Muscles in Winter: The Epigenetics of Metabolic Arrest
Abstract
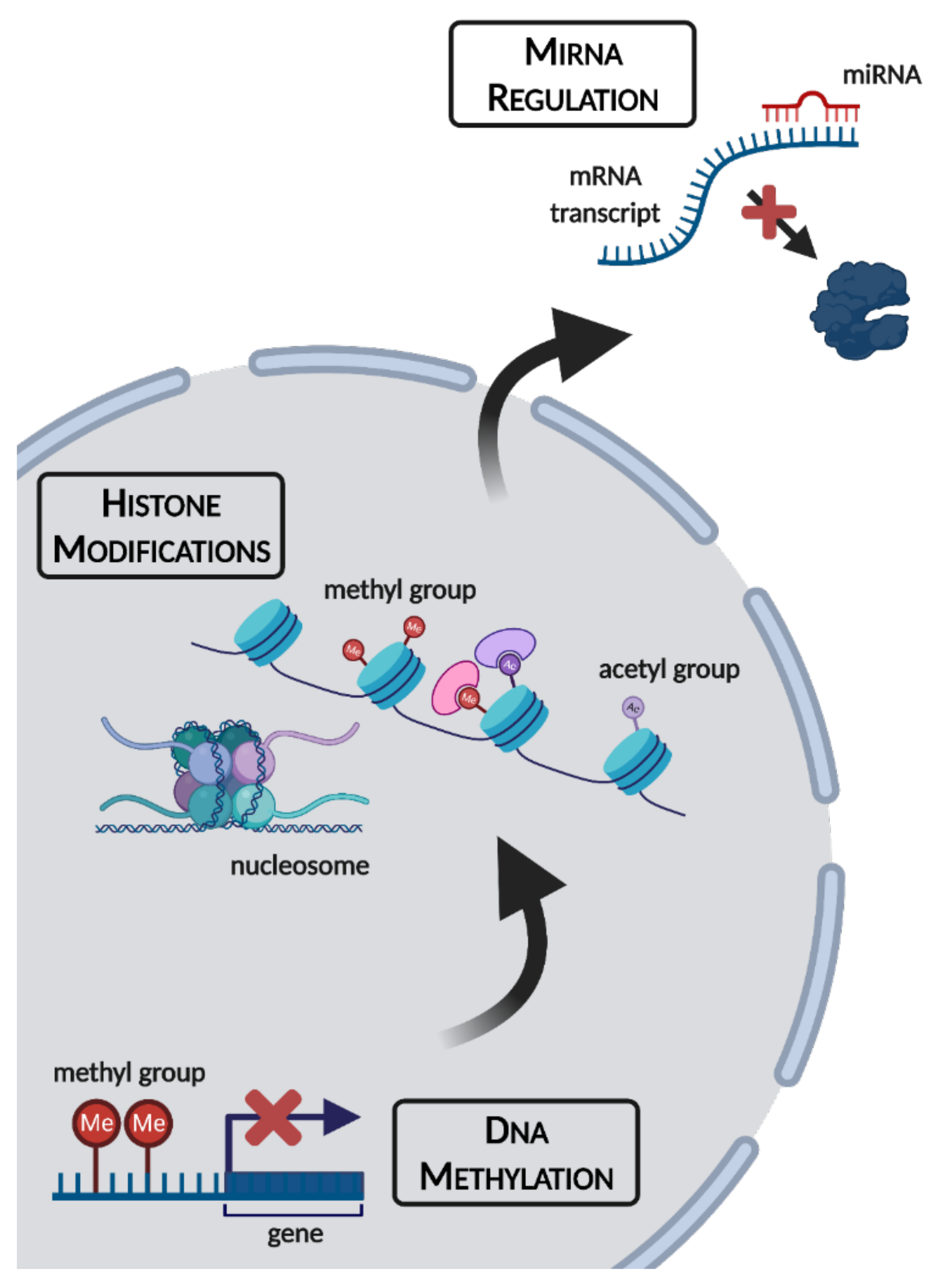
:1. Introduction
2. Myoprotection and Regeneration
3. Fuel Use: Glucose and Lipids
4. Transcriptional and Translational Suppression
5. Future Directions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Storey, K.B.; Storey, J.M. Metabolic Rate Depression. The Biochemistry of Mammalian Hibernation. In Advances in Clinical Chemistry; Makowski, G., Ed.; Academic Press: Burlington, MA, USA, 2010; pp. 77–78. [Google Scholar]
- Storey, K.B.; Storey, J.M. Aestivation: Signaling and Hypometabolism. J. Exp. Biol. 2012, 215, 1425–1433. [Google Scholar] [CrossRef] [Green Version]
- Krivoruchko, A.; Storey, K.B. Turtle Anoxia Tolerance: Biochemistry and Gene Regulation. Biochim. Et Biophys. Acta 2015, 1850, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Storey, K.B.; Storey, J.M. Molecular Physiology of Freeze Tolerance in Vertebrates. Physiol. Rev. 2017, 97, 623–665. [Google Scholar] [CrossRef] [PubMed]
- Ring, R.A. Freezing-Tolerant Insects with Low Supercooling Points. Comp. Biochem. Physiol. 1982, 73, 605–612. [Google Scholar] [CrossRef]
- Thomashow, M.F. Plant Cold Acclimation: Freezing Tolerance Genes and Regulatory Mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 571–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costanzo, J.P.; Lee, R.E.; Ultsch, G.R. Physiological Ecology of Overwintering in Hatchling Turtles. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2008, 309, 297–379. [Google Scholar] [CrossRef] [PubMed]
- Holmstrup, M. The Ins and Outs of Water Dynamics in Cold Tolerant Soil Invertebrates. J. Therm. Biol. 2014, 45, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.J. Freezing Resistance in Intertidal Invertebrates. Annu. Rev. Physiol. 1983, 45, 289–299. [Google Scholar] [CrossRef]
- Bird, A.P. CpG-Rich Islands and the Function of DNA Methylation. Nature 1986, 321, 209–213. [Google Scholar] [CrossRef]
- Lyko, F. The DNA Methyltransferase Family: A Versatile Toolkit for Epigenetic Regulation. Nat. Rev. Genet. 2018, 19, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Chédin, F.; Lieber, M.R.; Hsieh, C.L. The DNA Methyltransferase-like Protein DNMT3L Stimulates de Novo Methylation by DNMT3A. Proc. Natl. Acad. Sci. USA 2002, 99, 16916–16921. [Google Scholar] [CrossRef] [Green Version]
- Suetake, I.; Shinozaki, F.; Miyagawa, J.; Takeshima, H.; Tajima, S. DNMT3L Stimulates the DNA Methylation Activity of DNMT3A and DNMT3B through a Direct Interaction. J. Biol. Chem. 2004, 279, 27816–27823. [Google Scholar] [CrossRef] [Green Version]
- Kouzarides, T. Chromatin Modifications and Their Function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef] [Green Version]
