Insights into Cardiovascular Defects and Cardiac Epigenome in the Context of COVID-19
Abstract
:1. Introduction
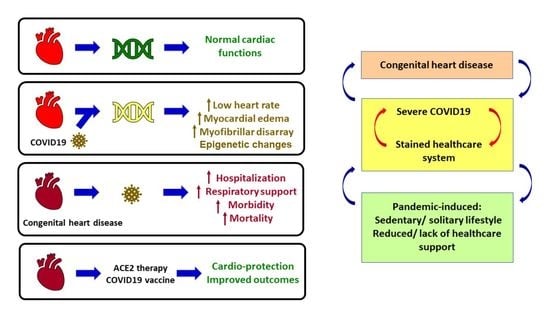
2. Altered Epigenomic Signature of DNA Methylation in Heart Impacts Transcriptome during SARS-CoV-2 Infection
3. COVID-19 and ACE2
ACE2/Ang1-7/Mas Receptor Axis Regulates Development, Physiology, and Organ Protection
4. Cardiovascular Damage during SARS-CoV-2 Infection
5. Congenital Heart Disease
6. Diagnosis of CHD
7. Management of CHD
8. CHD during the COVID-19 Pandemic
9. Vaccination for Improving Outcomes in CHD
10. Conclusions
Funding
Conflicts of Interest
References
- Gates, B. Responding to COVID-19—A Once-in-a-Century Pandemic? N. Engl. J. Med. 2020, 382, 1677–1679. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Dhama, K.; Khan, S.; Tiwari, R.; Sircar, S.; Bhat, S.; Malik, Y.S.; Singh, K.P.; Chaicumpa, W.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. Coronavirus Disease 2019-COVID-19. Clin. Microbiol. Rev. 2020, 33, e00028-20. [Google Scholar] [CrossRef] [PubMed]
- Tay, M.Z.; Poh, C.M.; Renia, L.; MacAry, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Ocaranza, M.P.; Michea, L.; Chiong, M.; Lagos, C.F.; Lavandero, S.; Jalil, J.E. Recent insights and therapeutic perspectives of angiotensin-(1-9) in the cardiovascular system. Clin. Sci. 2014, 127, 549–557. [Google Scholar] [CrossRef]
- Paul, M.; Poyan Mehr, A.; Kreutz, R. Physiology of local renin-angiotensin systems. Physiol. Rev. 2006, 86, 747–803. [Google Scholar] [CrossRef]
- Kanda, T.; Itoh, H. The ACE2/Ang(1-7)/Mas receptor axis in cardiovascular and renal diseases. Nihon Rinsho 2012, 70, 1487–1491. [Google Scholar]
- Chappell, M.C. Nonclassical renin-angiotensin system and renal function. Compr. Physiol. 2012, 2, 2733–2752. [Google Scholar] [CrossRef] [Green Version]
- McKinney, C.A.; Fattah, C.; Loughrey, C.M.; Milligan, G.; Nicklin, S.A. Angiotensin-(1-7) and angiotensin-(1-9): Function in cardiac and vascular remodelling. Clin. Sci. 2014, 126, 815–827. [Google Scholar] [CrossRef] [Green Version]
- Varagic, J.; Ahmad, S.; Nagata, S.; Ferrario, C.M. ACE2: Angiotensin II/angiotensin-(1-7) balance in cardiac and renal injury. Curr. Hypertens Rep. 2014, 16, 420. [Google Scholar] [CrossRef]
- Capettini, L.S.; Montecucco, F.; Mach, F.; Stergiopulos, N.; Santos, R.A.; da Silva, R.F. Role of renin-angiotensin system in inflammation, immunity and aging. Curr. Pharm. Des. 2012, 18, 963–970. [Google Scholar] [CrossRef] [PubMed]
- South, A.M.; Shaltout, H.A.; Washburn, L.K.; Hendricks, A.S.; Diz, D.I.; Chappell, M.C. Fetal programming and the angiotensin-(1-7) axis: A review of the experimental and clinical data. Clin. Sci. 2019, 133, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Oakes, J.M.; Fuchs, R.M.; Gardner, J.D.; Lazartigues, E.; Yue, X. Nicotine and the renin-angiotensin system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R895–R906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peiris, S.; Mesa, H.; Aysola, A.; Manivel, J.; Toledo, J.; Borges-Sa, M.; Aldighieri, S.; Reveiz, L. Pathological findings in organs and tissues of patients with COVID-19: A systematic review. PLoS ONE 2021, 16, e0250708. