Tribochemistry: A Review of Reactive Molecular Dynamics Simulations
Abstract
:1. Introduction
2. Review
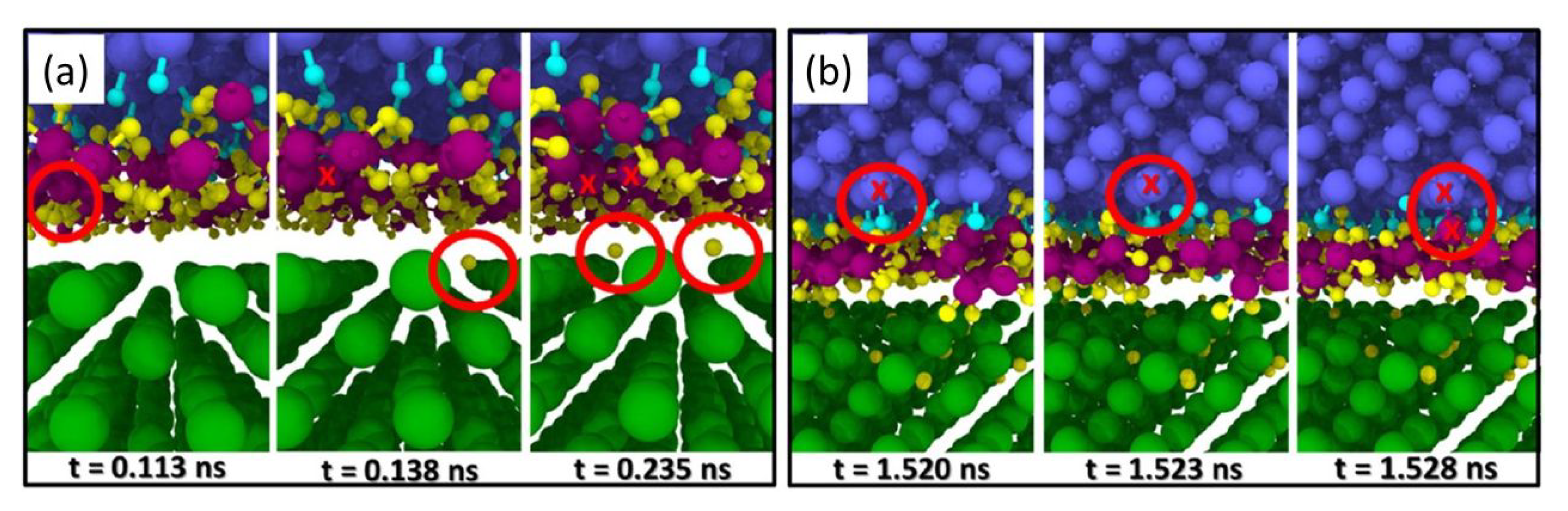
2.1. Reactions between Solid Surfaces
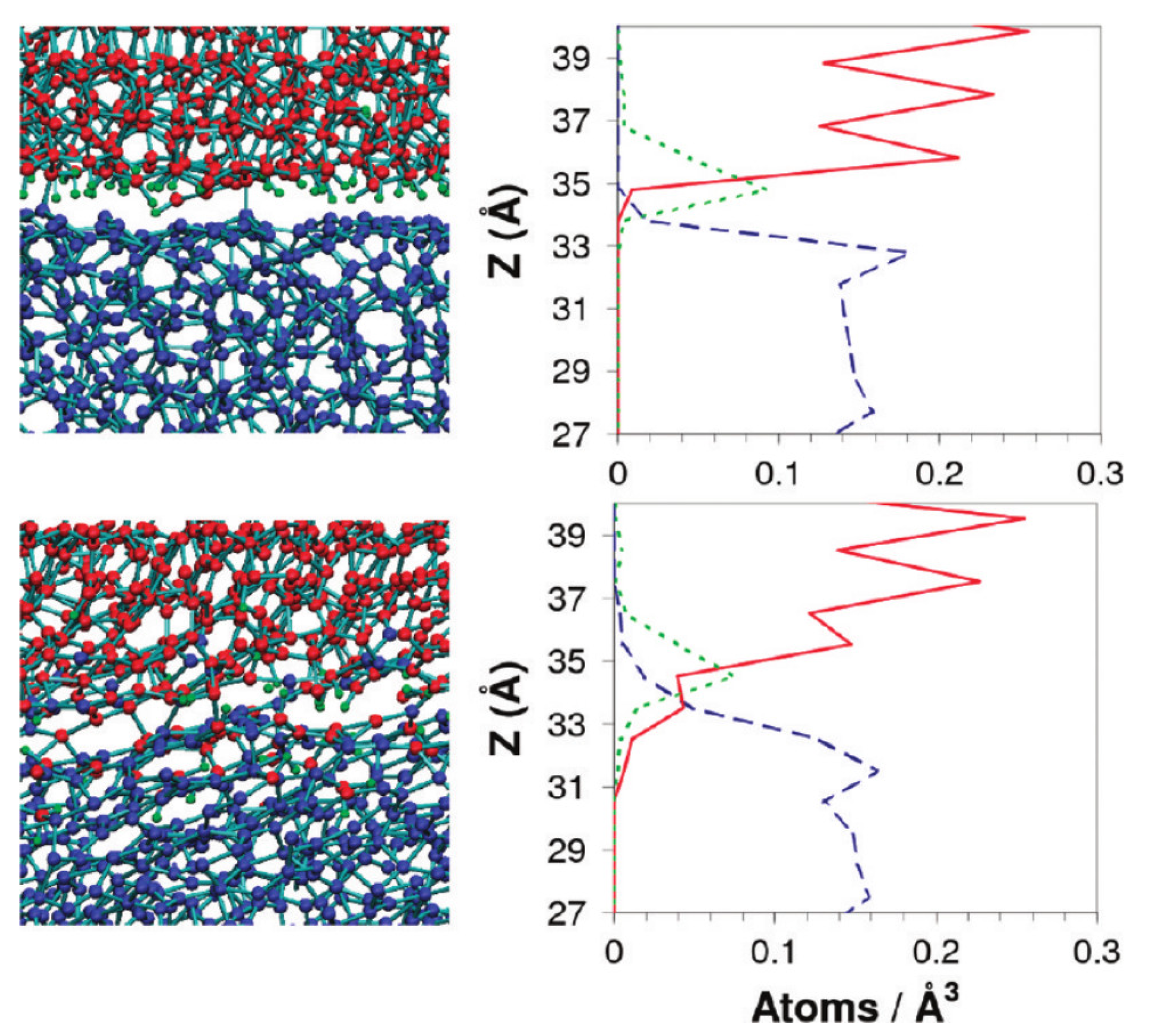
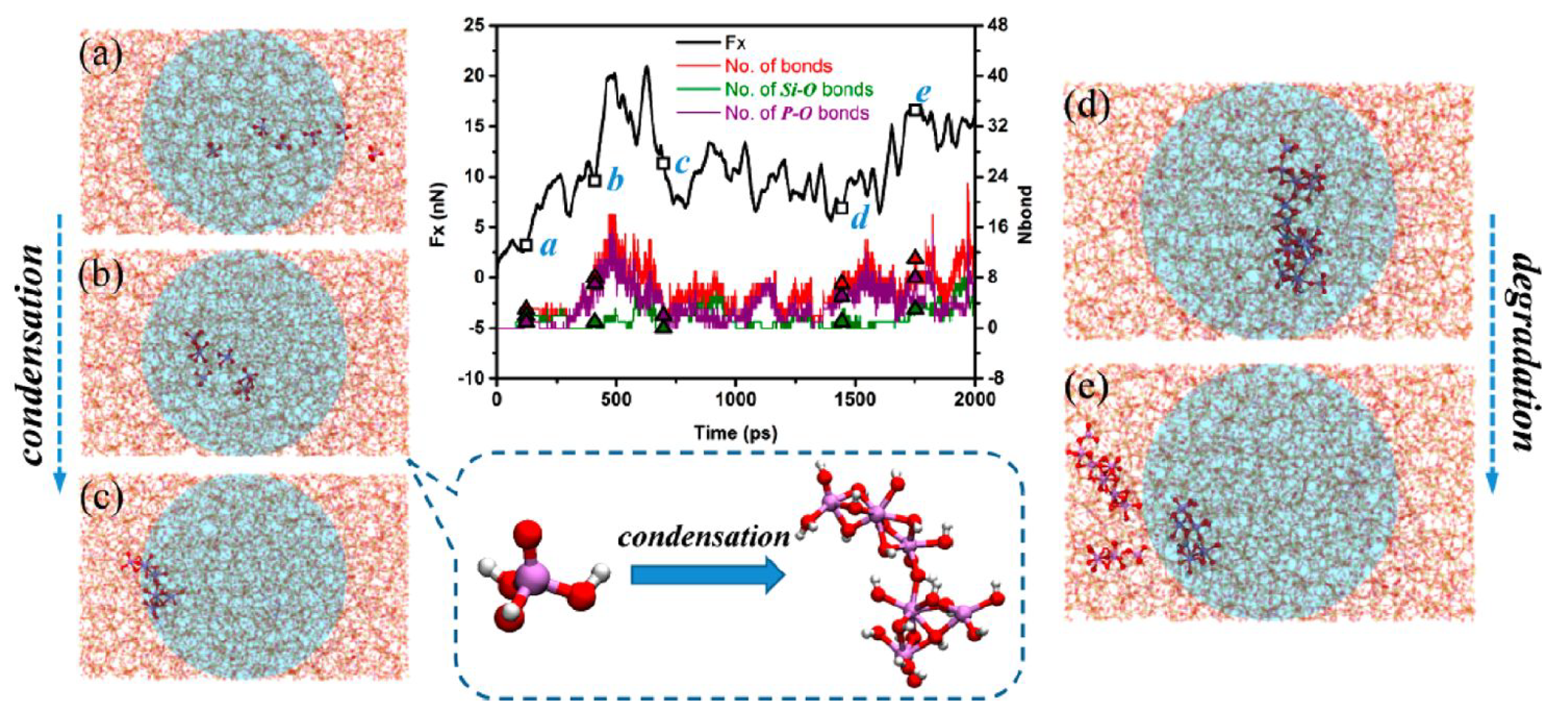
2.2. Reactions between Lubricants and Surfaces
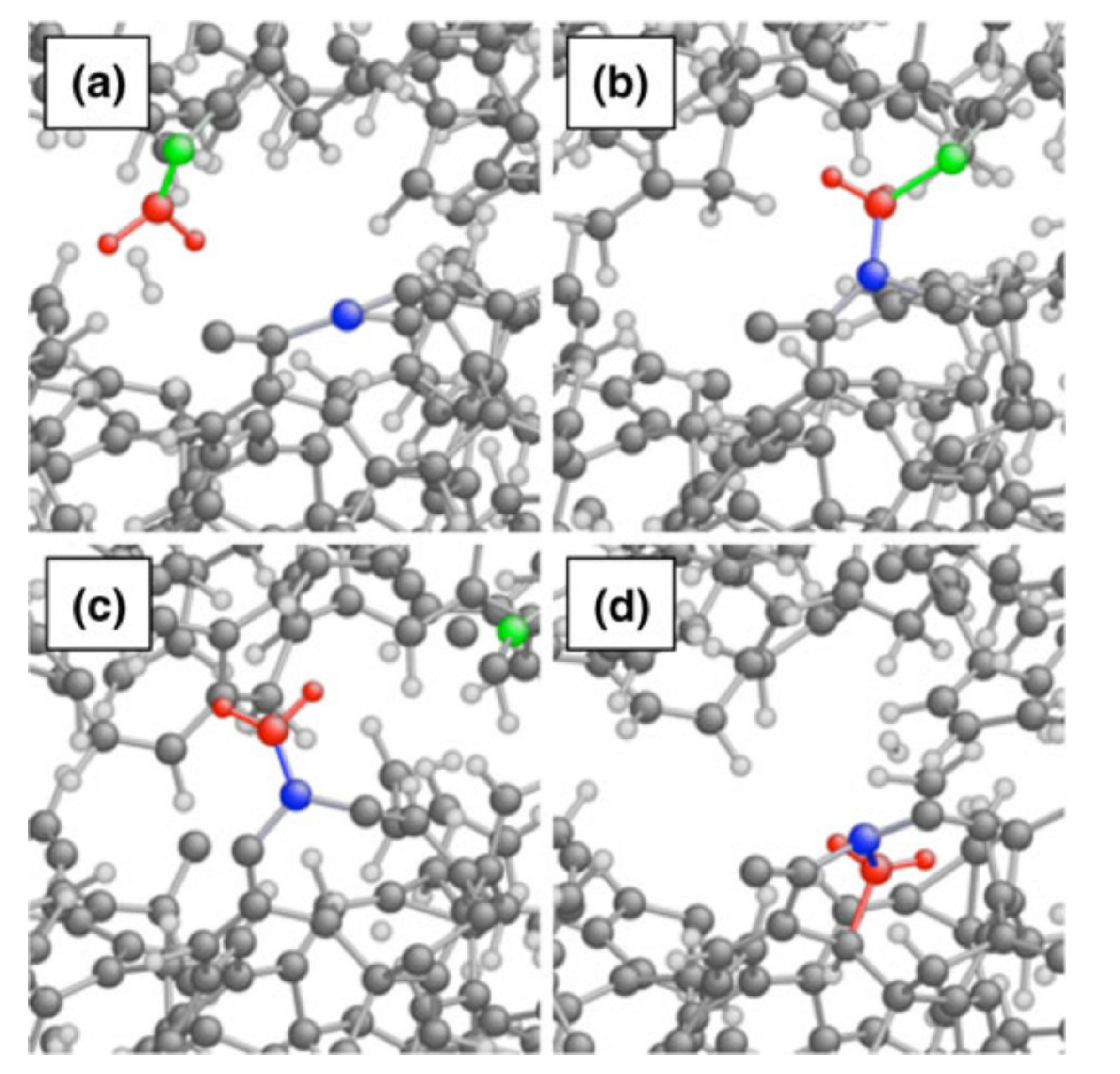
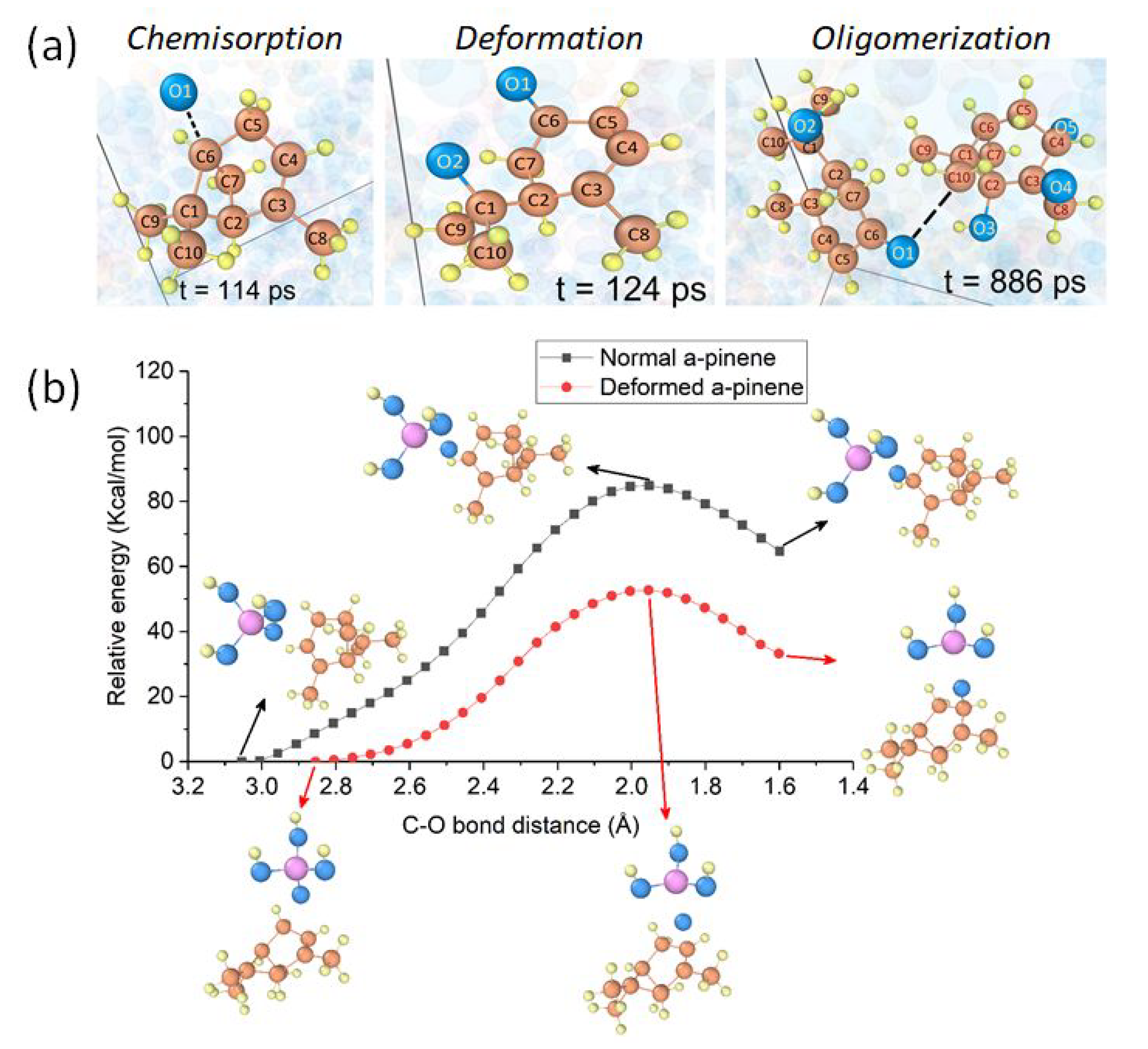
2.3. Reactions within Lubricants
3. Challenges and Opportunities
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fischer, T.E. Tribochemistry. Annu. Rev. Mater. Sci. 1988, 18, 303–323. [Google Scholar] [CrossRef]
- Hsu, S.; Zhang, J.; Yin, Z. The Nature and Origin of Tribochemistry. Tribol. Lett. 2002, 13, 131–139. [Google Scholar] [CrossRef]
- Kajdas, C. General Approach to Mechanochemistry and Its Relation to Tribochemistry. In Tribology in Engineering; Pihtili, H., Ed.; InTechOpen: London, UK, 2013; Chapter 12. [Google Scholar]
- Spikes, H. Stress-augmented Thermal Activation: Tribology Feels the Force. Friction 2018, 6, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Minami, I. Molecular science of lubricant additives. Appl. Sci. 2017, 7, 445. [Google Scholar] [CrossRef]
- Nicholls, M.; Do, T.; Norton, P.; Kasrai, M.; Bancroft, G. Review of the lubrication of metallic surfaces by zinc dialkyldithiophosphates. Tribol. Int. 2005, 38, 15–39. [Google Scholar] [CrossRef]
- Spikes, H. The history and mechanisms of ZDDP. Tribol. Lett. 2004, 17, 469–489. [Google Scholar] [CrossRef]
- De Barros Bouchet, M.I.; Martin, J.M.; Le-Mogne, T.; Vacher, B. Boundary lubrication mechanisms of carbon coatings by MoDTC and ZDDP additives. Tribol. Int. 2005, 38, 257–264. [Google Scholar] [CrossRef]
- Fontaine, J.; Donnet, C.; Erdemir, A. Fundamentals of the Tribology of DLC Coatings. In Tribology of Diamond-Like Carbon Films; Donnet, C., Erdemir, A., Eds.; Springer: Boston, MA, USA, 2008. [Google Scholar]
- Martin, J.M.; Erdemir, A. Superlubricity: Friction’s vanishing act. Phys. Today 2018, 71, 40. [Google Scholar] [CrossRef]
- Baykara, M.Z.; Vazirisereshk, M.R.; Martini, A. Emerging superlubricity: A review of the state of the art and perspectives on future research. Appl. Phys. Rev. 2018, 5, 041102. [Google Scholar] [CrossRef] [Green Version]
- Totolin, V.; Minami, I.; Gabler, C.; Brenner, J.; Dörr, N. Lubrication mechanism of phosphonium phosphate ionic liquid additive in alkylborane–imidazole complexes. Tribol. Lett. 2014, 53, 421–432. [Google Scholar] [CrossRef]
- Zhou, Y.; Qu, J. Ionic liquids as lubricant additives: A review. ACS Appl. Mater. Interfaces 2017, 9, 3209–3222. [Google Scholar] [CrossRef] [PubMed]
- Tannous, J.; Dassenoy, F.; Lahouij, I.; LeMogne, T.; Vacher, B.; Bruhács, A.; Tremel, W. Understanding the Tribochemical Mechanisms of IF-MoS2 Nanoparticles Under Boundary Lubrication. Tribol. Lett. 2011, 41, 55–64. [Google Scholar] [CrossRef]
