Nanocomposite of Poly(l-Lactic Acid) with Inorganic Nanotubes of WS2
Abstract
1. Introduction
2. Experimental
2.1. Preparation of PLLA/PLLA–INT–WS2 Nanocomposites
2.2. Functionalization of the INT-WS2
- PEI: INT–WS2 powder was sonicated at room temperature in a mixture of INT–WS2: 2% aqueous solution of PEI in a ratio of 2 mg: 1 mL for 10 min. Subsequently, the suspension was centrifuged at 6000 rpm for 15 min and washed with distilled water. Finally, the suspension was dried at 50 °C for 24 h. The PLLA 24 films were prepared according to the description in Section 2.1 but with chloroform as a solvent instead of DCM.
- NMP: INT–WS2 powder was sonicated for 30 min in a mixture of INT–WS2: NMP: distilled water in a ratio of 2 mg:1 mL:2 mL. Subsequently, the suspension was annealed for 6 h at 120 C. The PLLA 38 films were prepared according to the description in Section 2.1.
- PEG: INT–WS2 solution was mixed at room temperature in a mixture of INT–WS2:DCM:PEG distilled water in a ratio of 1 mg:1 mL: 1 mg:20 mL. Subsequently, the solution was treated in ultrasonic bath for 30 min followed by centrifugation at 6000 rpm for 5 min and washed with distilled water to eliminate the excess polymer. The PLLA 24 films were prepared according to the description in Section 2.1.
2.3. Characterization Techniques
2.3.1. High-Resolution Scanning Electron Microscopy (HRSEM)
2.3.2. Mechanical Properties
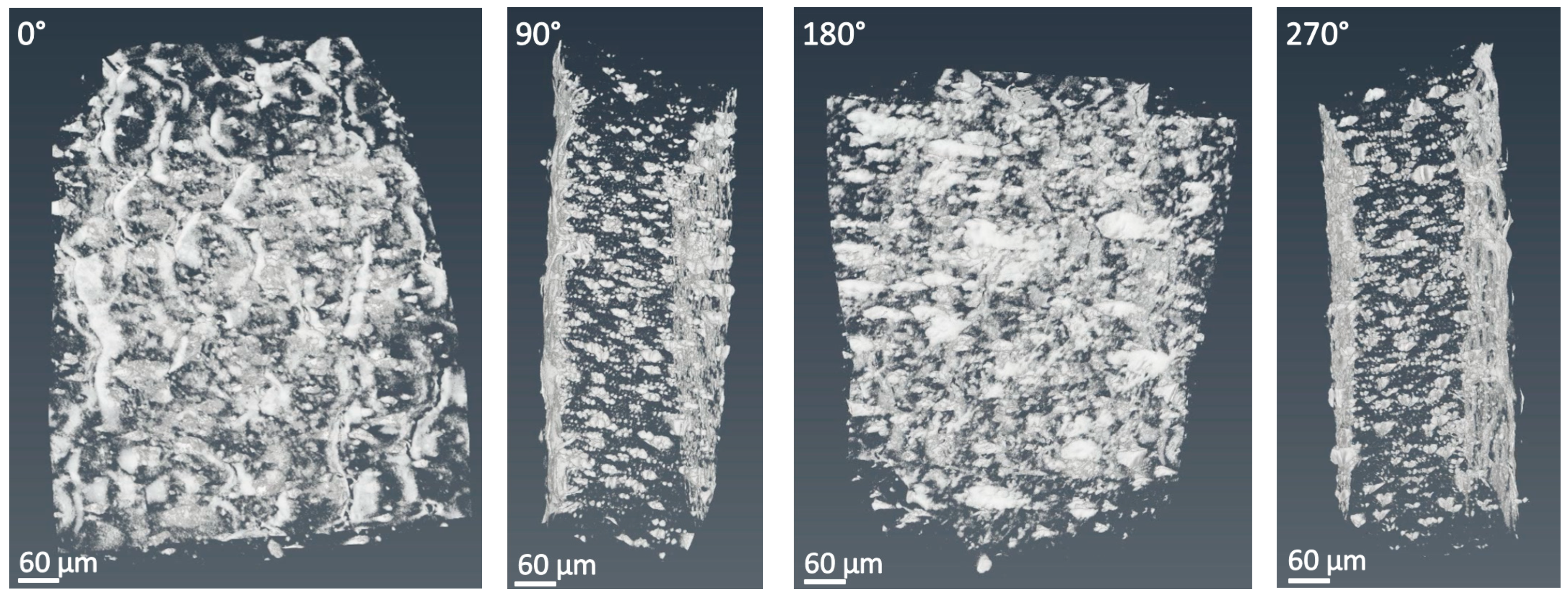
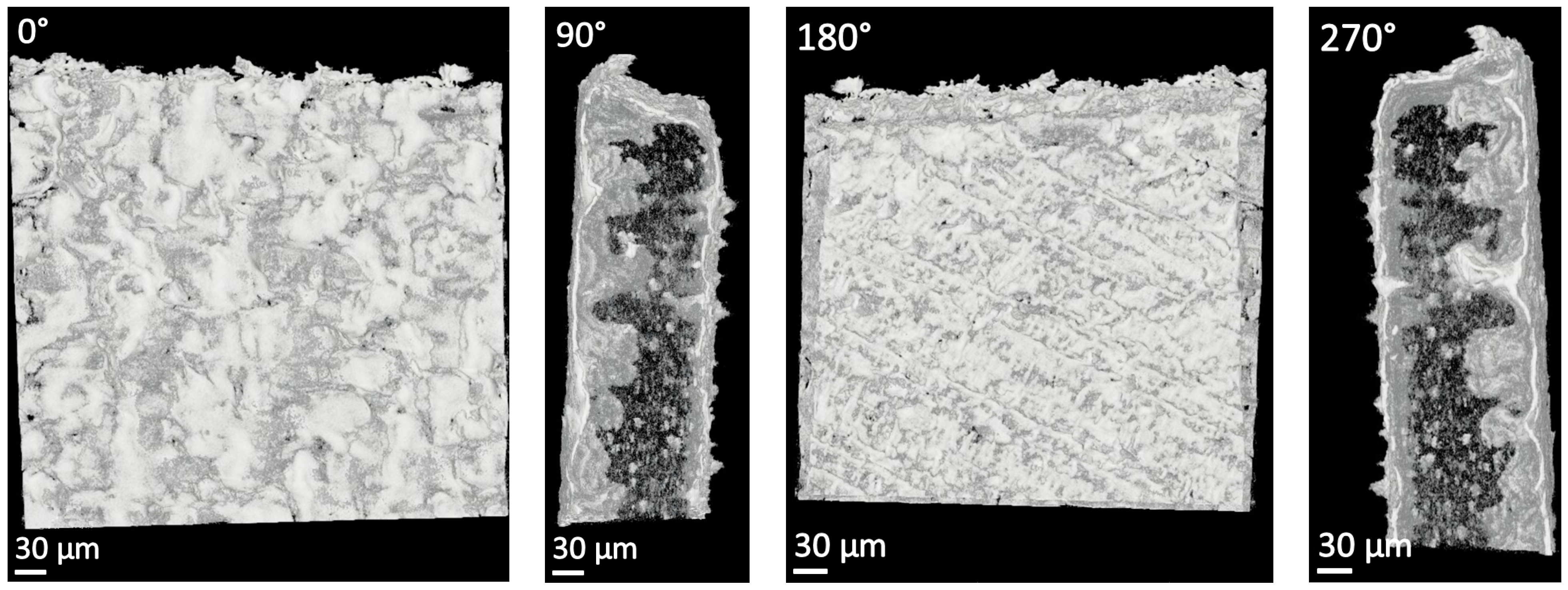
2.3.3. X-ray Tomographic Microscopy (Micro-XCT)
2.3.4. Differential Scanning Calorimetry (DSC)
2.3.5. X-ray Diffraction
2.3.6. Rheological Characterization
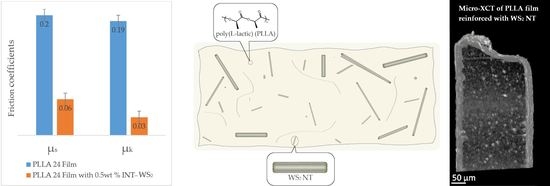
2.3.7. Friction Coefficient
- PLLA 24 film and PLLA 24 film with 0.5 wt % INT–WS2 were run 20 times on the steel table (the first 16 times served for running-in). The results of the last 4 measurements are reported.
- PLLA 24 film and PLLA 24 + 0.5 wt % INT–WS2 film was rubbed 5 times on a silicon–carbide (SiC) paper (1200 grit) and then measured 3 times using the steel table.
- PLLA 24 film and PLLA 24 + 0.5 wt % INT–WS2 film was rubbed 5 times on a SiC paper (150 grit) and then measured 3 times using the steel table.