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Escobar, L.G.; Hoffman, K.L.; Choi, J.J.; Borczuk, A.; Salvatore, S.; Alvarez-Mulett, S.L.; Galvan, M.D.; Zhao, Z.; Racine-Brzostek, S.E.; Yang, H.S.; et al. Cytokine signatures of end organ injury in COVID-19. Sci. Rep. 2021, 11, 12606. [Google Scholar] [CrossRef] [PubMed]
- Mueller, A.L.; McNamara, M.S.; Sinclair, D.A. Why does COVID-19 disproportionately affect older people? Aging 2020, 12, 9959–9981. [Google Scholar] [CrossRef]
- Booth, A.; Reed, A.B.; Ponzo, S.; Yassaee, A.; Aral, M.; Plans, D.; Labrique, A.; Mohan, D. Population risk factors for severe disease and mortality in COVID-19: A global systematic review and meta-analysis. PLoS ONE 2021, 16, e0247461. [Google Scholar] [CrossRef]
- The, L. Redefining vulnerability in the era of COVID-19. Lancet 2020, 395, 1089. [Google Scholar] [CrossRef]
- Viner, R.M.; Mytton, O.T.; Bonell, C.; Melendez-Torres, G.J.; Ward, J.; Hudson, L.; Waddington, C.; Thomas, J.; Russell, S.; van der Klis, F.; et al. Susceptibility to SARS-CoV-2 Infection Among Children and Adolescents Compared With Adults: A Systematic Review and Meta-analysis. JAMA Pediatr. 2020, 175, 143–156. [Google Scholar] [CrossRef]
- Ludvigsson, J.F. Children are unlikely to be the main drivers of the COVID-19 pandemic—A systematic review. Acta Paediatr. 2020, 109, 1525–1530. [Google Scholar] [CrossRef]
- Tsabouri, S.; Makis, A.; Kosmeri, C.; Siomou, E. Risk Factors for Severity in Children with Coronavirus Disease 2019: A Comprehensive Literature Review. Pediatr. Clin. N. Am. 2021, 68, 321–338. [Google Scholar] [CrossRef] [PubMed]
- Miller, I.F.; Becker, A.D.; Grenfell, B.T.; Metcalf, C.J.E. Disease and healthcare burden of COVID-19 in the United States. Nat. Med. 2020, 26, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, D.; Fowler, E.J.; Abrams, M.; Collins, S.R. COVID-19—Implications for the Health Care System. N. Engl. J. Med. 2020, 383, 1483–1488. [Google Scholar] [CrossRef] [PubMed]
- Bernacki, K.; Keister, A.; Sapiro, N.; Joo, J.S.; Mattle, L. Impact of COVID-19 on patient and healthcare professional attitudes, beliefs, and behaviors toward the healthcare system and on the dynamics of the healthcare pathway. BMC Health Serv. Res. 2021, 21, 1309. [Google Scholar] [CrossRef]
- Sarkar, S.; Sen, R. COVID-19 and Cardiovascular Diseases: The Vicious Cycle. BJSTR 2020, 30, 4. [Google Scholar] [CrossRef]
- Li, S.; Ma, F.; Yokota, T.; Garcia, G., Jr.; Palermo, A.; Wang, Y.; Farrell, C.; Wang, Y.C.; Wu, R.; Zhou, Z.; et al. Metabolic reprogramming and epigenetic changes of vital organs in SARS-CoV-2-induced systemic toxicity. JCI Insight 2021, 6, e145027. [Google Scholar] [CrossRef]
- Castro de Moura, M.; Davalos, V.; Planas-Serra, L.; Alvarez-Errico, D.; Arribas, C.; Ruiz, M.; Aguilera-Albesa, S.; Troya, J.; Valencia-Ramos, J.; Velez-Santamaria, V.; et al. Epigenome-wide association study of COVID-19 severity with respiratory failure. EBioMedicine 2021, 66, 103339. [Google Scholar] [CrossRef]
