1. Introduction
Organophosphorus compounds are commonly used as anti-wear additives in automotive and aviation lubrication systems [
1,
2,
3]. Anti-wear additives have vital importance under extreme pressure (EP) conditions where the oil film between contacting surfaces is not sufficiently thick to prevent direct contact between the surface asperities. In such conditions, ideal additives form robust anti-wear films that adhere strongly to the surfaces and also withstand higher temperatures, particularly in aviation engines that operate at higher temperatures and higher speeds [
4,
5]. Phosphate esters and (metal) thiophosphate esters have been used as lubricant additives for more than 50 years in both the automotive and aviation industries. Phosphate esters, such as tricresyl phosphate (TCP), tributyl phosphate (TBP), triphenyl phosphate (TPP), etc., were initially used for aviation engines. Despite the health concerns that they pose [
6,
7], their use has expanded, and they are presently employed in a wide range of applications, including automotive engines [
1].
Although environmental and health concerns place limitations on the amount of phosphorus in lubricants, the additives are expected to be utilized well into the future. Indeed, one common requirement for many systems is that newly developed lubricant additives must be compatible with existing phosphate esters additives, rather than outright replacing them. An appropriate strategy to limit their use levels while also retaining their function is to blend them with a synergistic and environmentally friendly additive. The overarching goal here is to add the synergistic additive without increasing the amount of phosphorus in the system until it is required to do so. This strategy would be completely practical to implement and applicable in various fields, such as aviation, automobiles, manufacturing, defense, etc., that implement a condition-based maintenance schedule [
8].
Nanomaterials are emerging as environmentally friendly and effective additives for controlling friction and wear in various applications [
9]. Several physical mechanisms such as change in friction mode (sliding to rolling), surface polishing/mending, reduction in cohesive forces via layered materials, etc., and chemical mechanisms, including material delivery/transfer for catalysis, tribofilm formation by tribochemical reactions, etc., have been identified as lubrication mechanisms for nanomaterials [
10]. However, nanomaterials should also be able to withstand tough environments under EP conditions during boundary lubrication and the high operating temperatures of aircraft engines if they are to be successfully blended with currently used additives. This requires that nanomaterials have, among other properties, a high melting point, high hardness, and favorable chemical reactions to allow rigid bonding onto the surface, together with the anti-wear film produced by the phosphate ester [
5].
Among the nanomaterials discovered and studied so far, nanodiamonds are the leading candidates from the point of view of hardness, high melting point, and the ability to withstand extreme environments. However, their impact on the performance of existing phosphate ester anti-wear additives is unknown. Several studies of nanodiamond additives have revealed their ability to improve tribological performance in various applications as well as various lubrication regimes including boundary lubrication [
11,
12,
13]. However, their direct influence on the performance of existing additives has not been extensively studied to date. Since nanodiamonds have been reported to suppress reaction kinetics in certain systems by raising the activation energy for reactions [
14], it is critically important to assess their direct effect on the reaction activities of existing anti-wear additives. Prior studies on various reaction mechanism paths through which phosphate esters form films on Fe surfaces have revealed that there is a requirement to overcome an activation energy to break either P–O or O–C bonds on the phosphate esters, depending on whether they are aliphatic or aromatic [
15,
16,
17]. The activation energy can be attained with thermal energy, resulting in a thermal reaction film, or by other means, such as rubbing the contacts to form a tribofilm, etc. Among the various cases, the films formed are similar in chemistry, but the formation temperature varies significantly [
18]. Nanodiamonds, as a proposed synergistic additive to phosphate esters, should clearly not have unfavorable effects on the reaction kinetics.
