Tribological Behaviour of PVD Coatings Lubricated with a FAP− Anion-Based Ionic Liquid Used as an Additive
Abstract
:1. Introduction
2. Experimental Section
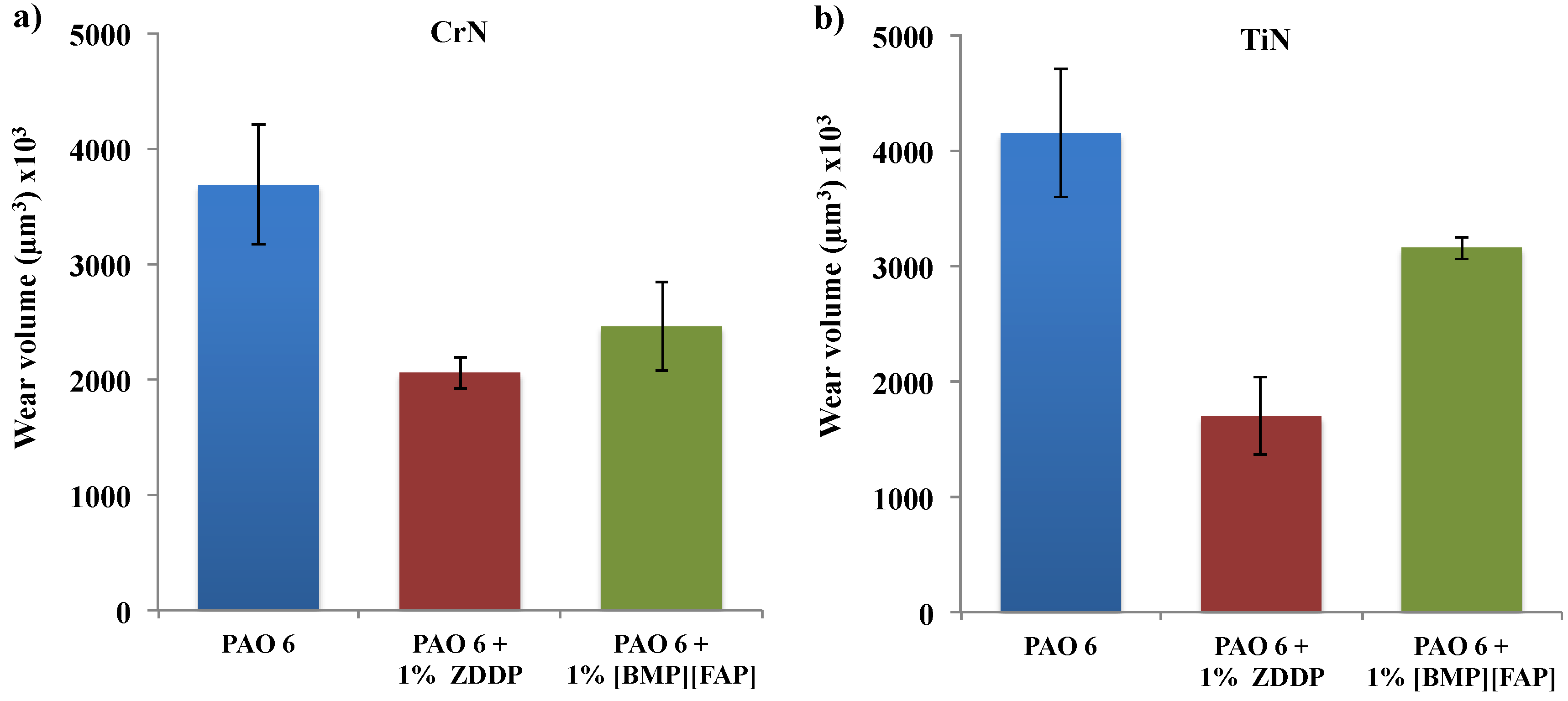
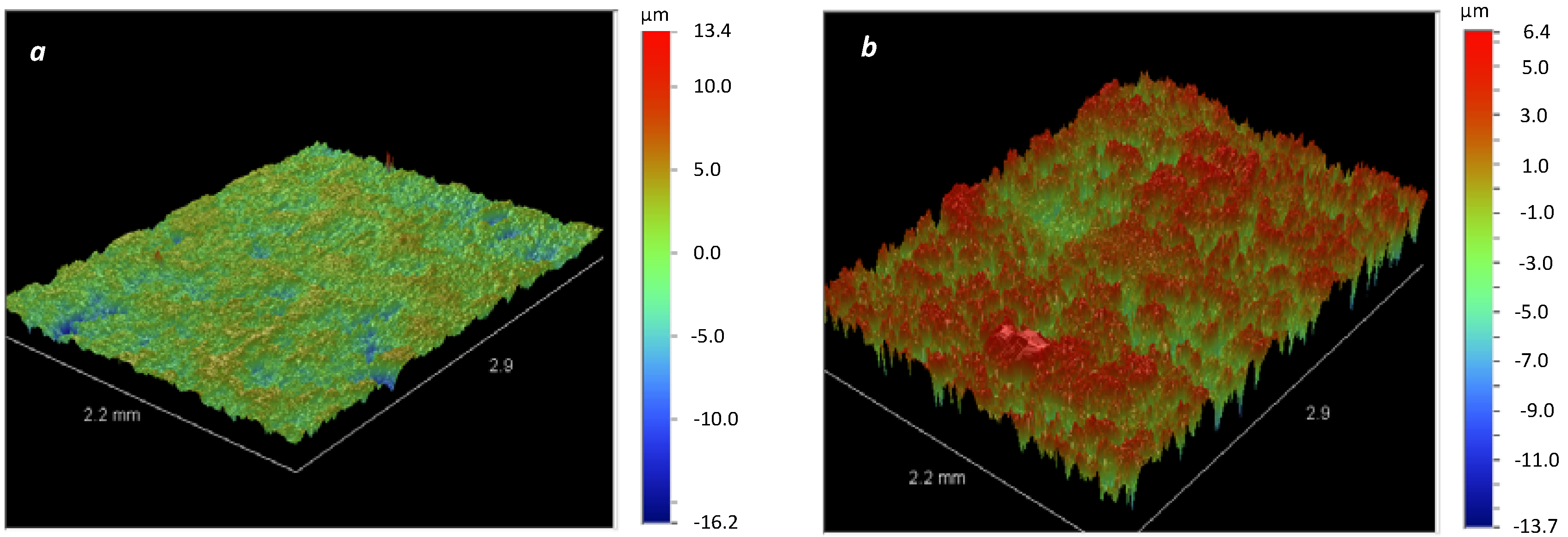
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Arora, H.; Cann, P.M. Lubricant film formation properties of alkyl imidazolium tetrafluoroborate and hexafluorophosphate ionic liquids. Tribol. Int. 2010, 43, 1908–1916. [Google Scholar]
- Wassercheid, P.; Welton, T. Ionic Liquid in Synthesis; Wiley-Vch: Weinheim, Germany, 2008. [Google Scholar]
- Rensselar, J.V. Unleashing the potential of ionic liquids. Tribol. Lubr. Technol. 2010, 24–31. [Google Scholar]
- Liu, W.; Liang, Y.; Zhou, F. Ionic liquid lubricants: designed chemistry for engineering applications. Chem. Soc. Rev. 2009, 38, 2590–2599. [Google Scholar]
- Minami, I. Ionic liquids in Tribology. Molecules 2009, 14, 2286–2305. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez, M.D.; Jiménez, A.E.; Sanes, J.; Carrión, F.J. Ionic liquids as advanced lubricant fluids. Molecules 2009, 14, 2888–2908. [Google Scholar] [CrossRef] [PubMed]
- Palacio, M.; Bhushan, B. A Review of Ionic Liquids for Green Molecular Lubrication in Nanotechnology. Tribol. Lett. 2010, 40, 247–268. [Google Scholar] [CrossRef]
- Jiménez, A.E.; Bermúdez, M.D. Ionic liquids as lubricants of titanium-steel contact. Tribol. Lett. 2009, 33, 111–126. [Google Scholar] [CrossRef]
- López Sánchez, F.; Otero, I.; López, E.R.; Fernández, J. Tribological Properties of Two Bis(trifluoromethylsulfonyl)Imide-Based Ionic Liquids on Steel-Steel Contact. Tribol. Trans. 2014, 57, 637–646. [Google Scholar] [CrossRef]
- Yao, M.; Liang, Y.; Xia, Y.; Zhou, F. Bisimidazolium Ionic Liquids as the High-Performance Anti-wear Additives in Poly (ethylene glycol) for Steel-Steel Contacts. ACS Appl. Mater. Interfaces 2009, 1, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Fan, M.; Liang, Y.; Zhou, F.; Xia, Y. Imidazolium hexafluorophospate ionic liquids as high temperature lubricants for steel-steel contacts. Wear 2010, 268, 67–71. [Google Scholar] [CrossRef]
- Reich, R.A.; Stewart, P.A.; Bohaychick, J.; Urbanski, J.A. Base oil properties of ionic liquids. Lubr. Eng. 2003, 59, 16–21. [Google Scholar]