- Balnis, J.; Madrid, A.; Hogan, K.J.; Drake, L.A.; Chieng, H.C.; Tiwari, A.; Vincent, C.E.; Chopra, A.; Vincent, P.A.; Robek, M.D.; et al. Blood DNA methylation and COVID-19 outcomes. Clin. Epigenet. 2021, 13, 118. [Google Scholar] [CrossRef]
- Kgatle, M.M.; Lawal, I.O.; Mashabela, G.; Boshomane, T.M.G.; Koatale, P.C.; Mahasha, P.W.; Ndlovu, H.; Vorster, M.; Rodrigues, H.G.; Zeevaart, J.R.; et al. COVID-19 Is a Multi-Organ Aggressor: Epigenetic and Clinical Marks. Front. Immunol. 2021, 12, 752380. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Pierouli, K.; Mitsis, T.; Diakou, K.I.; Palaiogeorgou, A.M.; Bacopoulou, F.; Chrousos, G.P.; Eliopoulos, E.; Vlachakis, D. COVID-19 global social lockdowns: Energy-related, psychological, epigenetic, health and environmental impacts (Review). Int. J. Epigenet. 2021, 1, 8. [Google Scholar] [CrossRef]
- Atlante, S.; Mongelli, A.; Barbi, V.; Martelli, F.; Farsetti, A.; Gaetano, C. The epigenetic implication in coronavirus infection and therapy. Clin. Epigenet. 2020, 12, 156. [Google Scholar] [CrossRef] [PubMed]
- Schmiedel, B.J.; Rocha, J.; Gonzalez-Colin, C.; Bhattacharyya, S.; Madrigal, A.; Ottensmeier, C.H.; Ay, F.; Chandra, V.; Vijayanand, P. COVID-19 genetic risk variants are associated with expression of multiple genes in diverse immune cell types. Nat. Commun. 2021, 12, 6760. [Google Scholar] [CrossRef] [PubMed]
- Chai, P.; Yu, J.; Ge, S.; Jia, R.; Fan, X. Genetic alteration, RNA expression, and DNA methylation profiling of coronavirus disease 2019 (COVID-19) receptor ACE2 in malignancies: A pan-cancer analysis. J. Hematol. Oncol. 2020, 13, 43. [Google Scholar] [CrossRef]
- Rath, S.; Perikala, V.; Jena, A.B.; Dandapat, J. Factors regulating dynamics of angiotensin-converting enzyme-2 (ACE2), the gateway of SARS-CoV-2: Epigenetic modifications and therapeutic interventions by epidrugs. Biomed. Pharmacother. 2021, 143, 112095. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.J.; McCuaig, R.D.; Melino, M.; Rawle, D.J.; Le, T.T.; Yan, K.; Suhrbier, A.; Johnston, R.L.; Koufariotis, L.T.; Waddell, N.; et al. Targeting novel LSD1-dependent ACE2 demethylation domains inhibits SARS-CoV-2 replication. Cell Discov. 2021, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Jit, B.P.; Qazi, S.; Arya, R.; Srivastava, A.; Gupta, N.; Sharma, A. An immune epigenetic insight to COVID-19 infection. Epigenomics 2021, 13, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Konwar, C.; Asiimwe, R.; Inkster, A.M.; Merrill, S.M.; Negri, G.L.; Aristizabal, M.J.; Rider, C.F.; MacIsaac, J.L.; Carlsten, C.; Kobor, M.S. Risk-focused differences in molecular processes implicated in SARS-CoV-2 infection: Corollaries in DNA methylation and gene expression. Epigenet. Chromatin 2021, 14, 54. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Albarran, M.; Navarro-Delgado, E.I.; Del Moral-Morales, A.; Alcaraz, N.; Baumbach, J.; Gonzalez-Barrios, R.; Soto-Reyes, E. Comparative transcriptome analysis reveals key epigenetic targets in SARS-CoV-2 infection. NPJ Syst. Biol. Appl. 2021, 7, 21. [Google Scholar] [CrossRef]