- Barski, A.; Cuddapah, S.; Cui, K.; Roh, T.Y.; Schones, D.E.; Wang, Z.; Wei, G.; Chepelev, I.; Zhao, K. High-Resolution Profiling of Histone Methylations in the Human Genome. Cell 2007, 129, 823–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikkelsen, T.S.; Ku, M.; Jaffe, D.B.; Issac, B.; Lieberman, E.; Giannoukos, G.; Alvarez, P.; Brockman, W.; Kim, T.K.; Koche, R.P.; et al. Genome-Wide Maps of Chromatin State in Pluripotent and Lineage-Committed Cells. Nature 2007, 448, 553–560. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [Green Version]
- Rice, S.A.; Ten Have, G.A.M.; Reisz, J.A.; Gehrke, S.; Stefanoni, D.; Frare, C.; Barati, Z.; Coker, R.H.; D’Alessandro, A.; Deutz, N.E.P.; et al. Nitrogen Recycling Buffers against Ammonia Toxicity from Skeletal Muscle Breakdown in Hibernating Arctic Ground Squirrels. Nat. Metab. 2020, 2, 1459–1471. [Google Scholar] [CrossRef]
- Tessier, S.N.; Storey, K.B. Lessons from Mammalian Hibernators: Molecular Insights into Striated Muscle Plasticity and Remodeling. Biomol. Concepts 2016, 7, 69–92. [Google Scholar] [CrossRef] [Green Version]
- Black, B.L.; Olson, E.N. Transcriptional Control of Muscle Development by Myocyte Enhancer Factor-2 (MEF2) Proteins. Annu. Rev. Cell Dev. Biol. 1998, 14, 167–196. [Google Scholar] [CrossRef] [PubMed]
- Tessier, S.N.; Storey, K.B. Expression of Myocyte Enhancer Factor-2 and Downstream Genes in Ground Squirrel Skeletal Muscle during Hibernation. Mol. Cell. Biochem. 2010, 344, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Carey, H.V.; Andrews, M.T.; Martin, S.L. Mammalian Hibernation: Cellular and Molecular Responses to Depressed Metabolism and Low Temperature. Physiol. Rev. 2003, 83, 1153–1181. [Google Scholar] [CrossRef] [Green Version]
- Srere, H.K.; Wang, L.C.H.; Martin, S.L. Central Role for Differential Gene Expression in Mammalian Hibernation. Proc. Natl. Acad. Sci. USA 1992, 89, 7119–7123. [Google Scholar] [CrossRef] [Green Version]
- Morin, P.; Storey, K.B. Evidence for a Reduced Transcriptional State during Hibernation in Ground Squirrels. Cryobiology 2006, 53, 310–318. [Google Scholar] [CrossRef]
- Wu, C.W.; Storey, K.B. Regulation of the MTOR Signaling Network in Hibernating Thirteen-Lined Ground Squirrels. J. Exp. Biol. 2012, 215, 1720–1727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abnous, K.; Dieni, C.A.; Storey, K.B. Suppression of MAPKAPK2 during Mammalian Hibernation. Cryobiology 2012, 65, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Tessier, S.N.; Storey, K.B. Myocyte Enhancer Factor-2 and Cardiac Muscle Gene Expression during Hibernation in Thirteen-Lined Ground Squirrels. Gene 2012, 501, 8–16. [Google Scholar] [CrossRef]
- Hershey, J.D.; Robbins, C.T.; Nelson, O.L.; Lin, D.C. Minimal Seasonal Alterations in the Skeletal Muscle of Captive Brown Bears. Physiol. Biochem. Zool. 2008, 81, 138–147. [Google Scholar] [CrossRef]