- Otero, I.; López, E.R.; Reichelt, M.; Villanueva, M.; Salgado, J.; Fernández, J. Ionic liquids based on phosphonium cations As neat lubricants or lubricant additives for a steel/steel contact. ACS Appl. Mater. Interfaces 2014, 6, 13115–13128. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.J.; Liu, X.Q.; Liang, Y.M.; Xue, Q.J. Effect of tetraalkylphosphonium based ionic liquids as lubricants on the tribological performance of a steel-on-steel system. Tribol. Lett. 2007, 26, 11–17. [Google Scholar] [CrossRef]
- Phillips, B.S.; John, G.; Zabinski, J.S. Surface chemistry of fluorine containing ionic liquids on steel substrates at elevated temperature using Mossbauer spectroscopy. Tribol. Lett. 2007, 26, 85–91. [Google Scholar] [CrossRef]
- Qu, J.; Blau, P.J.; Dai, S.; Luo, H.; Meyer, H.M., III; Truhan, J.J. Tribological characteristics of aluminum alloys sliding against steel lubricated by ammonium and imidazolium ionic liquids. Wear 2009, 267, 1226–1231. [Google Scholar] [CrossRef]
- Mo, Y.; Zhao, W.; Zhu, M.; Bai, M. Nano/Microtribological properties of ultrathin functionalized imidazolium wear-resistant ionic liquid films on single crystal silicon. Tribol. Lett. 2008, 32, 143–151. [Google Scholar] [CrossRef]
- Han, Y.; Qiao, D.; Zhang, L.; Feng, D. Study of tribological performance and mechanism of phosphonate ionic liquids for steel/aluminum contact. Tribol. Int. 2015, 84, 71–80. [Google Scholar] [CrossRef]
- Jimenez, A.E.; Bermudez, M.D.; Iglesias, P.; Carrion, F.J.; Martinez-Nicolas, G. 1-N-alkyl-3-methylimidazolium ionic liquids as neat lubricants and lubricant additives in steel–aluminium contacts. Wear 2006, 260, 766–782. [Google Scholar] [CrossRef]
- Jimenez, A.E.; Bermudez, M.D. Ionic liquids as lubricants for steel–aluminum contacts at low and elevated temperatures. Tribol. Lett. 2007, 26, 53–60. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, L.; Liu, X.; Quiao, Y. A comparative study on the tribological behavior of nanocrystalline nickel and coarse-grained nickel coatings under ionic liquid lubrication. Tribol. Lett. 2008, 30, 151–157. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, L.; Liu, X.; Quiao, Y. Tribological properties of phosphor bronze and nanocrystalline nickel coatings under PAO + MoDTC and ionic liquid lubricated condition. Tribol. Lett. 2008, 3, 149–158. [Google Scholar] [CrossRef]
- Xia, Y.; Sasaki, S.; Murakami, T.; Nakano, M.; Shi, L.; Wang, H. Ionic liquid lubrication of electrodeposited nickel-Si3N4 composite coatings. Wear 2007, 262, 765–771. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, S.; Zhou, F.; Wang, H.; Lin, Y.; Xu, T. Tribological properties of plasma nitrided stainless steel against SAE52100 steel under ionic liquid lubrication condition. Tribol. Int. 2006, 139, 635–640. [Google Scholar] [CrossRef]