- Gordon, D.E.; Jang, G.M.; Bouhaddou, M.; Xu, J.; Obernier, K.; White, K.M.; O’Meara, M.J.; Rezelj, V.V.; Guo, J.Z.; Swaney, D.L.; et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 2020, 583, 459–468. [Google Scholar] [CrossRef]
- Wu, M.; Chen, Y.; Xia, H.; Wang, C.; Tan, C.Y.; Cai, X.; Liu, Y.; Ji, F.; Xiong, P.; Liu, R.; et al. Transcriptional and proteomic insights into the host response in fatal COVID-19 cases. Proc. Natl. Acad. Sci. USA 2020, 117, 28336–28343. [Google Scholar] [CrossRef]
- Sen, R.; Garbati, M.; Bryant, K.; Lu, Y. Epigenetic mechanisms influencing COVID-19. Genome 2021, 64, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Sen, R. High-throughput approaches of diagnosis and therapies for COVID-19: Antibody panels, proteomics and metabolomics. Future Drug Discov. 2021, 3, FDD55. [Google Scholar] [CrossRef]
- Bouchard, B.A.; Colovos, C.; Lawson, M.A.; Osborn, Z.T.; Sackheim, A.M.; Mould, K.J.; Janssen, W.J.; Cohen, M.J.; Majumdar, D.; Freeman, K. Increased histone-DNA complexes and endothelial-dependent thrombin generation in severe COVID-19. Vascul. Pharmacol. 2022, 142, 106950. [Google Scholar] [CrossRef] [PubMed]
- Huckriede, J.; de Vries, F.; Hultstrom, M.; Wichapong, K.; Reutelingsperger, C.; Lipcsey, M.; Garcia de Frutos, P.; Frithiof, R.; Nicolaes, G.A.F. Histone H3 Cleavage in Severe COVID-19 ICU Patients. Front. Cell. Infect. Microbiol. 2021, 11, 694186. [Google Scholar] [CrossRef]
- Shirvaliloo, M. Epigenomics in COVID-19; the link between DNA methylation, histone modifications and SARS-CoV-2 infection. Epigenomics 2021, 13, 745–750. [Google Scholar] [CrossRef]
- Askari, N.; Hadizadeh, M.; Rashidifar, M. A new insight into sex-specific non-coding RNAs and networks in response to SARS-CoV-2. Infect. Genet. Evol. 2022, 97, 105195. [Google Scholar] [CrossRef]
- Badimon, L.; Robinson, E.L.; Jusic, A.; Carpusca, I.; deWindt, L.J.; Emanueli, C.; Ferdinandy, P.; Gu, W.; Gyongyosi, M.; Hackl, M.; et al. Cardiovascular RNA markers and artificial intelligence may improve COVID-19 outcome: A position paper from the EU-CardioRNA COST Action CA17129. Cardiovasc. Res. 2021, 117, 1823–1840. [Google Scholar] [CrossRef]
- Winkle, M.; El-Daly, S.M.; Fabbri, M.; Calin, G.A. Noncoding RNA therapeutics—Challenges and potential solutions. Nat. Rev. Drug Discov. 2021, 20, 629–651. [Google Scholar] [CrossRef]
- Greco, S.; Made, A.; Gaetano, C.; Devaux, Y.; Emanueli, C.; Martelli, F. Noncoding RNAs implication in cardiovascular diseases in the COVID-19 era. J. Transl. Med. 2020, 18, 408. [Google Scholar] [CrossRef]
- Yang, Q.; Lin, F.; Wang, Y.; Zeng, M.; Luo, M. Long Noncoding RNAs as Emerging Regulators of COVID-19. Front. Immunol. 2021, 12, 700184. [Google Scholar] [CrossRef]
- Natarelli, L.; Virgili, F.; Weber, C. SARS-CoV-2, Cardiovascular Diseases, and Noncoding RNAs: A Connected Triad. Int. J. Mol. Sci. 2021, 22, 12243. [Google Scholar] [CrossRef] [PubMed]