- Dai, W.; Kheireddin, B.; Gao, H.; Liang, H. Roles of nanoparticles in oil lubrication. Tribol. Int. 2016, 102, 88–98. [Google Scholar] [CrossRef]
- Brinksmeier, E.; Meyer, D.; Huesmann-Cordes, A.; Herrmann, C. Metalworking fluids—Mechanisms and performance. CIRP Ann. Manuf. Technol. 2015, 64, 605–628. [Google Scholar] [CrossRef] [Green Version]
- Byers, J. Metalworking Fluids, 3rd ed.; Taylor & Francis, CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Zhang, J.; Spikes, H. On the Mechanism of ZDDP Antiwear Film Formation. Tribol. Lett. 2016, 63, 24. [Google Scholar] [CrossRef] [Green Version]
- Tysoe, W.T. On Stress-Induced Tribochemical Reaction Rates. Tribol. Lett. 2017, 65, 48. [Google Scholar] [CrossRef]
- Zhang, J.; Ewen, J.P.; Ueda, M.; Wong, J.S.S.; Spikes, H.A. Mechanochemistry of Zinc Dialkyldithiophosphate on Steel Surfaces under Elastohydrodynamic Lubrication Conditions. ACS Appl. Mater. Interfaces 2020, 12, 6662–6676. [Google Scholar] [CrossRef]
- McNaught, A.D.; Wilkinson, A. IUPAC. Compendium of Chemical Terminology, 2nd ed.; Blackwell Scientific Publications: Oxford, UK, 2019. [Google Scholar]
- Boldyreva, E. Mechanochemistry of Inorganic and Organic Systems: What is Similar, What is Different? Chem. Soc. Rev. 2013, 42, 7719–7738. [Google Scholar] [CrossRef]
- James, S.; Friščić, T. Mechanochemistry. Chem. Soc. Rev. 2013, 42, 7494–7496. [Google Scholar] [CrossRef]
- Takacs, L. The historical development of mechanochemistry. Chem. Soc. Rev. 2013, 42, 7449–7459. [Google Scholar] [CrossRef]
- Tian, Y.; Boulatov, R. Comparison of the Predictive Performance of the Bell–Evans, Taylor-expansion and Statistical-mechanics Models of Mechanochemistry. Chem. Commun. 2013, 49, 4187–4189. [Google Scholar] [CrossRef] [PubMed]
- Spikes, H.; Tysoe, W.T. On the Commonality Between Theoretical Models for Fluid and Solid Friction, Wear and Tribochemistry. Tribol. Lett. 2015, 59, 21. [Google Scholar] [CrossRef]
- Dörr, N.; Brenner, J.; Ristić, A.; Ronai, B.; Besser, C.; Pejaković, V.; Frauscher, M. Correlation between engine oil degradation, tribochemistry, and tribological behavior with focus on ZDDP deterioration. Tribol. Lett. 2019, 67, 62. [Google Scholar]
- Sharma, V.; Dörr, N.; Erdemir, A.; Aswath, P. Antiwear properties of binary ashless blend of phosphonium ionic liquids and borate esters in partially formulated oil (No Zn). Tribol. Lett. 2019, 67, 42. [Google Scholar] [CrossRef]
- Crobu, M.; Rossi, A.; Mangolini, F.; Spencer, N. Chain-length identification strategy in zinc polyphosphate glasses by means of XPS and ToF-SIMS. Anal. Bioanal. Chem. 2012, 403, 1415–1432. [Google Scholar] [CrossRef]
- Dawczyk, J.; Ware, E.; Ardakani, M.; Russo, J.; Spikes, H. Use of FIB to study ZDDP tribofilms. Tribol. Lett. 2018, 66, 155. [Google Scholar] [CrossRef] [Green Version]
- Dorgham, A.; Neville, A.; Ignatiyev, K.; Mosselmans, F.; Morina, A. An In Situ Synchrotron XAS Methodology for Surface Analysis Under High Temperature, Pressure, and Shear. Rev. Sci. Instrum. 2017, 88, 015101. [Google Scholar] [CrossRef] [Green Version]
