The products obtained from the conversion of waste plastics can be employed in various applications. Distillation of the liquid yield will result in the formation of hydrocarbons similar to that of gasoline, kerosene, and diesel, to name a few. Lee et al. [
4] and Rehan et al. [
107] developed an electric current by employing obtained pyrolyzed oil in a diesel engine generator. Saptoadi et al. [
108] employed pyrolyzed oil as a substitute in a kerosene stove for heating. Some of the aromatic compounds produced from the pyrolysis process could be employed as raw material pertaining to the polymerization process in many chemical industries [
109,
110]. Several researchers have employed the pyrolyzed oil as a transportation fuel after mixing it with conventional diesel in various ratios. Mixed pyrolyzed oil and traditional diesel in a specific ratio were noted to provide similar engine performance compared with that of traditional diesel and the mixed fuel was relatively economical [
4,
111]. A limitation, as noted in one study, was that there was an increase in the exhaust emission from the engine when there was an increase in the mixture amount of pyrolyzed oil [
112].
There are various integral parameters that influence the conversion of waste plastics into lubricants. Parameters like the condition of the waste plastics and the existence of impurities in the waste plastics should be optimized to obtain better output products. There should be more studies carried out to optimize operational pressure, temperature, and residence time of the conversion process, which can lead to a superior and higher yields. Design and type of reactors need to be further explored to obtain better output yields [
113]. In the future, there should be research for new catalysts possessing lower acidity, which will generate a greater amount of desired output products. Studies on multifunctional catalysts need to be carried out that can enhance the yield from the conversion process [
114]. While it has been observed from studies that an acceptable product yield and configuration is achievable in a laboratory scale, it is difficult to obtain the desired result in the industrial scale. More research should be carried out to explore the feasibility of obtaining optimum results in industrial fields. Microwave assisted pyrolysis is an effective treatment for mixed plastic wastes. However, more research is required relating to this process on its viability to be applicable during the recycling of mixed plastic wastes. Further, the dissolution of plastic wastes in appropriate suitable solvents before carrying out the pyrolysis process may help overcome the limitation of the feasibility of the process [
115,
116]. Attempts need to be made to obtain hydrogen from waste plastics that can act as a green fuel for the automobile industry. Further research is required to help optimize the capital and operating costs necessary to improve output yield from the pyrolysis conversion process. It is important to reduce the moisture content and production of solid residues obtained from the pyrolysis process to obtain better liquid products. New techniques need to be investigated that can help overcome these limitations. Tribological tests on liquid and solid yield converted from waste plastics is another field where experiments can be performed to explore the feasibility of yields as potential lubricants. The next section discusses a study conducted to investigate the viscosity, density, and friction properties of a newly mixed waste polymer pyrolyzed oil to gauge the potential of waste plastics as potential sources of efficient lubricants. Further, the section details various experiments that can be used to study and analyse waste polymer pyrolyzed oils for tribological applications.
6.1. Commercial Conversion of Waste Plastics to Oil, Wax, and Chemical Feedstocks
The conversion of plastics to oil has been studied for many years. Beginning in the 1960s, efforts were made to convert plastics to a more basic hydrocarbon form using pyrolysis and a number of patents were issued for the process. In subsequent years, additional efforts were made to refine the process and produce materials ranging from oil and wax to what is considered “drop in” fuels and oils that could be used directly in engines [
1]. The principal was and still continues to be executed profitably on a commercial scale.
Today, companies employ the same basic pyrolysis process that has been explored and developed over many years and discussed in this article. The commercial system uses a process known as thermal depolymerization, where plastic polymers are subjected to heating within an oxygen-free environment. While the heat inside the reactor is easily sufficient to combust the plastic feedstock, without oxygen, the plastic polymer composed of carbon and hydrogen components essentially breaks apart into smaller “pieces” or hydrocarbon chains that exit the reactor and condense into a range of hydrocarbon products such as gases, oils, and waxes [
95].