- Harlow, H.J.; Lohuis, T.; Beck, T.D.I.; Iaizzo, P.A. Muscle Strength in Overwintering Bears. Nature 2001, 409, 997. [Google Scholar] [CrossRef] [PubMed]
- Tinker, D.B.; Harlow, H.J.; Beck, T.D.I. Protein Use and Muscle-fiber Changes in Free-ranging, Hibernating Black Bears. Physiol. Zool. 2015, 71, 414–424. [Google Scholar] [CrossRef] [Green Version]
- Luu, B.E.; Lefai, E.; Giroud, S.; Swenson, J.E.; Chazarin, B.; Gauquelin-Koch, G.; Arnemo, J.M.; Evans, A.L.; Bertile, F.; Storey, K.B. MicroRNAs Facilitate Skeletal Muscle Maintenance and Metabolic Suppression in Hibernating Brown Bears. J. Cell. Physiol. 2020, 235, 3984–3993. [Google Scholar] [CrossRef] [PubMed]
- Cannataro, R.; Carbone, L.; Petro, J.L.; Cione, E.; Vargas, S.; Angulo, H.; Forero, D.A.; Odriozola-Martínez, A.; Kreider, R.B.; Bonilla, D.A. Sarcopenia: Etiology, Nutritional Approaches, and MiRNAs. Int. J. Mol. Sci. 2021, 22, 9724. [Google Scholar] [CrossRef]
- Kornfeld, S.F.; Biggar, K.K.; Storey, K.B. Differential Expression of Mature MicroRNAs Involved in Muscle Maintenance of Hibernating Little Brown Bats, Myotis Lucifugus: A Model of Muscle Atrophy Resistance. Genom. Proteom. Bioinform. 2012, 10, 295–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.W.; Biggar, K.K.; Luu, B.E.; Szereszewski, K.E.; Storey, K.B. Analysis of MicroRNA Expression during the Torpor-Arousal Cycle of a Mammalian Hibernator, the 13-Lined Ground Squirrel. Physiol. Genom. 2016, 48, 388–396. [Google Scholar] [CrossRef] [Green Version]
- Oliveira-Carvalho, V.; Carvalho, V.O.; Bocchi, E.A. The Emerging Role of MiR-208a in the Heart. DNA Cell Biol. 2013, 32, 8–12. [Google Scholar] [CrossRef]
- Luu, B.E.; Biggar, K.K.; Wu, C.-W.; Storey, K.B. Torpor-Responsive Expression of Novel MicroRNA Regulating Metabolism and Other Cellular Pathways in the Thirteen-Lined Ground Squirrel, Ictidomys Tridecemlineatus. FEBS Lett. 2016, 590, 3574–3582. [Google Scholar] [CrossRef]
- Hadj-Moussa, H.; Zhang, J.; Pifferi, F.; Perret, M.; Storey, K.B. Profiling Torpor-Responsive MicroRNAs in Muscles of the Hibernating Primate Microcebus Murinus. Biochim. Biophys. Acta 2020, 1863, 194473. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.J. The MyomiR Network in Skeletal Muscle Plasticity. Exerc. Sport Sci. Rev. 2011, 39, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Lu, Y.; Pan, Z.; Chu, W.; Luo, X.; Lin, H.; Xiao, J.; Shan, H.; Wang, Z.; Yang, B. The Muscle-Specific MicroRNAs MiR-1 and MiR-133 Produce Opposing Effects on Apoptosis by Targeting HSP60, HSP70 and Caspase-9 in Cardiomyocytes. J. Cell Sci. 2007, 120, 3045–3052. [Google Scholar] [CrossRef] [Green Version]
- Storey, K.B. Out Cold: Biochemical Regulation of Mammalian Hibernation—A Mini-Review. Gerontology 2010, 56, 220–230. [Google Scholar] [CrossRef]