- Dorgham, A.; Parsaeian, P.; Neville, A.; Ignatiyev, K.; Mosselmans, F.; Masuko, M.; Morina, A. In Situ Synchrotron XAS Study of the Decomposition Kinetics of ZDDP Triboreactive Interfaces. RSC Adv. 2018, 8, 34168–34181. [Google Scholar] [CrossRef] [Green Version]
- Gosvami, N.N.; Bares, J.A.; Mangolini, F.; Konicek, A.R.; Yablon, D.G.; Carpick, R.W. Mechanisms of Antiwear Tribofilm Growth Revealed in Situ by Single-Asperity Sliding Contacts. Science 2015, 348, 102–106. [Google Scholar] [CrossRef] [Green Version]
- Jaiswal, V.; Rastogi, R.B.; Maurya, J.L.; Singh, P.; Tewari, A.K. Quantum chemical calculation studies for interactions of antiwear lubricant additives with metal surfaces. RSC Adv. 2014, 4, 13438–13445. [Google Scholar] [CrossRef]
- Adams, H.L.; Garvey, M.T.; Ramasamy, U.S.; Ye, Z.; Martini, A.; Tysoe, W.T. Shear-Induced Mechanochemistry: Pushing Molecules Around. J. Phys. Chem. C 2015, 119, 7115. [Google Scholar] [CrossRef]
- Fatti, G.; Righi, M. Selenium Chemisorption Makes Iron Surfaces Slippery. Tribol. Lett. 2019, 67, 125. [Google Scholar] [CrossRef]
- Ewen, J.P.; Fernández, E.R.; Smith, E.R.; Dini, D. Nonequilibrium Molecular Dynamics Simulations of Tribological Systems. In Modeling and Simulation of Tribological Problems in Technology; Paggi, M., Hills, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 95–130, Chapter 3. [Google Scholar]
- Senftle, T.P.; Hong, S.; Islam, M.M.; Kylasa, S.B.; Zheng, Y.; Shin, Y.K.; Junkermeier, C.; Engel-Herbert, R.; Janik, M.J.; Aktulga, H.M.; et al. The ReaxFF reactive force-field: Development, applications and future directions. NPJ Comput. Mater. 2016, 2, 15011. [Google Scholar] [CrossRef]
- Sinnott, S.B.; Brenner, D.W. Three decades of many-body potentials in materials research. MRS Bull. 2012, 37, 469–473. [Google Scholar] [CrossRef] [Green Version]
- Liang, T.; Shin, Y.K.; Cheng, Y.T.; Yilmaz, D.E.; Vishnu, K.G.; Verners, O.; Zou, C.; Phillpot, S.R.; Sinnott, S.B.; van Duin, A.C.T. Reactive potentials for advanced atomistic simulations. Annu. Rev. Mater. Res. 2013, 43, 109–129. [Google Scholar] [CrossRef]
- Harrison, J.A.; Schall, J.D.; Maskey, S.; Mikulski, P.T.; Knippenberg, M.T.; Morrow, B.H. Review of force fields and intermolecular potentials used in atomistic computational materials research. Appl. Phys. Rev. 2018, 5, 031104. [Google Scholar] [CrossRef]
- Abell, G. Empirical chemical pseudopotential theory of molecular and metallic bonding. Phys. Rev. B 1985, 31, 6184. [Google Scholar] [CrossRef]
- Tersoff, J. New empirical approach for the structure and energy of covalent systems. Phys. Rev. B 1988, 37, 6991. [Google Scholar] [CrossRef]
- Brenner, D.W. Empirical potential for hydrocarbons for use in simulating the chemical vapor deposition of diamond films. Phys. Rev. B 1990, 42, 9458. [Google Scholar] [CrossRef]