- Espinosa, T.; Jimenez, M.; Sanes, J.; Jimenez, A.E.; Iglesias, M.; Bermudez, M.D. Ultra-low friction with a protic ionic liquid boundary film at the water-lubricated sapphire-stainless steel interface. Tribol. Lett. 2014, 53, 1–9. [Google Scholar] [CrossRef]
- Jiménez, A.E.; Bermúdez, M.D.; Iglesias, P. Lubrication of Inconel 600 with ionic liquids at high temperature. Tribol. Int. 2009, 42, 1744–1751. [Google Scholar] [CrossRef]
- Nooruddin, N.S.; Wahlbeck, P.G.; Carper, W.R. Semi-empirical molecular modeling of ionic liquid tribology: Ionic liquid-hydroxylated silicon surface interactions. Tribol. Lett. 2009, 36, 147–156. [Google Scholar] [CrossRef]
- Battez, A.H.; González, R.; Viesca, J.L.; Blanco, D.; Asedegbega, E.; Osorio, A. Tribological behaviour of two imidazolium ionic liquids as lubricant additives for steel/steel contacts. Wear 2009, 266, 1224–1228. [Google Scholar] [CrossRef]
- Viesca, J.L.; Battez, A.H.; González, R.; Reddyhoff, T.; Pérez, A.T.; Spikes, H.A. Assessing boundary film formation of lubricant additivised with 1-hexyl-3-methylimidazolium tetrafluoroborate using ECR as qualitative indicator. Wear 2010, 269, 112–117. [Google Scholar] [CrossRef]
- Jin, C.M.; Ye, C.F.; Phillips, B.S.; Zabinski, J.S.; Liu, X.Q.; Liu, W.M. Polyethylene glycol functionalized dicationic ionic liquids with alkyl or polyfluoroalkyl substituents as high temperature lubricants. J. Mater. Chem. 2006, 16, 1529–1535. [Google Scholar] [CrossRef]
- Jiménez, A.E.; Bermúdez, M.D. Ionic liquids as lubricants of titanium-steel contact. Part 2: Friction, wear and surface interactions at high temperature. Tribol. Lett. 2010, 37, 431–443. [Google Scholar] [CrossRef]
- Masuko, M.; Terawaki, T.; Kobayashi, K.; Aoki, S.; Suzuki, A.; Fujinami, Y.; Nogi, T.; Obara, S. Contrasting lubrication properties of imidazolium-based ionic liquids affected by the nature of the surface under high vacuum. Tribol. Lett. 2014, 55, 235–244. [Google Scholar] [CrossRef]
- Zhang, H.; Xia, Y.; Yao, M.; Jia, Z.; Liu, Z. The influences of methyl group at C2 position in imidazolium ring on tribological properties. Tribol. Lett. 2009, 36, 105–111. [Google Scholar] [CrossRef]
- Qu, J.; Blau, P.J.; Dai, S.; Luo, H.; Meyer, H.M., III. Ionic liquids as novel lubricants and additives for diesel engine applications. Tribol. Lett. 2009, 35, 181–189. [Google Scholar] [CrossRef]