- Zhou, T.; Meng, X.; Che, H.; Shen, N.; Xiao, D.; Song, X.; Liang, M.; Fu, X.; Ju, J.; Li, Y.; et al. Regulation of Insulin Resistance by Multiple MiRNAs via Targeting the GLUT4 Signalling Pathway. Cell. Physiol. Biochem. 2016, 38, 2063–2078. [Google Scholar] [CrossRef] [PubMed]
- Vargas, E.; Podder, V.; Sepulveda, M.A.C. Physiology, Glucose Transporter Type 4. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Ke, R.; Xu, Q.; Li, C.; Luo, L.; Huang, D. Mechanisms of AMPK in the Maintenance of ATP Balance during Energy Metabolism. Cell Biol. Int. 2018, 42, 384–392. [Google Scholar] [CrossRef] [Green Version]
- Chung, D.; Dzal, Y.A.; Seow, A.; Milsom, W.K.; Pamenter, M.E. Naked Mole Rats Exhibit Metabolic but Not Ventilatory Plasticity Following Chronic Sustained Hypoxia. Proc. R. Soc. B 2016, 283, 20160216. [Google Scholar] [CrossRef] [Green Version]
- Edrey, Y.H.; Hanes, M.; Pinto, M.; Mele, J.; Buffenstein, R. Successful Aging and Sustained Good Health in the Naked Mole Rat: A Long-Lived Mammalian Model for Biogerontology and Biomedical Research. ILAR J. 2011, 52, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Hadj-Moussa, H.; Chiasson, S.; Cheng, H.; Eaton, L.; Storey, K.B.; Pamenter, M.E. MicroRNA-Mediated Inhibition of AMPK Coordinates Tissue-Specific Downregulation of Skeletal Muscle Metabolism in Hypoxic Naked Mole-Rats. J. Exp. Biol. 2021, 224. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Brozinick, J.T.; Valladares, O.; Bucan, M.; Birnbaum, M.J. A Role for AMP-Activated Protein Kinase in Contraction- and Hypoxia-Regulated Glucose Transport in Skeletal Muscle. Mol. Cell 2001, 7, 1085–1094. [Google Scholar] [CrossRef]
- Siques, P.; Brito, J.; Flores, K.; Ordenes, S.; Arriaza, K.; Pena, E.; León-Velarde, F.; de Pablo, Á.L.L.; Gonzalez, M.C.; Arribas, S. Long-Term Chronic Intermittent Hypobaric Hypoxia Induces Glucose Transporter (GLUT4) Translocation Through AMP-Activated Protein Kinase (AMPK) in the Soleus Muscle in Lean Rats. Front. Physiol. 2018, 9, 799. [Google Scholar] [CrossRef]
- Marsin, A.S.; Bertrand, L.; Rider, M.H.; Deprez, J.; Beauloye, C.; Vincent, M.F.; van den Berghe, G.; Carling, D.; Hue, L. Phosphorylation and Activation of Heart PFK-2 by AMPK Has a Role in the Stimulation of Glycolysis during Ischaemia. Curr. Biol. 2000, 10, 1247–1255. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Lu, C.; Chu, W.; Zhang, B.; Zhen, Q.; Wang, R.; Zhang, Y.; Li, Z.; Lv, B.; Li, H.; et al. MicroRNA-124 Suppresses Proliferation and Glycolysis in Non-Small Cell Lung Cancer Cells by Targeting AKT-GLUT1/HKII. Tumor Biol. 2017, 39, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, X.; Wang, H.; Ye, Y.; Shu, Y.; Deng, Y.; He, X.; Lu, G.; Zhang, S. MiR-124 Regulates Cell Apoptosis and Autophagy in Dopaminergic Neurons and Protects Them by Regulating AMPK/MTOR Pathway in Parkinson’s Disease. Am. J. Transl. Res. 2016, 8, 2127. [Google Scholar]