To explore this issue, we studied the effects of nanodiamonds on the formation of thermal reaction films on Fe and air baked Fe surfaces by TCP in a dibasic ester basestock. The TCP thermal reaction film formation temperatures in the presence and absence of nanodiamonds were recorded in-situ by monitoring of the reaction film formation on the surfaces using a quartz crystal microbalance (QCM) [
19]. We recently reported the in-situ TCP thermal reaction film formation temperatures for Fe to be 210 °C [
19]. Fe samples that were baked in air beforehand did not, however, react with TCP to form the thermal reaction film. It is therefore of interest to explore how the presence of nanodiamonds in the blend would influence whether or not thermal reaction films form on these substrates and whether the resulting reaction films, if present, are impacted by the nanodiamonds. The surface morphology, roughness, and thickness of the thermal reaction films that did form were studied with an atomic force microscope (AFM), and their chemical compositions were studied with Electron Dispersive X-ray Spectroscopy (EDS). The TCP-derived films were regarded as anti-wear films, rather than materials having the property of decreasing the coefficient of friction. Since the QCMs are fragile, the thermal reaction films formed on the QCM substrates could not withstand the rigorous tribo-testing procedure that is regularly performed for such anti-wear films. We, therefore, as a simple gauge, employed a basic friction measurement approach to provide information on the coefficient of sliding friction at the dry solid–solid contact between the thermal reaction films and the stainless-steel balls. The contact angles between the thermal reaction films and the base oil were measured to examine any changes in the solid–liquid wetting property of the surfaces after the TCP uptake. Change in contact angles are associated with changes in the physical and chemical properties of the surfaces after formation of the thermal reaction films. In particular, it is important to identify changes in the slip condition at the QCM’s solid–liquid interface [
20].
2. Materials and Methods
Diisodecyl Adipate (Sigma-Aldrich Co. LLC, St. Louis, MO, USA) was used as the base oil. The TCP (Sigma-Aldrich Co. LLC) was of a technical grade and was a mixture of isomers. One-inch diameter, 5 MHz, AT-cut QCMs with liquid-facing surface electrodes comprised of Fe (INFICON Inc., East Syracuse, NY, USA, P/N 149252-1) were employed for QCM measurements. These Fe-coated QCMs consisted of 800 nm Fe films deposited onto 60 nm Cr adhesion layers deposited onto the QCM surfaces. The air baked Fe samples were obtained by heating the Fe-coated QCMs in air. Nanodiamonds with 30 nm diameters dispersed in oil at a concentration of 1 wt % were obtained from Adamas Nanotechnologies. The nanodiamond dispersion was diluted to the desired concentration in the base oil and then used for further measurements.
The QCM measurement techniques employed here have been previously described in great detail [
19,
21]. The QCM crystals were housed in a custom made holder and then immersed in oil containing either TCP or a TCP–nanodiamond blend. The holder was then mounted within a temperature-controlled chamber and heated from room temperature to ~240 °C over 2 h. Data were collected using a QCM100 (Stanford Research Systems, Sunnyvale, CA, USA) system, comprised of an electronic oscillator and a controller [
22]. The frequency and conductance of the crystal were measured by a frequency counter (HP 53181A Frequency Counter, Palo Alto, CA, USA) and a multimeter (TEKTRONIX, INC., Beaverton, OR, USA, Keithley 2000 Series), respectively. The frequency shifts of the QCM provided information on film uptake, liquid viscosity, and film stress. Conductance measurements provided information on dissipative losses associated with the drag forces of the surrounding liquid and/or losses within the reaction film itself. A second multimeter measured the resistance of an RTD attached to the holder. A LabVIEW program (National Instrument, Austin, TX, USA, LabVIEW 7.1) recorded the frequency and conductance of the QCM and resistance of the RTD at 5 s intervals. The conductance, initially measured in volts, was converted to the motional resistance of the QCM using the relationship,
R = 10
(4−V/5) − 75 [
22], where
V is the conductance in volts and
R is the motional resistance of QCM in Ohms. The RTD resistance was converted to temperature by employing the Callendar–Van Dusen equation [
23].
To correct the data for the temperature effects, the crystal was first heated in air to obtain the temperature dependence of the frequency and the motional resistance response. The crystal was then held at 225 °C for 2 h, allowing the iron to oxidize, and is referred to as the “baked Fe” sample herein. Both the “baked Fe” samples and fresh (unheated) samples of Fe were employed for the TCP thermal reaction film monitoring studies. Since bare metal surfaces always have a few layers of chemisorbed oxide, a native oxide was presumed to be present on the unheated Fe samples. Thermal reaction film formation temperatures were recorded in-situ by monitoring the reaction film formation on the Fe and “baked Fe” by immersing the QCM samples in the basestock with 5% TCP in the absence or presence of 0.1 wt % nanodiamonds and then heating them from room temperature to ~240 °C. QCM responses were then corrected for temperature effects to isolate the effects of oil and thermal reaction films (if formed).