- Plowman, T.; Lagos, D. Non-Coding RNAs in COVID-19: Emerging Insights and Current Questions. Noncoding RNA 2021, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Robinson, E.L.; McKinsey, T.A. COVID-19 and BRD4: A stormy and cardiotoxic bromo-romance. J. Cardiovasc. Aging 2022, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Marchiano, S.; Hsiang, T.Y.; Khanna, A.; Higashi, T.; Whitmore, L.S.; Bargehr, J.; Davaapil, H.; Chang, J.; Smith, E.; Ong, L.P.; et al. SARS-CoV-2 Infects Human Pluripotent Stem Cell-Derived Cardiomyocytes, Impairing Electrical and Mechanical Function. Stem Cell Rep. 2021, 16, 478–492. [Google Scholar] [CrossRef] [PubMed]
- Downes, D.J.; Cross, A.R.; Hua, P.; Roberts, N.; Schwessinger, R.; Cutler, A.J.; Munis, A.M.; Brown, J.; Mielczarek, O.; de Andrea, C.E.; et al. Identification of LZTFL1 as a candidate effector gene at a COVID-19 risk locus. Nat. Genet. 2021, 53, 1606–1615. [Google Scholar] [CrossRef]
- Chung, M.K.; Zidar, D.A.; Bristow, M.R.; Cameron, S.J.; Chan, T.; Harding, C.V., 3rd; Kwon, D.H.; Singh, T.; Tilton, J.C.; Tsai, E.J.; et al. COVID-19 and Cardiovascular Disease: From Bench to Bedside. Circ. Res. 2021, 128, 1214–1236. [Google Scholar] [CrossRef]
- Unudurthi, S.D.; Luthra, P.; Bose, R.J.C.; McCarthy, J.R.; Kontaridis, M.I. Cardiac inflammation in COVID-19: Lessons from heart failure. Life Sci. 2020, 260, 118482. [Google Scholar] [CrossRef]
- Utrero-Rico, A.; Gonzalez-Cuadrado, C.; Chivite-Lacaba, M.; Cabrera-Marante, O.; Laguna-Goya, R.; Almendro-Vazquez, P.; Diaz-Pedroche, C.; Ruiz-Ruigomez, M.; Lalueza, A.; Folgueira, M.D.; et al. Alterations in Circulating Monocytes Predict COVID-19 Severity and Include Chromatin Modifications Still Detectable Six Months after Recovery. Biomedicines 2021, 9, 1253. [Google Scholar] [CrossRef]
- Herbein, G. An epigenetic signature to fight COVID-19. EBioMedicine 2021, 67, 103385. [Google Scholar] [CrossRef]
- Ono, R.; Nakamura, K.; Inoue, K.; Naruse, M.; Usami, T.; Wakisaka-Saito, N.; Hino, T.; Suzuki-Migishima, R.; Ogonuki, N.; Miki, H.; et al. Deletion of Peg10, an imprinted gene acquired from a retrotransposon, causes early embryonic lethality. Nat. Genet. 2006, 38, 101–106. [Google Scholar] [CrossRef]
- Akamatsu, S.; Wyatt, A.W.; Lin, D.; Lysakowski, S.; Zhang, F.; Kim, S.; Tse, C.; Wang, K.; Mo, F.; Haegert, A.; et al. The Placental Gene PEG10 Promotes Progression of Neuroendocrine Prostate Cancer. Cell Rep. 2015, 12, 922–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batra, J.; Hultquist, J.F.; Liu, D.; Shtanko, O.; Von Dollen, J.; Satkamp, L.; Jang, G.M.; Luthra, P.; Schwarz, T.M.; Small, G.I.; et al. Protein Interaction Mapping Identifies RBBP6 as a Negative Regulator of Ebola Virus Replication. Cell 2018, 175, 1917–1930.e1913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanagisawa, H.; Yanagisawa, M.; Kapur, R.P.; Richardson, J.A.; Williams, S.C.; Clouthier, D.E.; de Wit, D.; Emoto, N.; Hammer, R.E. Dual genetic pathways of endothelin-mediated intercellular signaling revealed by targeted disruption of endothelin converting enzyme-1 gene. Development 1998, 125, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, J.; Ferrero, P.; Chessa, M.; Bianco, F.; Ciliberti, P.; Secinaro, A.; Oreto, L.; Avesani, M.; Bucciarelli, V.; Calcaterra, G.; et al. COVID-19 and Congenital Heart Disease: Results from a Nationwide Survey. J. Clin. Med. 2020, 9, 1774. [Google Scholar] [CrossRef]