2.3.8. Friction Force
2.3.9. Raman Spectroscopy
3. Results and Discussion
3.1. X-ray Tomographic Microscopy (Micro-XCT)
3.2. Functionalization of the INT-WS2
Functionalization of INT–WS2 with PEI (Polyethylenimine)
3.3. Differential Scanning Calorimetry (DSC)
3.4. X-ray Diffraction
3.5. Rheological Characterization
3.6. Friction Coefficient
3.7. Friction Force
3.8. Raman Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Schick, C. Differential scanning calorimetry (DSC) of semicrystalline polymers. Anal. Bioanal. Chem. 2009, 395, 1589–1611. [Google Scholar] [CrossRef] [PubMed]
- Androsch, R.; Zhuravlev, E.; Schick, C. Solid-state reorganization, melting and melt-recrystallization of conformationally disordered crystals (α’-phase) of poly (l-lactic acid). Polymer (Guildf.) 2014, 55, 4932–4941. [Google Scholar] [CrossRef]
- Righetti, M.C.; Gazzano, M.; Di Lorenzo, M.L.; Androsch, R. Enthalpy of melting of α′- and α-crystals of poly(L-lactic acid). Eur. Polym. J. 2015, 70, 215–220. [Google Scholar] [CrossRef]
- Androsch, R.; Di Lorenzo, M.L. Effect of molar mass on the α′/α-transition in poly(L-lactic acid). Polymer (Guildf.) 2017, 114, 144–148. [Google Scholar] [CrossRef]
- Righetti, M.C.; Tombari, E. Crystalline, mobile amorphous and rigid amorphous fractions in poly(L-lactic acid) by TMDSC. Thermochim. Acta 2011, 522, 118–127. [Google Scholar] [CrossRef]
- Brás, A.R.; Viciosa, M.T.; Wang, Y.; Dionísio, M.; Mano, J.F. Crystallization of poly(L-lactic acid) probed with dielectric relaxation spectroscopy. Macromolecules 2006, 39, 6513–6520. [Google Scholar] [CrossRef]
- Van de Velde, K.; Kiekens, P. Biopolymers: Overview of several properties and consequences on their applications. Polym. Test. 2002, 21, 433–442. [Google Scholar] [CrossRef]
- Rezwana, K.; Chena, Q.Z.; Blakera, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-D.; Todo, M.; Arakawa, K. Effect of annealing on fracture mechanism of biodegradable poly(lactic acid). J. Mater. Sci. 2004, 39, 1113–1116. [Google Scholar] [CrossRef]
- Moon, S.I.; Jin, F.; Lee, C.J.; Tsutsumi, S.; Hyon, S.H. Novel carbon nanotube/poly(l-lactic acid) nanocomposites; their modulus, thermal stability, and electrical conductivity. Macromol. Symp. 2005, 224, 287–295. [Google Scholar] [CrossRef]
- Klonos, P.; Terzopoulou, Z.; Koutsoumpis, S.; Zidropoulos, S.; Kripotou, S.; Papageorgiou, G.Z.; Bikiaris, D.N.; Kyritsis, A.; Pissis, P. Rigid amorphous fraction and segmental dynamics in nanocomposites based on poly(l–lactic acid) and nano-inclusions of 1–3D geometry studied by thermal and dielectric techniques. Eur. Polym. J. 2016, 82, 16–34. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Klonos, P.A.; Kyritsis, A.; Tziolas, A.; Avgeropoulos, A.; Papageorgiou, G.Z.; Bikiaris, D.N. Interfacial interactions, crystallization and molecular mobility in nanocomposites of Poly(lactic acid) filled with new hybrid inclusions based on graphene oxide and silica nanoparticles. Polymer (Guildf.) 2019, 166, 1–12. [Google Scholar] [CrossRef]
- Leng, J.; Purohit, P.J.; Kang, N.; Wang, D.-Y.; Falkenhagen, J.; Emmerling, F.; Thünemann, A.F.; Schönhals, A. Structure–property relationships of nanocomposites based on polylactide and MgAl layered double hydroxides. Eur. Polym. J. 2015, 68, 338–354. [Google Scholar] [CrossRef]
- Saiter, A.; Delpouve, N.; Dargent, E.; Oberhauser, W.; Conzatti, L.; Cicogna, F.; Passaglia, E. Probing the chain segment mobility at the interface of semi-crystalline polylactide/clay nanocomposites. Eur. Polym. J. 2016, 78, 274–289. [Google Scholar] [CrossRef]
- Pluta, M.; Jeszka, J.K.; Boiteux, G. Polylactide/montmorillonite nanocomposites: Structure, dielectric, viscoelastic and thermal properties. Eur. Polym. J. 2007, 43, 2819–2835. [Google Scholar] [CrossRef]