The industries that produce waste plastic-based oils receive bulk plastic from a variety of sources and sort these materials to remove contaminants including paper, cardboard, metal, and materials such as PET and PVC plastics, as well as nylons, urethanes, thermoset plastics, and other types of “plastic” that either does not create oil or produce chemicals that can damage both the system hardware and the oil product. Polyvinyl chloride (PVC) is a good example of a material that is not well-suited to the oil production process because, as it is heated, it begins to decompose and releases gas and aqueous-phase hydrogen chloride [
117]. Hydrogen chloride can easily combine with the moisture that is always present in the feedstock and become extremely corrosive to process equipment (leading to premature failure), and it can become entrained in the oil, which can overall affect oil quality and create corrosion issues downstream with end-users.
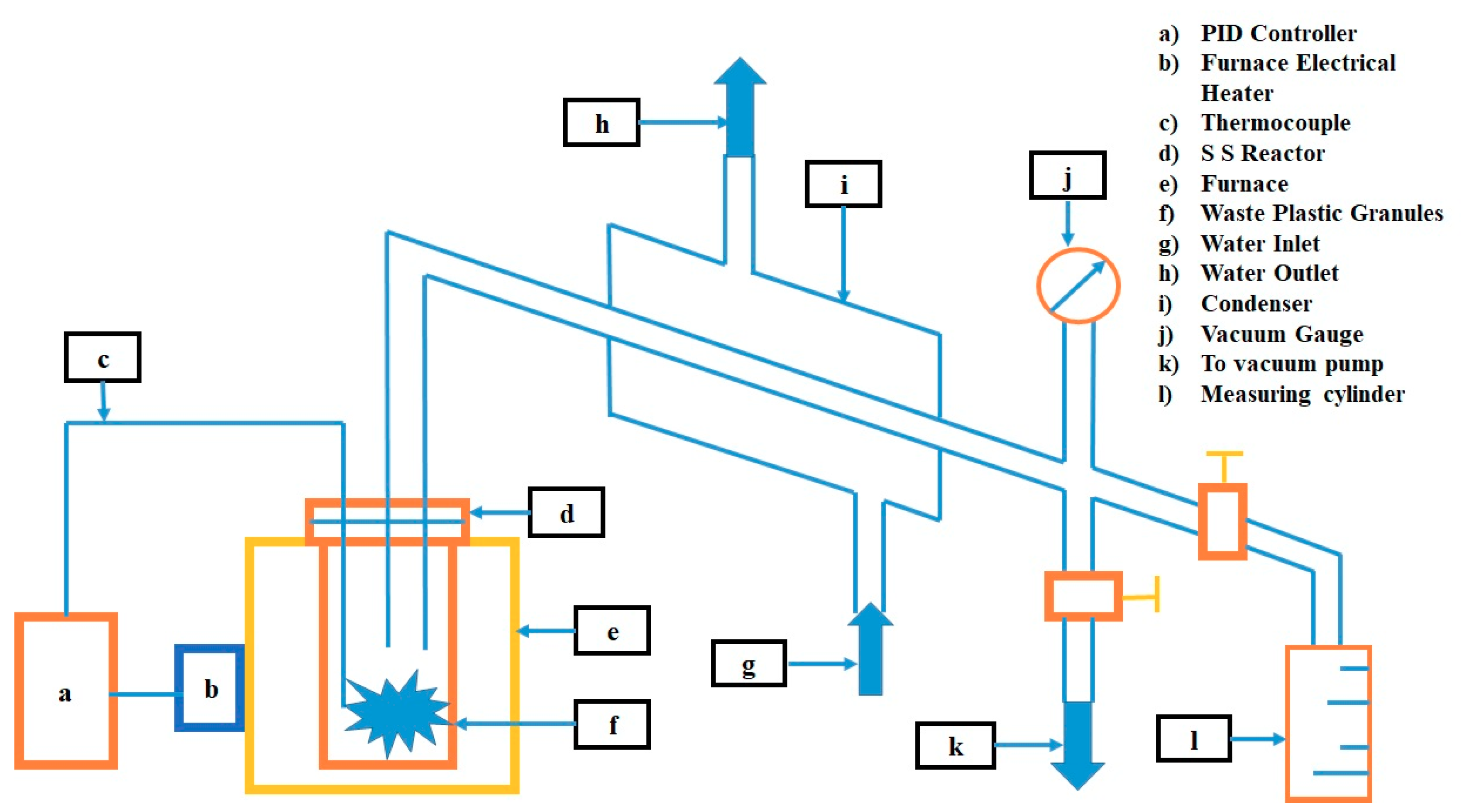
Once the incoming feedstock has been sorted and as many contaminants as possible are removed, the plastic is shredded. Shredding allows the size of the plastic to be reduced, which in turn allows it to feed properly into the downstream conveying and processing equipment. A schematic of a typical pyrolysis plant is shown in
Figure 8. Shredded plastic is moved into an equipment where it is heated and then moved to the primary processing reactors where it enters a “bath” of molten plastic and is heated to the temperatures necessary for achieving the pyrolysis reaction. (a) The PID (proportional integral derivative) controller in the plant is meant for controlling and maintaining temperatures along with the thermocouple. (b) The furnace electrical heater supplies heat continuously during the conversion process. Temperature control on the reactor’s heated surfaces is critical to avoid creating char from the plastic and the flow pattern of the molten plastic across the reactor’s heated surfaces is critical to maintaining the pyrolysis reaction, while at the same time preventing production of char. (c) The thermocouple monitors temperature in the plant during the conversion process. (d) The stainless steel (SS) reactor is a vacuum chamber that is supplied with heat from (e) the furnace used in the pyrolysis process. (f) The waste plastic granules accumulate at a chamber inside the furnace. (g) The water inlet connection supplies the required water for the plant to aid in the condensation of gases. The waste water leaves the plant by (h) water outlet pipes. (i) The condenser helps in cooling the gases. (j) Vacuum gauge helps in measurement and monitoring of pressure flow in the plant and (k) vacuum pump aids in the removal of excess gas molecules from the plant. (l) The measuring cylinder measures the volume of the pyrolyzed product obtained after the pyrolysis process. The next paragraph explains solid residues generated in a pyrolysis plant.