- Brenner, D.W.; Shenderova, O.A.; Harrison, J.A.; Stuart, S.J.; Ni, B.; Sinnott, S.B. A second-generation reactive empirical bond order (REBO) potential energy expression for hydrocarbons. J. Phys. Condens. Matter 2002, 14, 783. [Google Scholar] [CrossRef]
- Stuart, S.J.; Tutein, A.B.; Harrison, J.A. A reactive potential for hydrocarbons with intermolecular interactions. J. Chem. Phys. 2000, 112, 6472–6486. [Google Scholar] [CrossRef] [Green Version]
- Shin, Y.K.; Shan, T.R.; Liang, T.; Noordhoek, M.J.; Sinnott, S.B.; van Duin, A.C.T.; Phillpot, S.R. Variable charge many-body interatomic potentials. MRS Bull. 2012, 37, 504–512. [Google Scholar] [CrossRef]
- Yu, J.; Sinnott, S.B.; Phillpot, S.R. Charge optimized many-body potential for the Si/SiO2 system. Phys. Rev. B 2007, 75, 085311. [Google Scholar] [CrossRef]
- Shan, T.R.; Devine, B.D.; Hawkins, J.M.; Asthagiri, A.; Phillpot, S.R.; Sinnott, S.B. Second-generation charge-optimized many-body potential for Si/SiO2 and amorphous silica. Phys. Rev. B 2010, 82, 235302. [Google Scholar] [CrossRef]
- van Duin, A.C.T.; Dasgupta, S.; Lorant, F.; Goddard, W.A. ReaxFF: A reactive force field for hydrocarbons. J. Phys. Chem. A 2001, 105, 9396–9409. [Google Scholar] [CrossRef] [Green Version]
- Mohammadtabar, K.; Eder, S.J.; Bedolla, P.O.; Dörr, N.; Martini, A. Reactive Molecular Dynamics Simulations of Thermal Film Growth from Di-tert-butyl Disulfide on an Fe(100) Surface. Langmuir 2018, 34, 15681–15688. [Google Scholar] [CrossRef]
- Khajeh, A.; Hu, X.; Mohammadtabar, K.; Shin, Y.K.; van Duin, A.C.T.; Berkebile, S.; Martini, A. Statistical Analysis of Tri-Cresyl Phosphate Conversion on an Iron Oxide Surface Using Reactive Molecular Dynamics Simulations. J. Phys. Chem. C 2019, 123, 12886–12893. [Google Scholar] [CrossRef]
- Nicolini, P.; Capozza, R.; Restuccia, P.; Polcar, T. Structural Ordering of Molybdenum Disulfide Studied via Reactive Molecular Dynamics Simulations. ACS Appl. Mater. Interfaces 2018, 10, 8937–8946. [Google Scholar] [CrossRef] [Green Version]
- Berman, D.; Narayanan, B.; Cherukara, M.J.; Sankaranarayanan, S.K.R.S.; Erdemir, A.; Zinovev, A.; Sumant, A.V. Operando tribochemical formation of onion-like-carbon leads to macroscale superlubricity. Nat. Commun. 2018, 9, 1164. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, T.; Ryan, K.; Keating, P.; Grierson, D.S.; Lefever, J.A.; Turner, K.T.; Harrison, J.A.; Carpick, R.W. The Effect of Atomic-Scale Roughness on the Adhesion of Nanoscale Asperities: A Combined Simulation and Experimental Investigation. Tribol. Lett. 2013, 50, 81–93. [Google Scholar] [CrossRef]
- Khan, A.M.; Wu, H.; Ma, Q.; Chung, Y.W.; Wang, Q.J. Relating Tribological Performance and Tribofilm Formation to the Adsorption Strength of Surface-Active Precursors. Tribol. Lett. 2020, 68, 6. [Google Scholar] [CrossRef]