- Purohit, P.J.; Wang, D.-Y.; Wurm, A.; Schick, C.; Schönhals, A. Comparison of thermal and dielectric spectroscopy for nanocomposites based on polypropylene and Layered Double Hydroxide—Proof of interfaces. Eur. Polym. J. 2014, 55, 48–56. [Google Scholar] [CrossRef]
- Kuan, C.F.; Chen, C.H.; Kuan, H.C.; Lin, K.C.; Chiang, C.L.; Peng, H.C. Multi-walled carbon nanotube reinforced poly(L-lactic acid) nanocomposites enhanced by water-crosslinking reaction. J. Phys. Chem. Solids 2008, 69, 1399–1402. [Google Scholar] [CrossRef]
- Leal, C.V.; Martinez, D.S.T.; Más, B.A.; Alves, O.L.; Duek, E.A.R. Influence of purified multiwalled carbon nanotubes on the mechanical and morphological behavior in poly (L-lactic acid) matrix. J. Mech. Behav. Biomed. Mater. 2016, 59, 547–560. [Google Scholar] [CrossRef]
- Obarzanek-Fojt, M.; Elbs-Glatz, Y.; Lizundia, E.; Diener, L.; Sarasua, J.-R.; Bruinink, A. From implantation to degradation—Are poly (l-lactide)/multiwall carbon nanotube composite materials really cytocompatible? Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.W.; Gupta, R.K.; Bhattacharya, S.N.; Choi, H.J. Rheology and physical characteristics of synthetic biodegradable aliphatic polymer blends dispersed with MWNTs. Macromol. Mater. Eng. 2010, 295, 320–328. [Google Scholar] [CrossRef]
- Ivanova, R.; Kotsilkova, R. Rheological study of poly(lactic) acid nanocomposites with carbon nanotubes and graphene additives as a tool for materials characterization for 3D printing application. Appl. Rheol. 2018, 28, 54014–54023. [Google Scholar]
- Jonoobi, M.; Harun, J.; Mathew, A.P.; Oksman, K. Mechanical properties of cellulose nanofiber (CNF) reinforced polylactic acid (PLA) prepared by twin screw extrusion. Compos. Sci. Technol. 2010, 70, 1742–1747. [Google Scholar] [CrossRef]
- Huda, M.S.; Drzal, L.T.; Mohanty, A.K.; Misra, M. Chopped glass and recycled newspaper as reinforcement fibers in injection molded poly(lactic acid) (PLA) composites: A comparative study. Compos. Sci. Technol. 2006, 66, 1813–1824. [Google Scholar] [CrossRef]
- Luo, Y.-B.; Li, W.-D.; Wang, X.-L.; Xu, D.-Y.; Wang, Y.-Z. Preparation and properties of nanocomposites based on poly(lactic acid) and functionalized TiO2. Acta Mater. 2009, 57, 3182–3191. [Google Scholar] [CrossRef]
- Foruzanmehr, M.; Vuillaume, P.Y.; Elkoun, S.; Robert, M. Physical and mechanical properties of PLA composites reinforced by TiO2 grafted flax fibers. Mater. Des. 2016, 106, 295–304. [Google Scholar] [CrossRef]
- Tenne, R. Advances in the synthesis of inorganic nanotubes and fullerene-like nanoparticles. Angew. Chem. Int. Ed. 2003, 42, 5124–5132. [Google Scholar] [CrossRef]
- Kaplan-Ashiri, I.; Cohen, S.R.; Gartsman, K.; Ivanovskaya, V.; Heine, T.; Seifert, G.; Wiesel, I.; Wagner, H.D.; Tenne, R. On the mechanical behavior of WS2 nanotubes under axial tension and compression. Proc. Natl. Acad. Sci. USA 2006, 103, 523–528. [Google Scholar] [CrossRef]
- Rozenberg, B.A.; Tenne, R. Polymer-assisted fabrication of nanoparticles and nanocomposites. Prog. Polym. Sci. 2008, 33, 40–112. [Google Scholar] [CrossRef]
- Sedova, A.; Bar, G.; Goldbart, O.; Ron, R.; Achrai, B.; Kaplan-Ashiri, I.; Brumfeld, V.; Zak, A.; Gvishi, R.; Wagner, H.D.; et al. Reinforcing silica aerogels with tungsten disulfide nanotubes. J. Supercrit. Fluids 2015, 106, 9–15. [Google Scholar] [CrossRef]
- Sonker, A.K.; Wagner, H.D.; Bajpai, R.; Tenne, R.; Sui, X. Effects of tungsten disulphide nanotubes and glutaric acid on the thermal and mechanical properties of polyvinyl alcohol. Compos. Sci. Technol. 2016, 127, 47–53. [Google Scholar] [CrossRef]
- Goldbart, O.; Sedova, A.; Yadgarov, L.; Rosentsveig, R.; Shumalinsky, D.; Lobik, L.; Wagner, H.D.; Tenne, R. Lubricating medical devices with fullerene-like nanoparticles. Tribol. Lett. 2014, 55, 103–109. [Google Scholar] [CrossRef]
- Goldbart, O.; Elianov, O.; Shumalinsky, D.; Lobik, L.; Cytron, S.; Rosentsveig, R.; Wagner, H.D.; Tenne, R. Study of urological devices coated with fullerene-like nanoparticles. Nanoscale 2013, 5, 8526–8532. [Google Scholar] [CrossRef]