- Viesca, J.L.; García, A.; Hernández Battez, A.; González, R.; Monge, R.; Fernández-González, A.; Hadfield, M. FAP− Anion Ionic Liquids Used in the Lubrication of a Steel–Steel Contact. Tribol. Lett. 2013, 52, 431–437. [Google Scholar] [CrossRef]
- Nainaparampil, J.J.; Eapen, K.C.; Sanders, J.H.; Voevodin, A.A. Ionic-liquid lubrication of sliding MEMS contacts: Comparison of AFM liquid cell and device-level tests. J. Microelectrom. Syst. 2007, 16, 836–843. [Google Scholar] [CrossRef]
- Schrader, T.; Engel, U.; Merklein, M. Tribological characterization of PVD-coatings. Key Eng. Mater. 2010, 438, 179–186. [Google Scholar] [CrossRef]
- Harlin, P.; Carlsson, P.; Bexell, U.; Olsson, M. Influence of surface roughness of PVD coatings on tribological performance in sliding contacts. Surf. Coat. Technol. 2006, 201, 4253–4259. [Google Scholar] [CrossRef]
- Haque, T.; Morina, A.; Neville, A.; Kapadia, R.; Arrowsmith, S. Non-ferrous coating/lubricant interactions in tribological contacts: Assessment of tribofilms. Tribol. Int. 2007, 40, 1603–1612. [Google Scholar] [CrossRef]
- Mo, J.L.; Zhu, M.H. Sliding tribological behaviors of PVD CrN and AlCrN coatings against Si3N4 ceramic and pure titanium. Wear 2009, 267, 874–881. [Google Scholar] [CrossRef]
- Bozyazi, E.; Ürgen, M. Comparison of reciprocating wear behaviour of electrolytic hard chrome and arc-PVD CrN coatings. Wear 2004, 256, 832–839. [Google Scholar] [CrossRef]
- Haque, T.; Morina, A.; Neville, A.; Arrowsmith, S. Tribochemical Interactions of Friction Modifier and Antiwear Additives With CrN Coating Under Boundary Lubrication Conditions. J. Tribol. 2008, 130, 042302. [Google Scholar] [CrossRef]
- Hua, M.; Ma, H.Y.; Mok, C.K.; Li, J. Tribological behavior of patterned PVD TiN coatings on M2 steel. Tribol. Lett. 2004, 17, 645–653. [Google Scholar] [CrossRef]
- Zeng, Z.; Chen, Y.; Wang, D.; Zhang, J. Tribological behavior of amorphous Cr coatings electrodeposited from Cr (III) baths under ionic liquid lubrication. Electrochem. Solid State Lett. 2007, 10, D85–D87. [Google Scholar] [CrossRef]
- Chen, Y.M.; Zeng, Z.X.; Yang, S.R.; Zhang, J.Y. The tribological performance of BCN films under ionic liquids lubrication. Diamond Relat. Mater. 2009, 18, 20–26. [Google Scholar] [CrossRef]
- Jia, Z.; Xia, Y.; Li, J.; Pang, X.; Shao, X. Friction and wear behavior of diamond-like coating on plasma nitrided mild steel under boundary lubrication. Tribol. Int. 2010, 43, 474–482. [Google Scholar] [CrossRef]