Small amounts of paper and cardboard that make their way through the sorting process and end up in the reactor are converted into char, which accumulates within the molten bath and settles on the reactor bottom along with any inorganic material that enters the reactor. Char and other settled materials are drawn out of the reactor regularly to maintain the reactor char level within acceptable limits. As the plastic inside the reactor is depolymerized, it enters a vapour phase and exits the reactor. The vapour travels through a series of condensers where it is condensed at specific temperatures to yield the wax and oil in a liquid phase. The non-condensable gases formed during the pyrolysis reaction, such as butane and propane, are collected, compressed, and directed back to the main reactors, where it is consumed as a fuel for the reactor heating elements. Wax and oil that have been condensed from the vapours exiting the reactor are pumped to large storage tanks where they are stored until being pumped into commercial tanker trucks for highway transport to their end destination.
There have been and currently are a large number of companies attempting to scale plastic pyrolysis to a commercial level. Creating small amounts of oil from plastic in a laboratory or pilot scale setting is relatively easy. However, operating a commercial-scale system is quite different owing to the extremely large number of process variables that must be controlled while maintaining both operating and capital costs to the point where the company can be a viable and profitable entity. Some new companies have been able to commercially scale a very efficient and economical system, but they require further improvements. There is a need to work towards improvements that can allow such technologies to be deployed at a level that will make a genuine contribution to the widespread efforts to reduce plastic wastes in the environment.
In order to analyse the physical properties of the liquid oil obtained by a pyrolysis process, the tests and standards detailed in
Table 8 are employed.
A study was conducted on a mixed waste polymer pyrolyzed oil obtained from a local oil and lubricant industry that did not contain any catalyst or additives as part of its manufacturing process. A reactor-based manufacturing process as described earlier was used in the production of a waste plastic pyrolyzed oil. The oil has a viscosity range that is close to kerosene and other such crude petroleum products. Some of the physical and chemical properties of the waste plastic pyrolyzed oil are detailed in
Table 9. These oils were further tested for their kinematic viscosity, which was compared against other lubricants.
6.2. Kinematic Viscosity
To investigate kinematic viscosity (cSt) of the liquid oil, tests can be performed at different temperatures. As per many case studies, the kinematic viscosity for the pyrolyzed liquid oil is noted at room temperature, 40 °C, and 100 °C [
119,
120,
121]. The kinematic viscosity test can be carried out using a viscometer. To further investigate the dynamic nature of the liquid oils, the rpm of the spindle can be varied as per requirement. These steps are repeated with the liquid oil at temperatures of 40 °C and 100 °C for further insight into the working range of oils.