- Shneider, M.; Rapoport, L.; Moshkovich, A.; Dodiuk, H.; Kenig, S.; Tenne, R.; Zak, A. Tribological performance of the epoxy-based composite reinforced by WS2 fullerene-like nanoparticles and nanotubes. Phys. Status Solidi 2013, 210, 2298–2306. [Google Scholar] [CrossRef]
- Ke, S.; Lai, Y.; Zhou, T.; Li, L.; Wang, Y.; Ren, L.; Ye, S. Molybdenum disulfide nanoparticles resist oxidative stress-mediated impairment of autophagic flux and mitigate endothelial cell senescence and angiogenic dysfunctions. ACS Biomater. Sci. Eng. 2018, 4, 663–674. [Google Scholar] [CrossRef]
- Wu, X.; Tian, X.; Chen, T.; Zeng, A.; Yang, G. Inorganic fullerene-like molybdenum selenide with good biocompatibility synthesized by laser ablation in liquids. Nanotechnology 2018, 29, 295604. [Google Scholar] [CrossRef] [PubMed]
- Fojtů, M.; Teo, W.Z.; Pumera, M. Environmental impact and potential health risks of 2D nanomaterials. Environ. Sci. Nano 2017, 4, 1617–1633. [Google Scholar] [CrossRef]
- Pardo, M.; Shuster-Meiseles, T.; Levin-Zaidman, S.; Rudich, A.; Rudich, Y. Low cytotoxicity of inorganic nanotubes and fullerene-like nanostructures in human bronchial epithelial cells: Relation to inflammatory gene induction and antioxidant response. Environ. Sci. Technol. 2014, 48, 3457–3466. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.H.; Cuong, T.V.; Hur, S.H.; Oh, E.; Kim, E.J.; Shin, E.W.; Chung, J.S. Chemical functionalization of graphene sheets by solvothermal reduction of a graphene oxide suspension in N-methyl-2-pyrrolidone. J. Mater. Chem. 2011, 21, 3371–3377. [Google Scholar] [CrossRef]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.Y.; Han, T.H.; Hong, J.; Kim, J.E.; Lee, S.H.; Kim, H.W.; Kim, S.O. Noncovalent functionalization of graphene with end-functional polymers. J. Mater. Chem. 2010, 20, 1907–1912. [Google Scholar] [CrossRef]
- Giordani, S.; Bergin, S.D.; Nicolosi, V.; Lebedkin, S.; Kappes, M.M.; Blau, W.J.; Coleman, J.N. Debundling of single-walled nanotubes by dilution: Observation of large populations of individual nanotubes in amide solvent dispersions. J. Phys. Chem. B 2006, 110, 15708–15718. [Google Scholar] [CrossRef] [PubMed]
- Furtado, C.A.; Kim, U.J.; Gutierrez, H.R.; Pan, L.; Dickey, E.C.; Peter, C. Eklund Debundling and dissolution of single-walled carbon nanotubes in amide solvents. J. Am. Chem. Soc. 2004, 126, 6095–6105. [Google Scholar] [CrossRef] [PubMed]
- Sahu, M.; Narashimhan, L.; Prakash, O.; Raichur, A.M. Noncovalently functionalized tungsten disulfide nanosheets for enhanced mechanical and thermal properties of epoxy nanocomposites. ACS Appl. Mater. Interfaces 2017, 9, 14347–14357. [Google Scholar] [CrossRef]
- Kim, I.S.; Lee, S.K.; Park, Y.M.; Lee, Y.B.; Shin, S.C.; Lee, K.C.; Oh, I.J. Physicochemical characterization of poly(L-lactic acid) and poly(D,L-lactide-co-glycolide) nanoparticles with polyethylenimine as gene delivery carrier. Int. J. Pharm. 2005, 298, 255–262. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Y.; Tan, B.; Zhang, Y.; Chen, X.; Quan, X. PEGylated molybdenum dichalcogenide (PEG-MoS2) nanosheets with enhanced peroxidase-like activity for the colorimetric detection of H2O2. New J. Chem. 2017, 41, 6700–6708. [Google Scholar] [CrossRef]
- Sacchetti, C.; Motamedchaboki, K.; Magrini, A.; Palmieri, G.; Mattei, M.; Bernardini, S.; Rosato, N.; Bottini, N.; Bottini, M. Surface polyethylene glycol conformation influences the protein corona of polyethylene glycol-modified single-walled carbon nanotubes: Potential implications on biological performance. ACS Nano 2013, 7, 1974–1989. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Poly(propylene fumarate)/polyethylene glycol-modified graphene oxide nanocomposites for tissue engineering. ACS Appl. Mater. Interfaces 2016, 8, 17902–17914. [Google Scholar] [CrossRef]