- Harrison, J.A.; Brenner, D.W. Simulated Tribochemistry: An Atomic-Scale View of the Wear of Diamond. J. Am. Chem. Soc. 1994, 116, 10399–10402. [Google Scholar] [CrossRef]
- Gao, G.T.; Mikulski, P.T.; Harrison, J.A. Molecular-Scale Tribology of Amorphous Carbon Coatings: Effects of Film Thickness, Adhesion, and Long-Range Interactions. J. Am. Chem. Soc. 2002, 124, 7202–7209. [Google Scholar] [CrossRef] [PubMed]
- Schall, J.D.; Gao, G.; Harrison, J.A. Effects of Adhesion and Transfer Film Formation on the Tribology of Self-Mated DLC Contacts. J. Phys. Chem. C 2010, 114, 5321–5330. [Google Scholar] [CrossRef]
- Pastewka, L.; Moser, S.; Moseler, M. Atomistic Insights into the Running-in, Lubrication, and Failure of Hydrogenated Diamond-Like Carbon Coatings. Tribol. Lett. 2010, 39, 49–61. [Google Scholar] [CrossRef]
- Pastewka, L.; Moser, S.; Gumbsch, P.; Moseler, M. Anisotropic mechanical amorphization drives wear in diamond. Nat. Mater. 2011, 10, 34. [Google Scholar] [CrossRef]
- Moras, G.; Pastewka, L.; Gumbsch, P.; Moseler, M. Formation and Oxidation of Linear Carbon Chains and Their Role in the Wear of Carbon Materials. Tribol. Lett. 2011, 44, 355. [Google Scholar] [CrossRef]
- De Barros Bouchet, M.I.; Matta, C.; Vacher, B.; Le-Mogne, T.; Martin, J.M.; von Lautz, J.; Ma, T.; Pastewka, L.; Otschik, J.; Gumbsch, P.; et al. Energy filtering transmission electron microscopy and atomistic simulations of tribo-induced hybridization change of nanocrystalline diamond coating. Carbon 2015, 87, 317–0329. [Google Scholar] [CrossRef]
- Stoyanov, P.; Romero, P.A.; Jarvi, T.T.; Pastewka, L.; Scherge, M.; Stemmer, P.; Fischer, A.; Dienwiebel, M.; Moseler, M. Experimental and Numerical Atomistic Investigation of the Third Body Formation Process in Dry Tungsten/Tungsten-Carbide Tribo Couples. Tribol. Lett. 2013, 50, 67–80. [Google Scholar] [CrossRef]
- Stoyanov, P.; Stemmer, P.; Jarvi, T.T.; Merz, R.; Romero, P.A.; Scherge, M.; Kopnarski, M.; Fischer, A.; Dienwiebel, M. Friction and Wear Mechanisms of Tungsten–Carbon Systems: A Comparison of Dry and Lubricated Conditions. ACS Appl. Mater. Interfaces 2013, 5, 6123–6135. [Google Scholar] [CrossRef]
- Stoyanov, P.; Romero, P.A.; Merz, R.; Kopnarski, M.; Stricker, M.; Stemmer, P.; Dienwiebel, M.; Moseler, M. Nanoscale sliding friction phenomena at the interface of diamond-like carbon and tungsten. Acta Mater. 2013, 67, 395–408. [Google Scholar] [CrossRef]
- Juslin, N.; Erhart, P.; Träskelin, P.; Nord, J.; Henriksson, K.O.; Nordlund, K.; Salonen, E.; Albe, K. Analytical interatomic potential for modeling nonequilibrium processes in the W–C–H system. J. Appl. Phys. 2005, 98, 123520. [Google Scholar] [CrossRef]
- Kumagai, T.; Izumi, S.; Hara, S.; Sakai, S. Development of bond-order potentials that can reproduce the elastic constants and melting point of silicon for classical molecular dynamics simulation. Comput. Mater. Sci. 2007, 39, 457–464. [Google Scholar] [CrossRef]