The kinematic viscosity of the mixed waste polymer pyrolyzed oils detailed in
Table 9 was measured in a similar manner and compared with eight other lubricants currently used as bio-based lubricants [
4] and motor oils [
5,
6,
7].
Figure 9 shows the kinematic viscosity at 40 °C for waste plastic polymer pyrolyzed oils (1,2) compared with bio-lubricants (3,4), and motor oils (5–10). Further, the densities of the pyrolyzed oils, bio-lubricants, and motor oils are compared, as shown in
Figure 10.
The results indicate that the kinematic viscosity of the mixed waste polymer pyrolyzed oil (1) was 1.19 cSt, which was slightly lower than the minimum kinematic viscosity standard of petroleum oils as per ASTM D975 standard [
50], but close to the bio-lubricant viscosity as per ASTM D445 standards in the United States [
6]. The density of the same mixed waste polymer pyrolyzed oil was 0.75 × 10
−3 kg/cm
3, which was less than the conventional motor oils that had an average density of 0.83 × 10
−3 kg/cm
3 (
Figure 10). The kinematic viscosity of waste HDPE pyrolyzed oil (2) was 1.98 cSt at 40 °C, which was closer to the bio-lubricants viscosity, but still lower than conventional petroleum oils. The density of the small-scale commercially used HDPE pyrolyzed oil was similar to that of the mixed waste pyrolyzed oil (
Figure 10). The literature indicates that the density of these waste polymer plastic-based oils can be improved with further treatments as the lubricity of these oils are comparable to other petroleum crude oils, as discussed earlier in this article [
50]. The review finds that the pyrolyzed oil can also be blended with conventional petroleum oils in a specific volumetric ratio to enhance thermal efficiency [
4]. This indicates that this lubricant in its current state can be used as an additive to conventional lubricants, but with further treatment. They are found to be a suitable alternative to bio-lubricant sources of lubricant or for similar applications. It is recommended that the use of additives can enable control of the aspects of viscosity and density in addition to the treatments discussed in this article. Additionally, the sulphur and ash content in the pyrolyzed plastic oil is 0.246%, which is relatively low and reduces environmental issues [
5].
6.3. Tribological Properties
The mixed waste polymer pyrolyzed oil was subjected to tribological tests to understand its friction and wear properties. The oil is examined under a pin-on-disk set up, where the disk material is made up of AISI 1020 stainless steel having an average surface roughness value
(Ra) of 0.1 ± 0.04 µm after polishing. The pin material is made up of aluminium 2017 alloy. The tribological tests were performed over a sliding distance of 1000 m for a period of 60 minutes at room temperature condition and 50 N normal load. A friction study was performed using the pin-on-disk test where the coefficient of friction (COF) of the mixed waste plastic oil is measured for sliding between aluminium and steel tribo-pair. In order to explore physical nature of friction between pin and disk, it is important to establish the lubrication regime for which pin-on-disk experiments are performed. The Hamrock and Dowson equation [
122] can be employed to evaluate the film thickness at the lubricated contacts. The fluid film thickness parameter (
) can be calculated using the following equation.
where
is the minimum film thickness (m) and
and
are the root mean square (r.m.s) roughness of the pin and disk surface, respectively. Equation (1) will give information on the nature of lubricating regime by comparing the thickness of the lubricant fluid film and combined asperity heights of the tribo-pair taken into consideration for the experiment. Mathematically, the minimum fluid film thickness (
) can be obtained from the following equation [
123].
where
is the minimum film thickness (m) and
is the reduced radius of curvature, that is,
, where
and
are the reduced radius of curvatures in x and y directions, respectively.
is the entraining surface velocity (m/s), that is,
, where the subscripts A and B indicate the velocities of the pin and disk, respectively.
is the viscosity of the lubricant at atmospheric pressure (Pa s).
is the reduced Young’s modulus (Pa).
is the pressure-viscosity coefficient (m
2/N), that is,
is the constant load (N).
is the ellipticity parameter, defined as
=
, where
is the semi axis of the contact ellipse in the transverse direction (m) and
is the semi axis in the direction of motion (m).
Employing experimental variables of Equation (2) obtained from the experiment and substituting in Equation (1), the lubricating film thickness parameter (
for the experimental loading condition can be calculated. If the value obtained is less than 1, it will represent the boundary lubrication regime. If the obtained value is greater than 1, but less than 3, it will represent the mixed lubrication regime. If the obtained value is greater than 3, it will represent the hydrodynamic lubrication regime [
124].