- Huang, W.; Fernando, S.; Allard, L.F.; Sun, Y.P. Solubilization of single-walled carbon nanotubes with diamine-terminated oligomeric poly(ethylene glycol) in different functionalization reactions. Nano Lett. 2003, 3, 565–568. [Google Scholar] [CrossRef]
- Landi, B.J.; Ruf, H.J.; Worman, J.J.; Raffaelle, R.P. Effects of alkyl amide solvents on the dispersion of single-wall carbon nanotubes. J. Phys. Chem. B 2004, 108, 17089–17095. [Google Scholar] [CrossRef]
- Naffakh, M.; Marco, C.; Ellis, G. Development of novel melt-processable biopolymer nanocomposites based on poly(L-lactic acid) and WS2 inorganic nanotubes. CrystEngComm 2014, 16, 5062–5072. [Google Scholar] [CrossRef]
- Naffakh, M.; Marco, C.; Ellis, G. Inorganic WS2 nanotubes that improve the crystallization behavior of poly(3-hydroxybutyrate). CrystEngComm 2014, 16, 1126–1135. [Google Scholar] [CrossRef]
- Silverman, T.; Naffakh, M.; Marco, C.; Ellis, G.; Silverman, T.; Naffakh, M.; Marco, C.; Ellis, G. Effect of WS2 inorganic nanotubes on isothermal crystallization behavior and kinetics of poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Polymers (Basel) 2018, 10, 166. [Google Scholar] [CrossRef]
- Rapoport, L.; Fleischer, N.; Tenne, R. Applications of WS2(MoS2) inorganic nanotubes and fullerene-like nanoparticles for solid lubrication and for structural nanocomposites. J. Mater. Chem. 2005, 15, 1782–1788. [Google Scholar] [CrossRef]
- Rosentsveig, R.; Gorodnev, A.; Feuerstein, N.; Friedman, H.; Zak, A.; Fleischer, N.; Tannous, J.; Dassenoy, F.; Tenne, R. Fullerene-like MoS2 nanoparticles and their tribological behavior. Tribol. Lett. 2009, 36, 175–182. [Google Scholar] [CrossRef]
- Samorodnitzky-Naveh, G.R.; Redlich, M.; Rapoport, L.; Feldman, Y.; Tenne, R. Inorganic fullerene-like tungsten disulfide nanocoating for friction reduction of nickel–titanium alloys. Nanomedicine 2009, 4, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Goldbart, O.; Cohen, S.R.; Kaplan-Ashiri, I.; Glazyrina, P.; Wagner, H.D.; Enyashin, A.; Tenne, R. Diameter-dependent wetting of tungsten disulfide nanotubes. Proc. Natl. Acad. Sci. USA 2016, 113, 13624–13629. [Google Scholar] [CrossRef]
- Israelachvili, J.; Drummond, C.; Golan, Y.; Tenne, R.; Alcantar, N. Microtribology and friction-induced material transfer in WS2 nanoparticle additives. Adv. Funct. Mater. 2001, 11, 348–354. [Google Scholar]
- Maharaj, D.; Bhushan, B. Effect of MoS2 and WS2 nanotubes on nanofriction and wear reduction in dry and liquid environments. Tribol. Lett. 2013, 49, 323–339. [Google Scholar] [CrossRef]
- Yadgarov, L.; Petrone, V.; Rosentsveig, R.; Feldman, Y.; Tenne, R.; Senatore, A. Tribological studies of rhenium doped fullerene-like MoS2 nanoparticles in boundary, mixed and elasto-hydrodynamic lubrication conditions. Wear 2013, 297, 1103–1110. [Google Scholar] [CrossRef]
- Zak, A.; Sallacan-Ecker, L.; Margolin, A.; Feldman, Y.; Popovitz-Biro, R.; Albu-Yaron, A.; Genut, M.; Tenne, R. Scaling up of the WS2 nanotubes synthesis. Fuller. Nanotubes Carbon Nanostruct. 2011, 19, 18–26. [Google Scholar] [CrossRef]
- Steffe, J.F. Rheological Methods in Food Process Engineering; Freeman Press: East Lansing, MI, USA, 1996. [Google Scholar]
- Mata, D.; Horovistiz, A.L.; Branco, I.; Ferro, M.; Ferreira, N.M.; Belmonte, M.; Lopes, M.A.; Silva, R.F.; Oliveira, F.J. Carbon nanotube-based bioceramic grafts for electrotherapy of bone. Mater. Sci. Eng. C 2014, 34, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Androsch, R.; Iqbal, H.M.M.N.; Schick, C. Non-isothermal crystal nucleation of poly(L-lactic acid). Polymer (Guildf.) 2015, 81, 151–158. [Google Scholar] [CrossRef]
- Shenoy, A.V. Rheology of Filled Polymer Systems; Springer: Berlin, Germany, 1999. [Google Scholar]