- Moras, G.; Klemenz, A.; Reichenbach, T.; Gola, A.; Uetsuka, H.; Moseler, M.; Pastewka, L. Shear melting of silicon and diamond and the disappearance of the polyamorphic transition under shear. Phys. Rev. Mater. 2018, 2, 083601. [Google Scholar] [CrossRef]
- Hu, X.; Altoe, M.V.P.; Martini, A. Amorphization-assisted nanoscale wear during the running-in process. Wear 2016, 370–371, 46. [Google Scholar] [CrossRef] [Green Version]
- Barry, P.R.; Chiu, P.Y.; Perry, S.S.; Sawyer, W.G.; Phillpot, S.R.; Sinnott, S.B. The effect of normal load on polytetrafluoroethylene tribology. J. Phys. Condens. Matter 2009, 21, 144201. [Google Scholar] [CrossRef] [Green Version]
- Chiu, P.Y.; Barry, P.R.; Perry, S.S.; Sawyer, W.G.; Phillpot, S.R.; Sinnott, S.B. Influence of the molecular level structure of polyethylene and polytetrafluoroethylene on their tribological response. Tribol. Lett. 2011, 42, 193–201. [Google Scholar] [CrossRef]
- Barry, P.R.; Chiu, P.Y.; Perry, S.S.; Sawyer, W.G.; Sinnott, S.B.; Phillpot, S.R. Effect of temperature on the friction and wear of PTFE by atomic-level simulation. Tribol. Lett. 2015, 58, 50. [Google Scholar] [CrossRef]
- Erdemir, A.; Ramirez, G.; Eryilmaz, O.L.; Narayanan, B.; Liao, Y.; Kamath, G.; Sankaranarayanan, S.K.R.S. Carbon-based tribofilms from lubricating oils. Nature 2016, 536, 67–71. [Google Scholar] [CrossRef]
- Yue, D.C.; Ma, T.B.; Hu, Y.Z.; Yeon, J.; van Duin, A.C.T.; Wang, H.; Luo, J. Tribochemical Mechanism of Amorphous Silica Asperities in Aqueous Environment: A Reactive Molecular Dynamics Study. Langmuir 2015, 31, 1429–1436. [Google Scholar] [CrossRef]
- Wen, J.; Ma, T.; Zhang, W.; Psofogiannakis, G.; van Duin, A.C.T.; Chen, L.; Qian, L.; Hu, Y.; Lu, X. Atomic insight into tribochemical wear mechanism of silicon at the Si/SiO2 interface in aqueous environment: Molecular dynamics simulations using ReaxFF reactive force field. Appl. Surf. Sci. 2016, 390, 216–223. [Google Scholar] [CrossRef]
- Yeon, J.; van Duin, A.C.T.; Kim, S.H. Effects of Water on Tribochemical Wear of Silicon Oxide Interface: Molecular Dynamics (MD) Study with Reactive Force Field (ReaxFF). Langmuir 2016, 32, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Ma, T.; Zhang, W.; van Duin, A.C.T.; Van Duin, D.M.; Hu, Y.; Lu, X. Atomistic Insights into Cu Chemical Mechanical Polishing Mechanism in Aqueous Hydrogen Peroxide and Glycine: ReaxFF Reactive Molecular Dynamics Simulations. J. Phys. Chem. C 2019, 123, 26467–26474. [Google Scholar] [CrossRef]
- Wen, J.; Ma, T.; Zhang, W.; van Duin, A.C.T.; Lu, X. Atomistic Mechanisms of Si Chemical Mechanical Polishing in Aqueous H2O2: ReaxFF Reactive Molecular Dynamics Simulations. Comp. Mater. Sci. 2019, 131, 230–238. [Google Scholar] [CrossRef] [Green Version]
- Hahn, S.H.; Liu, H.; Kim, S.H.; van Duin, A.C.T. Atomistic understanding of surface wear process of sodium silicate glass in dry versus humid environments. J. Am. Ceram. Soc. 2020, 103, 3060–3069. [Google Scholar] [CrossRef]