After the QCM measurements, the crystals were cleaned with
iso-propanol to remove excess oil from the surface. The surface morphology, roughness, and thickness of the thermal reaction films were then studied with an AFM (MFP 3D, Asylum Research, an Oxford Instruments Company, Santa Barbara, CA, USA). Silicon tips were used to image the surfaces in tapping mode. Images with 512 × 512 pixels were recorded with a speed of 1 line per second. The scan sizes ranged from 2 to 6 µm. Their roughness was characterized by the root mean square (rms) roughness value,
and also, the average roughness value,
, which provide a measure of the magnitude of variation between the surface height profiles. Surface roughness was also characterized by a third parameter, the self-affine fractal roughness exponent H, which reflects the nature of the texture of surface irregularities on the surfaces. For a self-affine surface, H is related to
σ and scan size (
L) as
σ ∝
LH. It can be obtained from the slope of a linear fit of log(
σ) vs log(
L) plot for the scan sizes below the saturation length [
24]. The fractal dimension,
D = 3 − H, for the QCM surface electrodes before and after the TCP uptake were calculated after estimating the values of H for the respective surfaces. A surface with
D = 2 is a perfect 2D surface, while a
D value higher than 2 indicates surfaces with more texture that deviate from a 2D plane. The thicknesses of the thermal reaction films were measured by calculating the differences in AFM height profiles measured across the film–surface boundary after etching the thermal reaction film with 0.3 M oxalic acid (Sigma-Aldrich Co. LLC.). The chemical compositions of the thermal reaction films were studied with EDS (JSM-6010LA Analytical Scanning Electron Microscope, JEOL USA Inc., Peabody, MA, USA). The measurements were performed with a 20 kV acceleration voltage, a 10 mm working distance, and with 100× magnification. The contact angles of the base oil on the QCM electrode surfaces before and after the TCP uptake were measured using the drop shape analysis technique [
25].
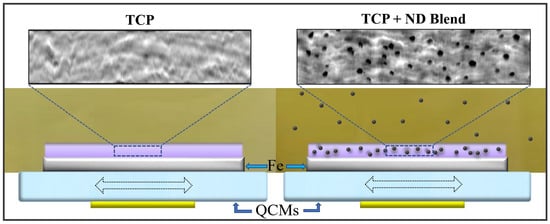
As mentioned earlier, the thermal reaction films formed on the QCM substrates could not be employed for a rigorous tribo-testing procedure because of the fragility of the quartz substrates. Therefore, the coefficients of sliding friction at the dry solid–solid contact between the thermal reaction film and 304 stainless-steel ball bearings were measured by a conventional Pasco laboratory instrument (Pasco Scientific, Roseville, CA, USA). Seven stainless-steels balls of 5/32′ diameter were glued onto a weight (in hexagonal close packed configuration). The weight was then placed on the surface electrode of a QCM glued on a platform, as shown in the
Figure 1. The weight was then pulled by another weight with a lightweight cord tied to it while measuring the force continuously until a constant lowest possible relative speed was maintained between the QCM surface and the sliding weight. The coefficient of sliding friction was then furnished from the applied force and the normal force.
4. Discussion
The nanodiamonds were observed to influence the formation of TCP thermal reaction films on both Fe and baked Fe surfaces. It is important to identify whether or not these effects are favorable.
Figure 9 shows the QCM responses of all film-forming systems presented in
Figure 2 and
Figure 3. TCP was observed to form a thermal reaction film on baked Fe surfaces in the presence of nanodiamonds which otherwise did not form the film. This is a notable characteristic of nanodiamonds. The changes in
f and
R in the presence of nanodiamonds observed on baked Fe starting at 190 °C suggested the adsorption of mass onto the surface. This is likely to be due to the nanodiamonds either making their way to the inner layers of the oxide film or causing wear to the oxide layers, thus allowing the TCP molecules to absorb into the inner layers of the oxide surface. This mechanism is conceivable since the oscillation of QCM at a frequency of 5 MHz would produce a maximum oscillating surface speed in the order of 10 cm/s, and nanodiamonds have been reported to have these characteristics under such conditions [
27]. The TCP molecules making their way to the Fe through the oxide layers then formed a thermal reaction film on the surface when the activation energy reached a temperature of 228 °C. This thermal reaction film was expected to have a higher oxygen content which was, indeed, observed during the EDS measurements. In the presence of nanodiamonds, the formation of the thermal reaction films was observed on both Fe and baked Fe surfaces at the same temperature of 228 °C (within the experimental error) which is higher by 18 °C than that observed on Fe surfaces in the absence of nanodiamonds. This result is not unexpected, because it was anticipated that the nanodiamonds would raise the activation energy in such reactions. The increase in activation energy is not, however, highly disadvantageous, as the observed increase in reaction temperature was modest and readily achievable in actual applications.