- Pötschke, P.; Fornes, T.D.; Paul, D.R. Rheological behavior of multiwalled carbon nanotube/polycarbonate composites. Polymer (Guildf.) 2002, 43, 3247–3255. [Google Scholar] [CrossRef]
- Song, Y.S.; Youn, J.R. Influence of dispersion states of carbon nanotubes on physical properties of epoxy nanocomposites. Carbon N. Y. 2005, 43, 1378–1385. [Google Scholar] [CrossRef]
- Rapoport, L.; Bilik, Y.; Feldman, Y.; Homyonfer, M.; Cohen, S.R.; Tenne, R. Hollow nanoparticles of WS2 as potential solid-state lubricants. Nature 1997, 387, 791–793. [Google Scholar] [CrossRef]
- Kister, G.; Cassanas, G.; Vert, M.; Pauvert, B.; Térol, A. Vibrational analysis of poly(L-lactic acid). J. Raman Spectrosc. 1995, 26, 307–311. [Google Scholar] [CrossRef]
- Kister, G.; Cassanas, G.; Vert, M. Effects of morphology, conformation and configuration on the IR and Raman spectra of various poly(lactic acid)s. Polymer (Guildf.) 1998, 39, 267–273. [Google Scholar] [CrossRef]
- Grinberg, O.; Deng, S.; Zussman, E.; Livneh, T.; Zak, A. Raman scattering from single WS2 nanotubes in stretched PVDF electrospun fibers. Phys. Chem. Chem. Phys. 2017, 19, 18443–18451. [Google Scholar] [CrossRef]
- Chen, S.J.H.; Schwartz, M. Raman study of vibrational relaxation in dichloromethane and [2H2]dichloromethane. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1985, 81, 235–243. [Google Scholar] [CrossRef]
- Cassanas, G.; Morssli, M.; Fabrègue, E.; Bardet, L. Vibrational spectra of lactic acid and lactates. J. Raman Spectrosc. 1991, 22, 409–413. [Google Scholar] [CrossRef]
- Eitan, A.; Fisher, F.T.; Andrews, R.; Brinson, L.C.; Schadler, L.S. Reinforcement mechanisms in MWCNT-filled polycarbonate. Compos. Sci. Technol. 2006, 66, 1162–1173. [Google Scholar] [CrossRef]
- Gersappe, D. Molecular Mechanisms of Failure in Polymer Nanocomposites. Phys. Rev. Lett. 2002, 89, 058301. [Google Scholar] [CrossRef]
- Dorigato, A.; Dzenis, Y.; Pegoretti, A. Filler aggregation as a reinforcement mechanism in polymer nanocomposites. Mech. Mater. 2013, 61, 79–90. [Google Scholar] [CrossRef]
- Wagner, H.D.; Vaia, R.A. Nanocomposites: Issues at the interface. Mater. Today 2004, 7, 38–42. [Google Scholar] [CrossRef]
- Lorenz, T.; Teich, D.; Joswig, J.O.; Seifert, G. Theoretical study of the mechanical behavior of individual TiS2 and MoS2 nanotubes. J. Phys. Chem. C 2012, 116, 11714–11721. [Google Scholar] [CrossRef]
- Zibouche, N.; Ghorbani-Asl, M.; Heine, T.; Kuc, A. Electromechanical Properties of Small Transition-Metal Dichalcogenide Nanotubes. Inorganics 2014, 2, 155–167. [Google Scholar] [CrossRef]









| Sample | Modulus (GPa) | Yield Strength (MPa) | Elongation (%) | Toughness (MPa*%) | Thickness (mm) |
|---|---|---|---|---|---|
| PLLA 38 film | 1.6 ± 0.1 | 26.6 ± 1.5 | 3.2 ± 0.4 | 0.6 ± 0.1 | 0.15 ± 0.1 |
| PLLA 38 film with 0.25 wt % INT–WS2 | 1.8 ± 0.02 | 29.7 ± 1.2 | 2.9 ± 0.3 | 0.6 ± 0.1 | 0.12 ± 0.1 |
| PLLA 38 film with 0.4 wt % INT–WS2 | 1.9 ± 0.1 | 39.5 ± 2.0 | 5.4 ± 0.7 | 1.5 ± 0.2 | 0.11 ± 0.1 |
| PLLA 38 film with 0.7 wt % INT–WS2 | 2.4 ± 0.1 | 46.1 ± 1.5 | 3.7 ± 0.5 | 1.1 ± 0.2 | 0.10 ± 0.1 |
| Sample | Modulus (GPa) | Yield Strength (MPa) | Elongation (%) | Toughness (MPa*%) | Thickness (mm) |
|---|---|---|---|---|---|
| PLLA 24 film | 1.4 ± 0.05 | 22.0 ± 4.1 | 3.5 ± 0.6 | 1.2 ± 0.3 | 0.14 ± 0.1 |
| PLLA 24 film with 0.25 wt % INT–WS2 | 1.8 ± 0.05 | 41.5 ± 1.4 | 8.1 ± 1.5 | 2.0 ± 0.4 | 0.11 ± 0.1 |
| PLLA 24 film with 0.5 wt % INT–WS2 | 2.8 ± 0.2 | 60.9 ± 2.8 | 18.4 ± 5.2 | 5.7 ± 0.7 | 0.10 ± 0.1 |
| PLLA 24 film with 0.8 wt % INT–WS2 | 2.2 ± 0.2 | 43.7 ± 3.5 | 10.3 ± 2.4 | 3.8 ± 1.2 | 0.10 ± 0.1 |
| Number | Sample | Modulus (GPa) | Yield Strength (MPa) | Elongation (%) | Toughness (MPa*%) | Thickness (mm) |
|---|---|---|---|---|---|---|
| 1 | PLLA 38 film | 1.6 ± 0.1 | 26.6 ± 1.5 | 3.2 ± 0.4 | 0.6 ± 0.1 | 0.15 ± 0.1 |
| 2 | PLLA 38 film with 0.5 wt % INT–WS2 | 2.4 | 49.3 | 14.0 | 6.1 | 0.14 |
