B. Lippincott Company: Philadelphia, PA, USA, 1954. [Google Scholar]
- Key, J.A. The reformation of synovial membrane in the knees of rabbits after synovectomy. J. Bone Joint Surg. 1925, 7, 793–813. [Google Scholar]
- Burkandt, A.; Katzer, A.; Thaler, K.; Von Baehr, V.; Friedrich, R.E.; Ruther, W.; Amling, M.; Zustin, J. Proliferation of the synovial lining cell layer in suggested metal hypersensitivity. In Vivo 2011, 25, 679–686. [Google Scholar] [PubMed]
- Goldring, S.R.; Jasty, M.; Roelke, M.S.; Rourke, C.M.; Bringhurst, F.R.; Harris, W.H. Formation of a synovial-like membrane at the bone-cement interface. Its role in bone resorption and implant loosening after total hip replacement. Arthritis Rheum. 1986, 29, 836–842. [Google Scholar]
- Goldring, S.R.; Schiller, A.L.; Roelke, M.; Rourke, C.M.; O’Neil, D.A.; Harris, W.H. The synovial-like membrane at the bone-cement interface in loose total hip replacements and its proposed role in bone lysis. J. Bone Joint Surg. 1983, 65, 575–584. [Google Scholar] [PubMed]
- Lalor, P.A.; Revell, P.A. The presence of a synovial layer at the bone-implant interface: An immunohistological study demonstrating the close similarity to true synovium. Clin. Mater. 1993, 14, 91–100. [Google Scholar] [CrossRef]
- Lennox, D.W.; Schofield, B.H.; McDonald, D.F.; Riley, L.H., Jr. A histologic comparison of aseptic loosening of cemented, press-fit, and biologic ingrowth prostheses. Clin. Orthop. Relat. Res. 1987, 171–191. [Google Scholar]
- Drachman, D.B.; Sokoloff, L. Role of movement in embryonic joint development. Dev. Biol. 1966, 14, 401–420. [Google Scholar] [CrossRef]
- Engh, C.A.; Oconnor, D.; Jasty, M.; Mcgovern, T.F.; Bobyn, J.D.; Harris, W.H. Quantification of implant micromotion, strain shielding, and bone-resorption with porous-coated anatomic medullary locking femoral prostheses. Clin. Orthop. Relat. Res. 1992, 285, 13–29. [Google Scholar] [PubMed]
- Konttinen, Y.T.; Li, T.F.; Mandelin, J.; Ainola, M.; Lassus, J.; Virtanen, I.; Santavirta, S.; Tammi, M.; Tammi, R. Hyaluronan synthases, hyaluronan, and its CD44 receptor in tissue around loosened total hip prostheses. J. Pathol. 2001, 194, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.C.; Sedgwick, A.D.; Willoughby, D.A. The formation of a structure with the features of synovial lining by subcutaneous injection of air: An in vivo tissue culture system. J. Pathol. 1981, 134, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Pap, G.; Machner, A.; Rinnert, T.; Horler, D.; Gay, R.E.; Schwarzberg, H.; Neumann, W.; Michel, B.A.; Gay, S.; Pap, T. Development and characteristics of a synovial-like interface membrane around cemented tibial hemiarthroplasties in a novel rat model of aseptic prosthesis loosening. Arthritis Rheum. 2001, 44, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, A.D.; Sin, Y.M.; Edwards, J.C.W.; Willoughby, D.A. Increased inflammatory reactivity in newly formed lining tissue. J. Pathol. 1983, 141, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Radin, E.L.; Paul, I.L.; Swann, D.A.; Schottstaedt, E.S. Lubrication of synovial membrane. Ann. Rheum. Dis. 1971, 30, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Konttinen, Y.T.; Zhao, D.; Beklen, A.; Ma, G.; Takagi, M.; Kivela-Rajamaki, M.; Ashammakhi, N.; Santavirta, S. The microenvironment around total hip replacement prostheses. Clin. Orthop. Relat. Res. 2005, 430, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Hills, B.A.; Butler, B.D. Surfactants identified in synovial fluid and their ability to act as boundary lubricants. Ann. Rheum. Dis. 1984, 43, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Seror, J.; Zhu, L.; Goldberg, R.; Day, A.J.; Klein, J. Supramolecular synergy in the boundary lubrication of synovial joints. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Radin, E.L.; Swann, D.A.; Weisser, P.A. Separation of a hyaluronate-free lubricating fraction from synovial fluid. Nature 1970, 228, 377–378. [Google Scholar] [CrossRef] [PubMed]