- He, H.; Hahn, H.; Yu, J.; Qiao, Q.; van Duin, A.C.T.; Kim, S.H. Friction-induced subsurface densification of glass at contact stress far below indentation damage threshold. Acta Mater. 2020, 189, 166–173. [Google Scholar] [CrossRef]
- Romero, P.A.; Mayrhofer, L.; Stoyanov, P.; Merz, R.; Kopnarski, M.; Dienwiebel, M.; Moseler, M. Atomistic Insights Into Lubricated Tungsten/Diamond Sliding Contacts. Front. Mech. Eng. 2019, 5, 6. [Google Scholar] [CrossRef]
- Mohammadtabar, K.; Eder, S.J.; Dörr, N.; Martini, A. Heat-, Load-, and Shear-Driven Reactions of Di-tert-butyl Disulfide on Fe(100). J. Phys. Chem. C 2019, 123, 19688. [Google Scholar] [CrossRef]
- Furlong, O.; Miller, B.; Kotvis, P.; Adams, H.; Tysoe, W.T. Shear and Thermal Effects in Boundary Film Formation During Sliding. RSC Adv. 2014, 4, 24059–24066. [Google Scholar] [CrossRef] [Green Version]
- Ewen, J.P.; Latorre, C.A.; Gattinoni, C.; Khajeh, A.; Moore, J.D.; Remias, J.E.; Martini, A.; Dini, D. Substituent Effects on the Thermal Decomposition of Phosphate Esters on Ferrous Surfaces. J. Phys. Chem. C 2020, in press. [Google Scholar]
- Ta, T.D.; Le, H.M.; Tieu, A.K.; Zhu, H.; Ta, H.T.T.; Tran, N.V.; Wan, S.; van Duin, A.C.T. Reactive Molecular Dynamics Study of Hierarchical Tribochemical Lubricant Films at Elevated Temperatures. ACS Appl. Nano Mater. 2020, 3, 2687–2704. [Google Scholar] [CrossRef]
- Asay, D.B.; Dugger, M.T.; Kim, S.H. In-situ Vapor-Phase Lubrication of MEMS. Tribol. Lett. 2008, 29, 67–74. [Google Scholar] [CrossRef]
- Yeon, J.; He, X.; Martini, A.; Kim, S. Mechanochemistry at Solid Surfaces: Polymerization of Adsorbed Molecules by Mechanical Shear at Tribological Interfaces. ACS Appl. Mater. Interfaces 2017, 9, 3142–3148. [Google Scholar] [CrossRef] [PubMed]
- Chateauneuf, G.M.; Mikulski, P.T.; Gao, G.T.; Harrison, J.A. Compression- and Shear-Induced Polymerization in Model Diacetylene-Containing Monolayers. J. Phys. Chem. B 2004, 108, 16626–16635. [Google Scholar] [CrossRef]
- Khajeh, A.; He, X.; Yeon, J.; Kim, S.H.; Martini, A. Mechanochemical Association Reaction of Interfacial Molecules Driven by Shear. Langmuir 2018, 34, 5971–5977. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, C.; Luo, J. Superlubricity Behavior with Phosphoric Acid-Water Network Induced by Rubbing. Langmuir 2011, 27, 9413–9417. [Google Scholar] [CrossRef]
- Yue, D.C.; Ma, T.B.; Hu, Y.Z.; Yeon, J.; van Duin, A.C.T.; Wang, H.; Luo, J. Tribochemistry of Phosphoric Acid Sheared between Quartz Surfaces: A Reactive Molecular Dynamics Study. J. Phys. Chem. C 2013, 117, 25604–25614. [Google Scholar] [CrossRef]
- Matta, C.; Joly-Pottuz, L.; Bouchet, M.I.D.B.; Martin, J.M. Superlubricity and tribochemistry of polyhydric alcohols. Phys. Rev. B 2008, 78, 085436. [Google Scholar] [CrossRef] [Green Version]
- Joshi, K.L.; Raman, S.; van Duin, A.C.T. Connectivity-Based Parallel Replica Dynamics for Chemically Reactive Systems: From Femtoseconds to Microseconds. J. Phys. Chem. Lett. 2013, 4, 3792–3797. [Google Scholar] [CrossRef]