- Li, Y.; Luan, Y.; Li, J.; Song, H.; Li, Y.; Qi, H.; Sun, B.; Zhang, P.; Wu, X.; Liu, X.; et al. Exosomal MiR-199a-5p Promotes Hepatic Lipid Accumulation by Modulating MST1 Expression and Fatty Acid Metabolism. Hepatol. Int. 2020, 14, 1057–1074. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, S.; Gao, X.; Zhao, Y.; Li, Y.; Yang, B.; Zhang, N.; Ma, L. Octreotide Alleviates Autophagy by Up-Regulation of MicroRNA-101 in Intestinal Epithelial Cell Line Caco-2. Cell. Physiol. Biochem. 2018, 49, 1352–1363. [Google Scholar] [CrossRef]
- Liu, P.; Ye, F.; Xie, X.; Li, X.; Tang, H.; Li, S.; Huang, X.; Song, C.; Wei, W.; Xie, X.; et al. Mir-101-3p Is a Key Regulator of Tumor Metabolism in Triple Negative Breast Cancer Targeting AMPK. Oncotarget 2016, 7, 35188–35198. [Google Scholar] [CrossRef] [Green Version]
- Pamenter, M.E.; Dzal, Y.A.; Thompson, W.A.; Milsom, W.K. Do Naked Mole Rats Accumulate a Metabolic Acidosis or an Oxygen Debt in Severe Hypoxia? J. Exp. Biol. 2019, 222, jeb191197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jornayvaz, F.R.; Shulman, G.I. Regulation of Mitochondrial Biogenesis. Essays Biochem. 2010, 47, 69–84. [Google Scholar] [CrossRef] [Green Version]
- Borralho, P.M.; Rodrigues, C.M.P.; Steer, C.J. MicroRNAs in Mitochondria: An Unexplored Niche. Adv. Exp. Med. Biol. 2015, 887, 31–51. [Google Scholar] [CrossRef]
- Das, S.; Ferlito, M.; Kent, O.A.; Fox-Talbot, K.; Wang, R.; Liu, D.; Raghavachari, N.; Yang, Y.; Wheelan, S.J.; Murphy, E.; et al. Nuclear MiRNA Regulates the Mitochondrial Genome in the Heart. Circ. Res. 2012, 110, 1596–1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Song, C.; Zhou, X.; Han, X.; Li, J.; Wang, Z.; Shang, H.; Liu, Y.; Cao, H. Mitochondria Associated MicroRNA Expression Profiling of Heart Failure. BioMed Res. Int. 2017, 2017, 4042509. [Google Scholar] [CrossRef]
- Machado, I.F.; Teodoro, J.S.; Palmeira, C.M.; Rolo, A.P. MiR-378a: A New Emerging MicroRNA in Metabolism. Cell. Mol. Life Sci. 2020, 77, 1947–1958. [Google Scholar] [CrossRef] [PubMed]
- Rippo, M.R.; Olivieri, F.; Monsurrò, V.; Prattichizzo, F.; Albertini, M.C.; Procopio, A.D. MitomiRs in Human Inflamm-Aging: A Hypothesis Involving MiR-181a, MiR-34a and MiR-146a. Exp. Gerontol. 2014, 56, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Tessier, S.N.; Ingelson-Filpula, W.A.; Storey, K.B. Epigenetic Regulation by DNA Methyltransferases during Torpor in the Thirteen-Lined Ground Squirrel Ictidomys Tridecemlineatus. Mol. Cell. Biochem. 2021, 476, 3975–3985. [Google Scholar] [CrossRef]
- Alvarado, S.; Mak, T.; Liu, S.; Storey, K.B.; Szyf, M. Dynamic Changes in Global and Gene-Specific DNA Methylation during Hibernation in Adult Thirteen-Lined Ground Squirrels, Ictidomys Tridecemlineatus. J. Exp. Biol. 2015, 218, 1787–1795. [Google Scholar] [CrossRef] [Green Version]