| 3 | PLLA 38 film with 1 wt % INT–WS2 | 4.0 | 68.25 | 29.8 | 20.0 | 0.11 |
| 4 | PLLA 38 film with 3 wt % INT–WS2 | 3.4 | 57.4 | 11.7 | 4.9 | 0.13 |
| 5 | PLLA 24 film | 1.4 ± 0.05 | 22.0 ± 4.1 | 3.5 ± 0.6 | 1.2 ± 0.3 | 0.14 ± 0.1 |
| 6 | PLLA 24 film with 0.5 wt % INT–WS2 | 2.4 | 60.7 | 75.7 | 40.5 | 0.12 |
| 7 | PLLA 24 film with 0.5 wt % INT–WS2 | 2.0 | 40.7 | 3.2 | 1.0 | 0.13 |
| Sample | Modulus (GPa) | Yield Strength (MPa) | Elongation (%) | Toughness (MPa*%) | Thickness (mm) |
|---|---|---|---|---|---|
| PLLA 24 film | 1.2 ± 0.1 | 22.0 ± 1.7 | 14.9 ± 6.7 | 1.5 ± 0.7 | 0.14 ± 0.1 |
| PLLA 24 film with 0.5 wt % INT–WS2 | 1.6 ± 0.1 | 31.5 ± 0.7 | 9.2 ± 1.4 | 2.3 ± 0.4 | 0.1 ± 0.1 |
| PLLA 24 film | 2.1 ± 0.3 | 39.0 ± 3.0 | 26.6 ± 6.2 | 9.2 ± 2.2 | 0.12 ± 0.1 |
| PLLA 24 film with 0.25 wt % INT–WS2 after functionalized with PEI | 1.2 ± 0.1 | 24.6 ± 3.0 | 58.3 ± 44.2 | 14.0 ± 12.0 | 0.14 ± 0.1 |
| PLLA 24 film with 0.5 wt % INT–WS2 after functionalized with PEI | 2.65 ± 0.2 | 47.3 ± 3.8 | 41.5 ± 29.5 | 16.3 ± 13.5 | 0.09 ± 0.1 |
| PLLA 24 film with 1 wt % INT–WS2 after functionalized with PEI | 2.15 ± 0.2 | 39.4 ± 1.9 | 15.1 ± 16.6 | 4.4 ± 5.35 | 0.1 ± 0.1 |
| Sample | Tg (°C) | Tcc (°C) | ΔHcc (J/g) | Tm (°C) | ΔHm (J/g) | Tc (°C) | ΔHc (J/g) | Xc (%) | (1 − λ)c (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| A | PLLA 24 | 61.7 ± 0.4 | 114.8 ± 0.3 | 32.4 ± 12.0 | 178.6 ± 0.4 | 33.9 ± 12.2 | 101.6 ± 0.4 | 2.0 ± 0.1 | 1.6 ± 0.2 | 2.1 ± 0.4 |
| PLLA 24 with 0.5 wt % INT-WS2 | 65.5 ± 1.7 | 107.9 ± 7.9 | 3.1 ± 0.6 | 178.9 ± 0.3 | 37.9 ± 1.5 | 103.0 ± 0.9 | 30.6 ± 6.1 | 37.4 ± 2.1 | 32.9 ± 6.6 | |
| B | PLLA 24 | 61.8 ± 0.3 | 114.6 ± 0.6 | 38.9 ± 3.4 | 178.8 ± 0.5 | 39.2 ± 3.4 | 101.8 ± 0.2 | 2.2 ± 0.4 | 0.3 ± 0.01 | 2.4 ± 0.4 |
| PLLA 24 with 0.5 wt % INT-WS2 | 62.2 ± 0.2 | 100.4 ± 0.8 | 6.7 ± 2.2 | 178.8 ± 0.4 | 35.4 ± 0.8 | 100.3 ± 0.2 | 23.9 ± 1.2 | 30.9 ± 2.5 | 25.6 ± 1.3 |
| Type of Test | PLLA 24 Film | PLLA 24 Film with 0.5wt % INT–WS2 | ||
|---|---|---|---|---|
| µs | µk | µs | µk | |
| Run 20 times on steel plate | 0.2 ± 0.01 | 0.19 ± 0.01 | 0.06 ± 0.01 | 0.03 ± 0.01 |
| After 5 (run-in) runs on 1200 SiC paper | 0.21 ± 0.01 | 0.18 ± 0.02 | 0.07 ± 0.05 | 0.03 ± 0.01 |
| After run 5 times on 150 SiC paper | 0.24 ± 0.02 | 0.21 ± 0.01 | 0.06 ± 0.01 | 0.03 ± 0.01 |
| Sample | PLLA 38 | PLLA 24 | PLLA (2.1 dL/g) | PDLLA (0.55 dL/g) | PDLGA 50/50 (0.4 dL/g) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | Dry | Wet | |
| SS rod | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Blank | 1.73 | 1.73 | 1.75 | 2.66 | 1.42 | 1.41 | 1.57 | 1.38 | 1.47 | 1.30 |
| 0.5 wt % INT–WS2 | 0.27 | 0.23 | 0.43 | 0.35 | 0.31 | 0.09 | 1.29 | 1.03 | 1.26 | 0.43 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shalom, H.; Sui, X.; Elianov, O.; Brumfeld, V.; Rosentsveig, R.; Pinkas, I.; Feldman, Y.; Kampf, N.; Wagner, H.D.; Lachman, N.; et al. Nanocomposite of Poly(l-Lactic Acid) with Inorganic Nanotubes of WS2. Lubricants 2019, 7, 28. https://doi.org/10.3390/lubricants7030028
Shalom H, Sui X, Elianov O, Brumfeld V, Rosentsveig R, Pinkas I, Feldman Y, Kampf N, Wagner HD, Lachman N, et al. Nanocomposite of Poly(l-Lactic Acid) with Inorganic Nanotubes of WS2. Lubricants. 2019; 7(3):28. https://doi.org/10.3390/lubricants7030028
Chicago/Turabian StyleShalom, Hila, XiaoMeng Sui, Olga Elianov, Vlad Brumfeld, Rita Rosentsveig, Iddo Pinkas, Yishay Feldman, Nir Kampf, H.D. Wagner, Noa Lachman, and et al. 2019. "Nanocomposite of Poly(l-Lactic Acid) with Inorganic Nanotubes of WS2" Lubricants 7, no. 3: 28. https://doi.org/10.3390/lubricants7030028
APA StyleShalom, H., Sui, X., Elianov, O., Brumfeld, V., Rosentsveig, R., Pinkas, I., Feldman, Y., Kampf, N., Wagner, H. D., Lachman, N., & Tenne, R. (2019). Nanocomposite of Poly(l-Lactic Acid) with Inorganic Nanotubes of WS2. Lubricants, 7(3), 28. https://doi.org/10.3390/lubricants7030028