- McCutchen, C.W. Joint lubrication. Bull. Hosp. Jt. Dis. Orthop. Inst. 1983, 43, 118–129. [Google Scholar]
- Balazs, E.A. The Physical Properties of Synovial Fluid and the Specific Role of Hyaluronic Acid; J B Lippincott: Philadelphia, PA, USA, 1982. [Google Scholar]
- Balazs, E.A.; Watson, D.; Duff, I.F.; Roseman, S. Hyaluronic acid in synovial fluid. I. Molecular parameters of hyaluronic acid in normal and arthritis human fluids. Arthritis Rheum. 1967, 10, 357–376. [Google Scholar]
- Ogston, A.G.; Stanier, J.E. The physiological function of hyaluronic acid in synovial fluid; viscous, elastic and lubricant properties. J. Physiol. 1953, 119, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, C.; Thormann, E.; Dedinaite, A. Hyaluronan and phospholipid association in biolubrication. Biomacromolecules 2013, 14, 4198–4206. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.; Bracco, P.; del Prever, E.B.; Luda, M.P.; Trossarelli, L. Analysis of products diffused into UHMWPE prosthetic components in vivo. Biomaterials 2001, 22, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Mazzucco, D.; McKinley, G.; Scott, R.D.; Spector, M. Rheology of joint fluid in total knee arthroplasty patients. J. Orthop. Res. 2002, 20, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Mazzucco, D.; Scott, R.; Spector, M. Composition of joint fluid in patients undergoing total knee replacement and revision arthroplasty: Correlation with flow properties. Biomaterials 2004, 25, 4433–4445. [Google Scholar] [CrossRef] [PubMed]
- Gale, L.R.; Chen, Y.; Hills, B.A.; Crawford, R. Boundary lubrication of joints: Characterization of surface-active phospholipids found on retrieved implants. Acta Orthop. 2007, 78, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, G.; Graichen, F.; Rohlmann, A.; Verdonschot, N.; van Lenthe, G.H. Frictional heating of total hip implants. Part 1: Measurements in patients. J. Biomech. 2001, 34, 421–428. [Google Scholar]
- Bergmann, G.; Graichen, F.; Rohlmann, A.; Verdonschot, N.; van Lenthe, G.H. Frictional heating of total hip implants. Part 2: Finite element study. J. Biomech. 2001, 34, 429–435. [Google Scholar]
- Ghosh, S.; Choudhury, D.; Das, N.S.; Pingguan-Murphy, B. Tribological role of synovial fluid compositions on artificial joints—A systematic review of the last 10 years. Lubr. Sci. 2014, 26, 387–410. [Google Scholar] [CrossRef]
- Bonnevie, E.D.; Baro, V.J.; Wang, L.; Burris, D.L. Fluid load support during localized indentation of cartilage with a spherical probe. J. Biomech. 2012, 45, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Caligaris, M.; Ateshian, G.A. Effects of sustained interstitial fluid pressurization under migrating contact area, and boundary lubrication by synovial fluid, on cartilage friction. Osteoarthritis Cartilage 2008, 16, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Delecrin, J.; Oka, M.; Takahashi, S.; Yamamuro, T.; Nakamura, T. Changes in joint fluid after total arthroplasty. A quantitative study on the rabbit knee joint. Clin. Orthop. Relat. Res. 1994, 307, 240–249. [Google Scholar]
- Campbell, P.; Ebramzadeh, E.; Nelson, S.; Takamura, K.; De Smet, K.; Amstutz, H.C. Histological features of pseudotumor-like tissues from metal-on-metal hips. Clin. Orthop. Relat. Res. 2010, 468, 2321–2327. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.P.; Willert, H.G.; Campbell, P.A.; Learmonth, I.D.; Case, C.P. An unusual lymphocytic perivascular infiltration in tissues around contemporary metal-on-metal joint replacements. J. Bone Joint Surg. 2005, 87, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Grammatopoulos, G.; Pandit, H.; Kamali, A.; Maggiani, F.; Glyn-Jones, S.; Gill, H.S.; Murray, D.W.; Athanasou, N. The correlation of wear with histological features after failed hip resurfacing arthroplasty. J. Bone Joint Surg. 2013, 95, e81. [Google Scholar] [CrossRef] [PubMed]