- González, R.; Hernández Battez, A.; Blanco, D.; Viesca, J.L.; Fernández-González, A. Lubrication of TiN, CrN and DLC PVD Coatings with 1-Butyl-1-Methylpyrrolidinium tris(pentafluoroethyl)trifluorophosphate. Tribol. Lett. 2010, 40, 269–277. [Google Scholar] [CrossRef]
- Blanco, D.; Battez, A.H.; Viesca, J.L.; González, R.; Fernández-González, A. Lubrication of CrN Coating With Ethyl-Dimethyl-2-Methoxyethylammonium Tris(pentafluoroethyl)Trifluorophosphate Ionic Liquid as Additive to PAO 6. Tribol. Lett. 2010, 41, 295–302. [Google Scholar] [CrossRef]
- Blanco, D.; González, R.; Hernández Battez, A.; Viesca, J.L.; Fernández-González, A. Use of ethyl-dimethyl-2-methoxyethylammonium tris(pentafluoroethyl)trifluorophosphate as base oil additive in the lubrication of TiN PVD coating. Tribol. Int. 2011, 44, 645–650. [Google Scholar] [CrossRef]
- Hernández Battez, A.; González, R.; Viesca, J.L.; Fernández-González, A.; Hadfield, M. Lubrication of PVD coatings with ethyl-dimethyl-2-methoxyethylammonium tris(pentafluoroethyl)trifluorophosphate. Tribol. Int. 2013, 58, 71–78. [Google Scholar] [CrossRef]
- González, R.; Hernández Battez, A.; Viesca, J.L.; Higuera-Garrido, A.; Fernández-González, A. Lubrication of DLC Coatings with TwoTris(pentafluoroethyl)trifluorophosphate Anion-Based Ionic Liquids. Tribol. Trans. 2013, 56, 887–895. [Google Scholar]
- Spikes, H. The history and mechanisms of ZDDP. Tribol. Lett. 2004, 17, 469–489. [Google Scholar] [CrossRef]
- Barnes, A.M.; Bartle, K.; Vincent, R.; Thibon, P. A review of zinc dialkyldithiophosphates (ZDDPS): Characterization and role in the lubricating oil. Tribol. Int. 2001, 34, 389–395. [Google Scholar] [CrossRef]
- Chang, J.H.; Jung, M.N.; Park, J.S.; Park, S.H.; Im, I.H.; Lee, H.J.; Ha, J.S.; Fujii, K.; Hanada, T.; Yao, T.; et al. X-ray photoelectron spectroscopy study on the CrN surface grown on sapphire substrate to control the polarity of ZnO by plasma-assisted molecular beam epitaxy. Appl. Surf. Sci. 2009, 255, 8582–8586. [Google Scholar] [CrossRef]
- Wagner, C.D.; Riggs, W.M.; Davis, L.E.; Moulder, J.F. Handbook of X-Ray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Waltham, MA, USA, 1979. [Google Scholar]
- Lippitz, A.; Hübert, T. XPS investigations on chromium nitride thin films. Surf. Coat. Technol. 2005, 200, 250–253. [Google Scholar] [CrossRef]
- Im, J.S.; Park, S.J.; Lee, Y.S. The metal-carbon-fluorine system for improving hydrogen storage by using metal and fluorine with different levels of electronegativity. Int. J. Hydrogen Energy 2009, 34, 1423–1428. [Google Scholar] [CrossRef]