In the present study, the Equation (2) variables for the mixed waste polymer pyrolyzed oil were as follows: the r.m.s roughness value of the pin before test was 0.2 ± 0.05 μm and, for the disk, was 0.1 ± 0.05 μm, respectively. The other variables that were calculated from the experiment are the pin’s tip radius = 3.175 mm, sliding speed = 0.277 mm/s, kinematic viscosity of the mixed waste polymer pyrolyzed oil at atmospheric pressure ranging between 5 and 10 mPa s, and Young’s modulus of the pin = 72 Gpa and that of the disk = 205 GPa, in order to obtain the reduced Young’s modulus, respectively. Substituting these values into Equation (2) and then into Equation (1) gave the film thickness parameter () as ranging between 0.0002 and 0.0004. As the value of was less than 1, the lubrication regime in which the mixed waste polymer pyrolyzed oil existed was boundary lubrication, for the normal load of 50 N applied. The COF obtained from the experiments pertaining to the mixed waste polymer pyrolyzed oil also confirms the same lubrication regime.
The was also calculated for jatropha-based bio-lubricant and Silkolene Comp 4 motor oil. The value for the jatropha-based bio-lubricant was found to range between 0.0112 and 0.0276, and for Silkolene Comp 4 motor oil, it was found to range between 0.0927 and 0.1710, respectively, for the normal load of 50 N applied. As the value was less than 1 in both lubricants, it could be concluded that the lubrication regime here was boundary lubrication.
In a study conducted by Syahrullail et al. [
125], different bio-lubricants such as jatropha, Refined Bleached and Deodorized (RBD) kernel oil, palm oil, and soya bean oil, as well as mineral oils such as commercial stamping and commercial hydraulic oil, were investigated to compare their tribological properties using four ball tester equipment. It was observed that palm oil exhibited the least COF of 0.025 among all the bio-lubricants and commercial stamping oil produced the highest COF of 0.11 among the mineral oils. The commercial stamping oil exhibited a maximum wear scar diameter of 800 μm among the mineral oils, whereas jatropha and soya bean oil exhibited the highest wear scar diameter of 500 μm and 550 μm, respectively, among the bio-lubricants. Studies have indicated that, above a threshold load, the oxidation stability of the mineral oil degrades, leading to an abrasive form of wear. The large wear scar diameter in bio-lubricants could be attributed to the phenomenon of hydrogenation occurring during the experiment. Hydrogenation enhances the oxidation rate and volume of free fatty acid in the bio-lubricant that accelerates corrosion rate in the tribo system. Additionally, during the run-in process, the ball’s protective layer gets worn out and the unworn part is exposed to free fatty acid of the bio-lubricants. As a result of wear-initiated reactions with the fatty acids, the unworn area on the ball is oxidized, rendering the surface brittle and leading to corrosive wear. The wear also causes an increase in the surface roughness of the balls. The results indicate that the susceptibility of surfaces to wear and corrosion in bio-lubricants causes the surfaces to become brittle, which can be mitigated by employing the mixed waste polymer pyrolyzed oil. The mixed waste polymer pyrolyzed oil does not undergo hydrogenation, and hence can provide high resilience to wear and corrosion.
Moreover, as the viscosity and density of mixed waste polymer pyrolyzed oil are found to be similar to the bio-lubricants (from
Figure 9 and
Figure 10), the observed friction for this mixed waste polymer pyrolyzed oil was compared with bio-lubricants and a motor oil lubricant, as shown in
Figure 11.
The observed results for the COF behaviour indicate that the friction behaviour of the waste plastic oil is similar to, or in some cases better than, the bio-lubricants (
Figure 11). This can be attributed to the long-chain polymers used in the waste plastics for recycling and conversion to oil. The long chain polymer-based oils such as synthetic oils have been found to provide enhanced lubrication by reducing the friction drastically [
126,
127]. Similar sliding mechanisms are expected to occur at the tribo interface lubricated with mixed waste polymer pyrolyzed oils. These basic insights into a new source of lubricant should drive the tribological research towards studying environmentally beneficial products. Increased research in this area can promote and shape the market needs and demands for the petroleum lubricant alternatives and recycling of waste plastics for tribological needs.