- Cheng, T.; Jaramillo-Botero, A.; Goddard, W.A.; Sun, H. Adaptive Accelerated ReaxFF Reactive Dynamics with Validation from Simulating Hydrogen Combustion. J. Am. Chem. Soc. 2014, 136, 9434–9442. [Google Scholar] [CrossRef] [Green Version]
- Vashisth, A.; Ashraf, C.; Zhang, W.; Bakis, C.E.; van Duin, A.C.T. Accelerated ReaxFF Simulations for Describing the Reactive Cross-Linking of Polymers. J. Phys. Chem. A 2018, 122, 6633–6642. [Google Scholar] [CrossRef] [PubMed]
- Ganeshan, K.; Hossain, M.J.; van Duin, A.C.T. Multiply accelerated ReaxFF molecular dynamics: Coupling parallel replica dynamics with collective variable hyper dynamics. Mol. Simulat. 2019, 45, 14–15. [Google Scholar] [CrossRef]
- Voter, A.F.; Montalenti, F.; Germann, T.C. Extending the Time Scale in Atomistic Simulation of Materials. Annu. Rev. Mater. Res. 2002, 32, 321–346. [Google Scholar] [CrossRef] [Green Version]
- Martini, A.; Dong, Y.; Perez, D.; Voter, A.F. Low Speed Atomistic Simulation of Stick-Slip Friction using Parallel Replica Dynamics. Tribol. Lett. 2009, 36, 63–68. [Google Scholar] [CrossRef]
- Jaramillo-Botero, A.; Naserifar, S.; Goddard, W.A. General Multiobjective Force Field Optimization Framework, with Application to Reactive Force Fields for Silicon Carbide. J. Chem. Theor. Comput. 2014, 10, 1426–1439. [Google Scholar] [CrossRef] [Green Version]
- Larsson, H.; Hartke, B.; van Duin, A.C.T. Global optimization of parameters in the reactive force field ReaxFF for SiOH. J. Comput. Chem. 2013, 34, 2178–2189. [Google Scholar] [CrossRef] [Green Version]
- Rice, B.M.; Larentzos, J.P.; Byrd, E.F.C.; Weingarten, N.S. Parameterizing complex reactive force fields using multiple objective evolutionary strategies (MOES): Part 2: Transferability of ReaxFF models to C-H-N-O energetic materials. J. Theor. Comput. Chem. 2015, 11, 392–405. [Google Scholar] [CrossRef]
- Dittner, M.; Muller, J.; Aktulga, H.M.; Hartke, B. Efficient global optimization of reactive force-field parameters. J. Comput. Chem. 2015, 36, 1550–1561. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martini, A.; Eder, S.J.; Dörr, N. Tribochemistry: A Review of Reactive Molecular Dynamics Simulations. Lubricants 2020, 8, 44. https://doi.org/10.3390/lubricants8040044
Martini A, Eder SJ, Dörr N. Tribochemistry: A Review of Reactive Molecular Dynamics Simulations. Lubricants. 2020; 8(4):44. https://doi.org/10.3390/lubricants8040044
Chicago/Turabian StyleMartini, Ashlie, Stefan J. Eder, and Nicole Dörr. 2020. "Tribochemistry: A Review of Reactive Molecular Dynamics Simulations" Lubricants 8, no. 4: 44. https://doi.org/10.3390/lubricants8040044
APA StyleMartini, A., Eder, S. J., & Dörr, N. (2020). Tribochemistry: A Review of Reactive Molecular Dynamics Simulations. Lubricants, 8(4), 44. https://doi.org/10.3390/lubricants8040044