- Lang-Ouellette, D.; Morin, P.J. Differential Expression of MiRNAs with Metabolic Implications in Hibernating Thirteen-Lined Ground Squirrels, Ictidomys Tridecemlineatus. Mol. Cell. Biochem. 2014, 394, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Feng, X. TGF-Beta Receptor Signaling. Biochim. Biophys. Acta 1997, 1333, F105–F150. [Google Scholar] [CrossRef]
- Li, Z.; Lan, X.; Han, R.; Wang, J.; Huang, Y.; Sun, J.; Guo, W.; Chen, H. MiR-2478 Inhibits TGFβ1 Expression by Targeting the Transcriptional Activation Region Downstream of the TGFβ1 Promoter in Dairy Goats. Sci. Rep. 2017, 7, 42627. [Google Scholar] [CrossRef]
- Biggar, K.K.; Storey, K.B. Functional Impact of MicroRNA Regulation in Models of Extreme Stress Adaptation. J. Mol. Cell Biol. 2018, 10, 93–101. [Google Scholar] [CrossRef]
- Logan, S.M.; Wu, C.W.; Storey, K.B. The Squirrel with the Lagging EIF2: Global Suppression of Protein Synthesis during Torpor. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2019, 227, 161–171. [Google Scholar] [CrossRef]
- Hadj-Moussa, H.; Moggridge, J.A.; Luu, B.E.; Quintero-Galvis, J.F.; Gaitán-Espitia, J.D.; Nespolo, R.F.; Storey, K.B. The Hibernating South American Marsupial, Dromiciops Gliroides, Displays Torpor-Sensitive MicroRNA Expression Patterns. Sci. Rep. 2016, 6, 24627. [Google Scholar] [CrossRef] [Green Version]
- Storey, K.B.; Storey, J.M. Freeze Tolerance in Animals. Physiol. Rev. 1988, 68, 27–84. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Luu, B.E.; Storey, K.B. MicroRNA Regulation in Heart and Skeletal Muscle over the Freeze–Thaw Cycle in the Freeze Tolerant Wood Frog. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2016, 186, 229–241. [Google Scholar] [CrossRef]
- Cooley, N.; Cowley, M.J.; Lin, R.C.Y.; Marasco, S.; Wong, C.; Kaye, D.M.; Dart, A.M.; Woodcock, E.A. Influence of Atrial Fibrillation on MicroRNA Expression Profiles in Left and Right Atria from Patients with Valvular Heart Disease. Physiol. Genom. 2012, 44, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Storey, K.B. Akt Signaling and Freezing Survival in the Wood Frog, Rana Sylvatica. Biochim. Et Biophys. Acta Gen. Subj. 2013, 1830, 4828–4837. [Google Scholar] [CrossRef]
- Hadj-Moussa, H.; Storey, K.B. Micromanaging Freeze Tolerance: The Biogenesis and Regulation of Neuroprotective MicroRNAs in Frozen Brains. Cell. Mol. Life Sci. 2018, 75, 3635–3647. [Google Scholar] [CrossRef]
- Zhang, J.; Hawkins, L.J.; Storey, K.B. DNA Methylation and Regulation of DNA Methyltransferases in a Freeze Tolerant Vertebrate. Biochem. Cell Biol. 2019, 98, 145–153. [Google Scholar] [CrossRef]