- Howie, D.W.; Cain, C.M.; Cornish, B.L. Pseudo-abscess of the psoas bursa in failed double-cup arthroplasty of the hip. J. Bone Joint Surg. 1991, 73, 29–32. [Google Scholar]
- Willert, H.G.; Buchhorn, G.H.; Fayyazi, A.; Flury, R.; Windler, M.; Koster, G.; Lohmann, C.H. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints. A clinical and histomorphological study. J. Bone Joint Surg. 2005, 87, 28–36. [Google Scholar] [CrossRef]
- Hirohata, K.; Kobayashi, I. Fine structures of the synovial tissues in rheumatoid arthritis. Kobe J. Med. Sci. 1964, 10, 195–225. [Google Scholar] [PubMed]
- Haraoui, B.; Pelletier, J.P.; Cloutier, J.M.; Faure, M.P.; Martel-Pelletier, J. Synovial membrane histology and immunopathology in rheumatoid arthritis and osteoarthritis. In vivo effects of antirheumatic drugs. Arth. Rheum. 1991, 34, 153–163. [Google Scholar]
- Walker, P.S. A comparison of normal and artificial human joints. Acta Orthop. Belg. 1973, 39, 43–54. [Google Scholar] [PubMed]
- Jay, G.D.; Waller, K.A. The biology of lubricin: Near frictionless joint motion. Matrix Boil. J. Int. Soc. Matrix Biol. 2014, 39, 17–24. [Google Scholar] [CrossRef]
- Swann, D.A.; Silver, F.H.; Slayter, H.S.; Stafford, W.; Shore, E. The molecular structure and lubricating activity of lubricin isolated from bovine and human synovial fluids. Biochem. J. 1985, 225, 195–201. [Google Scholar] [PubMed]
- Fan, J.; Myant, C.; Underwood, R.; Cann, P. Synovial fluid lubrication of artificial joints: Protein film formation and composition. Faraday Discuss. 2012, 156, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Gispert, M.P.; Serro, A.P.; Colaco, R.; Saramago, B. Friction and wear mechanisms in hip prosthesis: Comparison of joint materials behaviour in several lubricants. Wear 2006, 260, 149–158. [Google Scholar] [CrossRef]
- Roba, M.; Bruhin, C.; Ebneter, U.; Ehrbar, R.; Crockett, R.; Spencer, N.D. Latex on glass: An appropriate model for cartilage-lubrication studies? Tribol. Lett. 2010, 38, 267–273. [Google Scholar] [CrossRef]
- Wang, A.; Essner, A.; Schmidig, G. The effects of lubricant composition on in vitro wear testing of polymeric acetabular components. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 68, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.M.; Briere, L.K.; Marr, J.; MacDonald, S.J.; Bourne, R.B.; Medley, J.B. Biochemical comparisons of osteoarthritic human synovial fluid with calf sera used in knee simulator wear testing. J. Biomed. Mater. Res. A 2010, 94, 961–971. [Google Scholar] [PubMed]
- Reinders, J.; Sonntag, R.; Kretzer, J.P. Synovial fluid replication in knee wear testing: An investigation of the fluid volume. J. Orthop. Res. 2015, 33, 92–97. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kung, M.S.; Markantonis, J.; Nelson, S.D.; Campbell, P. The Synovial Lining and Synovial Fluid Properties after Joint Arthroplasty. Lubricants 2015, 3, 394-412. https://doi.org/10.3390/lubricants3020394
Kung MS, Markantonis J, Nelson SD, Campbell P. The Synovial Lining and Synovial Fluid Properties after Joint Arthroplasty. Lubricants. 2015; 3(2):394-412. https://doi.org/10.3390/lubricants3020394
Chicago/Turabian StyleKung, Michael Shang, John Markantonis, Scott D. Nelson, and Patricia Campbell. 2015. "The Synovial Lining and Synovial Fluid Properties after Joint Arthroplasty" Lubricants 3, no. 2: 394-412. https://doi.org/10.3390/lubricants3020394
APA StyleKung, M. S., Markantonis, J., Nelson, S. D., & Campbell, P. (2015). The Synovial Lining and Synovial Fluid Properties after Joint Arthroplasty. Lubricants, 3(2), 394-412. https://doi.org/10.3390/lubricants3020394