- Riyadh, M.A.; Datar, A.; Bhorsakar, S.V.; Bhoraskar, V.N. Surface modification of polymers by atomic oxygen using ECR plasma. Nucl. Instrum. Methods Phys. Res. B 2007, 258, 345–351. [Google Scholar]
- Li, D.; Haneda, H.; Hishita, S.; Ohashi, N.; Labhsetwar, N.K. Fluorine-doped TiO2 powders prepared by spray pyrolysis and their improved photocatalytic activity for decomposition of gas-phase acetaldehyde. J. Fluorine Chem. 2005, 126, 69–77. [Google Scholar] [CrossRef]
- Galvanetto, E.; Galliano, F.P.; Borgioli, F.; Bardi, U.; Lavacchi, A. XRD and XPS study on reactive plasma sprayed titanium–titanium nitride coatings. Thin Solid Films 2001, 384, 223–229. [Google Scholar] [CrossRef]
- Kang, B.S.; Sul, Y.T.; Oh, S.J.; Lee, H.J.; Albrektsson, T. XPS, AES and SEM analysis of recent dental implants. Acta Biomater. 2009, 5, 2222–2229. [Google Scholar] [CrossRef] [PubMed]
- Lahoz, R.; Espinós, J.P.; De la Fuente, G.F.; González-Elipe, A.R. “In situ” XPS studies of laser induced surface cleaning and nitridation of Ti. Surf. Coat. Tech. 2008, 202, 1486–1492. [Google Scholar] [CrossRef]
- Yu, B.; Zhou, F.; Mu, Z.; Liang, Y.; Liu, W. Tribological properties of ultra-thin ionic liquid films on single-crystal silicon wafers with functionalized surfaces. Tribol. Int. 2006, 39, 879–887. [Google Scholar] [CrossRef]

| Materials | Properties | |||
|---|---|---|---|---|
| Base Oil/Additive | Density (g/cm3) ASTM D 4052 (15 °C) | Kinematic Viscosity (mm2/s) ASTM D 445 | Viscosity Index ASTM D 2270 | |
| 40 °C | 100 °C | |||
| PAO 6 | 0.826 | 31.0 | 5.90 | 135 |
| ZDDP | 2.320 | 320 | 14 | - |
| [BMP][FAP] | 1.590 | 58.8 | 8.6 | 118 |
| PVD Coatings | Thickness (µm) | Roughness (µm) | Hardness (Vickers) | |
| TiN | 2.9 | 0.27 | 1633 | |
| CrN | 3 | 0.26 | 2137 | |
| Ionic Liquid | ||||
| IUPAC name | Purity (%) | Water content (%) | ||
| 1-Butyl-1-methylpyrrolidinium tris(pentafluoroethyl)trifluorophosphate | 98 | <1 (Karl Fisher) | ||
| Cation | Anion | |||
| (C4H8)(CH3)(CH2CH2CH2CH3)N+ | F3(C2F5)3P− | |||
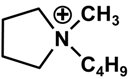 | 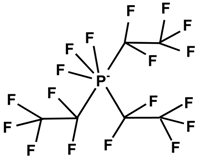 | |||
| [BMP] | [FAP] | |||
| Load (N) | Coating | Lubricant | Average | SD | COF Reduction (%) |
|---|---|---|---|---|---|
| 20 | CrN | PAO 6 | 0.122 | 0.0061 | - |
| +1% ZDDP | 0.077 | 0.0033 | 36.85 | ||
| +1% IL | 0.099 | 0.0030 | 18.38 | ||