The change in motional resistance observed for formation of the thermal reaction film is associated with changes in the QCM’s dissipative properties, such as a change in the slip condition at the solid–liquid boundary, an increase in energy dissipation within the viscoelastic film on the QCM’s surface, a change in surface roughness, etc. The observed shift in
R was not in the direction expected from the smoother films and/or surfaces with higher contact angle values (c.a.
Figure 5) [
20,
28]. Therefore, the higher increase in
R during the film formation on Fe surfaces in the absence of nanodiamonds suggested that this film is relatively softer or more viscous compared to the films formed in the presence of nanodiamonds [
29]. This implies that the films formed in the presence of nanodiamonds are more rigid which is to be expected if the nanodiamonds are embedded into the film, as they are potentially capable of exhibiting more outward stress.
The increase in frequency observed during the formation of thermal reaction films is in fact consistent with stress developing within the film. The rigidly-bonded thermal reaction film on the surface acts to stretch the surface and causes stress on the crystal. The positive
f shift for the AT-cut quartz indicates that the tensile stress was isotropic and outward. The average stresses on the crystal and the thermal reaction film on the Fe surface in the absence of nanodiamonds (corresponding to the associated
δf ≈ 5 kHz) have been reported to be
TQ ≈ 40 MPa and
Tf ≈ 200 GPa, respectively [
19]. The value of this internal stress for the thermal reaction film is in the same order of the effective modulus as the TCP tribofilms, and the stress on the crystal is in the same order as the shear modulus of quartz [
3]. However, the frequency shifts associated with the film formation in the presence of nanodiamonds were significantly higher compared to the film formed in the absence of nanodiamonds (c.a.
Figure 9). These higher frequency shifts are consistent with the idea that increased stress is present when nanodiamonds are present in the film. The average stresses on the crystals and the thermal reaction films formed on the Fe and baked Fe surfaces in the presence of nanodiamonds (corresponding to the associated
δf > 10 kHz) would be higher than their corresponding elastic moduli. These stresses greatly inhibit the ability of the crystal to oscillate. During the measurements, the crystals indeed stopped oscillating about 2–3 min after the film formation in the presence of nanodiamonds. The
δf and
δR data shown in
Figure 3,
Figure 4, and
Figure 9 for both surfaces in the presence of nanodiamonds were recorded until the crystals oscillated after the film was formed. It is expected that the
δf would have increased further until it plateaued once the reaction was nearly exhausted, as seen in the case of the Fe surfaces without nanodiamonds. These steeply increasing values of
δf in the presence of nanodiamonds meanwhile confirmed that although these nanodiamonds increase the thermal reaction film formation temperature by raising the activation energy, the reaction rate may not necessarily be slower, as the reaction for the phosphate esters, being exothermic, readily provides the required activation energy to continue the reaction once it is initiated [
15,
16].
The films formed in the presence of nanodiamonds were observed to be stiffer and exhibited higher levels of internal stress. The internal stress of the reaction films is an integral part of their performance, since it counteracts external load forces, thus enhancing the load-bearing capacity of the substrate [
30]. The nanodiamonds also changed the texture of the thermal reaction films (c.a.
Figure 5c) while increasing their wettability with the lubricant (c.a.
Figure 5d). These effects are beneficial, especially in extreme pressure conditions, whence the lubricants can easily be squeezed out from the contact at the surfaces with lower wettability, lowering the resistance to scuffing [
31,
32].
The thermal reaction film on the baked Fe surfaces in dry conditions in an ambient environment was observed not to have the signature characteristics of a typical anti-wear film. One plausible reason for this could be that the film formed on baked Fe surfaces with a different mechanism and has a higher oxygen content, as observed in the EDS measurements. The higher amount of oxygen probably has an effect on the strength of the film during dry contact, because the oxygen can absorb the moisture/humidity from the ambient air rather easily and deteriorate the film strength [
33]. A thorough investigation is necessary to confirm this suggestion and also, to find other probable causes. While the nanodiamonds did not favor the dry contact on the baked Fe surfaces, it might be beneficial in other situations, especially under mixed lubrication on surfaces with higher oxide contents. Having the film is always more beneficial than having a bare surface. In real applications, in oil environments, the nanodiamonds would be able to perform the special role of enabling film formation and replenishing it continuously from the fresh supply of oil while the tribo-pairs are in action. The rate of film formation, however, must not be exceeded by the wear rate [
34].