- Bashtrykov, P.; Jankevicius, G.; Smarandache, A.; Jurkowska, R.Z.; Ragozin, S.; Jeltsch, A. Specificity of Dnmt1 for Methylation of Hemimethylated CpG Sites Resides in Its Catalytic Domain. Chem. Biol. 2012, 19, 572–578. [Google Scholar] [CrossRef] [Green Version]
- Symmank, J.; Zimmer, G. Regulation of Neuronal Survival by DNA Methyltransferases. Neural Regen. Res. 2017, 12, 1768. [Google Scholar] [CrossRef]
- Aapola, U.; Liiv, I.; Peterson, P. Imprinting Regulator DMNT3L Is a Transcriptional Repressor with Histone Deacetylase Activity. Nucleic Acids Res. 2002, 30, 3602–3608. [Google Scholar] [CrossRef]
- Deplus, R. Dnmt3L Is a Transcriptional Repressor That Recruits Histone Deacetylase. Nucleic Acids Res. 2002, 30, 3831–3838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, D.C.; Ultsch, G.R. Physiology of Hibernation under the Ice by Turtles and Frogs. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2010, 313, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Wijenayake, S.; Storey, K.B. The Role of DNA Methylation during Anoxia Tolerance in a Freshwater Turtle (Trachemys Scripta Elegans). J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2016, 186, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Biggar, K.K.; Storey, K.B. Evidence for Cell Cycle Suppression and MicroRNA Regulation of Cyclin D1 during Anoxia Exposure in Turtles. Cell Cycle 2012, 11, 1705–1713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biggar, K.K.; Storey, K.B. Exploration of Low Temperature MicroRNA Function in an Anoxia Tolerant Vertebrate Ectotherm, the Red Eared Slider Turtle (Trachemys Scripta Elegans). J. Therm. Biol. 2017, 68, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Watts, A.J.; Storey, K.B. Hibernation Impacts Lysine Methylation Dynamics in the 13-Lined Ground Squirrel, Ictidomys Tridecemlineatus. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2019, 331, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, L.J.; Storey, K.B. Histone Methylation in the Freeze-Tolerant Wood Frog (Rana Sylvatica). J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2018, 188, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Wijenayake, S.; Hawkins, L.J.; Storey, K.B. Dynamic Regulation of Six Histone H3 Lysine (K) Methyltransferases in Response to Prolonged Anoxia Exposure in a Freshwater Turtle. Gene 2018, 649, 50–57. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ingelson-Filpula, W.A.; Storey, K.B. Muscles in Winter: The Epigenetics of Metabolic Arrest. Epigenomes 2021, 5, 28. https://doi.org/10.3390/epigenomes5040028
Ingelson-Filpula WA, Storey KB. Muscles in Winter: The Epigenetics of Metabolic Arrest. Epigenomes. 2021; 5(4):28. https://doi.org/10.3390/epigenomes5040028
Chicago/Turabian StyleIngelson-Filpula, W. Aline, and Kenneth B. Storey. 2021. "Muscles in Winter: The Epigenetics of Metabolic Arrest" Epigenomes 5, no. 4: 28. https://doi.org/10.3390/epigenomes5040028