| TiN | PAO 6 | 0.132 | 0.0061 | - | |
| +1% ZDDP | 0.072 | 0.0028 | 45.42 | ||
| +1% IL | 0.091 | 0.0033 | 36.33 | ||
| 40 | CrN | PAO 6 | 0.130 | 0.0061 | - |
| +1% ZDDP | 0.098 | 0.0035 | 24.14 | ||
| +1% IL | 0.110 | 0.0037 | 15.21 | ||
| TiN | PAO 6 | 0.164 | 0.0087 | - | |
| +1% ZDDP | 0.086 | 0.0046 | 47.43 | ||
| +1% IL | 0.137 | 0.0043 | 15.54 |
| Band | Load (N) | B.E. (eV) | % | Assigned to | Reference |
|---|---|---|---|---|---|
| F 1s | 20 | 687.9 | 88 | FAP− | [47] |
| 686.2 | 12 | Cr-F | [48] | ||
| Cr 2p | 574.9 | 17 | CrN | [54] | |
| 575.7 | 74 | Air exposed CrN | [54] | ||
| 579.8 | 9 | Cr-F | [55] | ||
| N 1s | 396.6 | 61 | CrN | [56] | |
| 402.5 | 39 | Cr nitrites/nitrates | [56] | ||
| F 1s | 40 | 688.1 | FAP− | [47] | |
| Cr 2p | 574.9 | 14 | CrN | [54] | |
| 575.7 | 86 | Air exposed CrN | [54] | ||
| N 1s | 396.7 | 57 | CrN | [56] | |
| 402.6 | 43 | Cr nitrites/nitrates | [56] |
| Band | Load (N) | B.E. (eV) | % | Assigned to | Reference |
|---|---|---|---|---|---|
| F 1s | 20 | 685.2 | 45 | TiOF2 | [59] |
| 686.1 | 55 | TiF | [59] | ||
| Ti 2p | 453.1 | 54 | Ti° | [62] | |
| 455.1 | 9 | TiO | [61] | ||
| 455.6 | 37 | TiN | [62] | ||
| F 1s | 40 | 686.9 | 70 | Monofluorinated C | [57,58] |
| 688.0 | 30 | [FAP−] | [63] | ||
| Ti 2p | 454.6 | 58 | TiN | [60] | |
| 456.9 | 42 | Ti2O3 | [61] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viesca, J.-L.; Anand, M.; Blanco, D.; Fernández-González, A.; García, A.; Hadfield, M. Tribological Behaviour of PVD Coatings Lubricated with a FAP− Anion-Based Ionic Liquid Used as an Additive. Lubricants 2016, 4, 8. https://doi.org/10.3390/lubricants4010008
Viesca J-L, Anand M, Blanco D, Fernández-González A, García A, Hadfield M. Tribological Behaviour of PVD Coatings Lubricated with a FAP− Anion-Based Ionic Liquid Used as an Additive. Lubricants. 2016; 4(1):8. https://doi.org/10.3390/lubricants4010008
Chicago/Turabian StyleViesca, José-Luis, Mayank Anand, David Blanco, Alfonso Fernández-González, Alberto García, and Mark Hadfield. 2016. "Tribological Behaviour of PVD Coatings Lubricated with a FAP− Anion-Based Ionic Liquid Used as an Additive" Lubricants 4, no. 1: 8. https://doi.org/10.3390/lubricants4010008
APA StyleViesca, J.-L., Anand, M., Blanco, D., Fernández-González, A., García, A., & Hadfield, M. (2016). Tribological Behaviour of PVD Coatings Lubricated with a FAP− Anion-Based Ionic Liquid Used as an Additive. Lubricants, 4(1), 8. https://doi.org/10.3390/lubricants4010008