- Guan, W.J.; Liang, W.H.; He, J.X.; Zhong, N.S. Cardiovascular comorbidity and its impact on patients with COVID-19. Eur. Respir. J. 2020, 55, 2001227. [Google Scholar] [CrossRef]
- Radke, R.M.; Frenzel, T.; Baumgartner, H.; Diller, G.P. Adult congenital heart disease and the COVID-19 pandemic. Heart 2020, 106, 1302–1309. [Google Scholar] [CrossRef]
- Oudit, G.Y.; Penninger, J.M. Recombinant human angiotensin-converting enzyme 2 as a new renin-angiotensin system peptidase for heart failure therapy. Curr. Heart Fail. Rep. 2011, 8, 176–183. [Google Scholar] [CrossRef]
- Wang, W.; Bodiga, S.; Das, S.K.; Lo, J.; Patel, V.; Oudit, G.Y. Role of ACE2 in diastolic and systolic heart failure. Heart Fail. Rev. 2012, 17, 683–691. [Google Scholar] [CrossRef]
- Patel, V.B.; Putko, B.; Wang, Z.; Zhong, J.C.; Oudit, G.Y. Manipulating angiotensin metabolism with angiotensin converting enzyme 2 (ACE2) in heart failure. Drug Discov. Today Ther. Strateg. 2012, 9, e141–e148. [Google Scholar] [CrossRef]
- Cole-Jeffrey, C.T.; Liu, M.; Katovich, M.J.; Raizada, M.K.; Shenoy, V. ACE2 and Microbiota: Emerging Targets for Cardiopulmonary Disease Therapy. J. Cardiovasc. Pharmacol. 2015, 66, 540–550. [Google Scholar] [CrossRef] [Green Version]
- Awwad, Z.M.; El-Ganainy, S.O.; ElMallah, A.I.; Khedr, S.M.; Khattab, M.M.; El-Khatib, A.S. Assessment of Pregabalin-Induced Cardiotoxicity in Rats: Mechanistic Role of Angiotensin 1-7. Cardiovasc. Toxicol. 2020, 20, 301–311. [Google Scholar] [CrossRef] [PubMed]
- South, A.M.; Diz, D.I.; Chappell, M.C. COVID-19, ACE2, and the cardiovascular consequences. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H1084–H1090. [Google Scholar] [CrossRef] [Green Version]
- Danilczyk, U.; Penninger, J.M. Angiotensin-converting enzyme II in the heart and the kidney. Circ. Res. 2006, 98, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Souza-Mello, V. Hepatic structural enhancement and insulin resistance amelioration due to AT1 receptor blockade. World J. Hepatol. 2017, 9, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.A., Jr.; Lazartigues, E.; Lucchesi, P.A. The angiotensin converting enzyme 2/Ang-(1-7) axis in the heart: A role for MAS communication? Circ. Res. 2008, 103, 1197–1199. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; He, W.; Guo, L.; Zhang, Y.; Li, H.; Han, S.; Shen, D. The ACE2-Ang (1-7)-Mas receptor axis attenuates cardiac remodeling and fibrosis in post-myocardial infarction. Mol. Med. Rep. 2017, 16, 1973–1981. [Google Scholar] [CrossRef]
- Uri, K.; Fagyas, M.; Kertesz, A.; Borbely, A.; Jenei, C.; Bene, O.; Csanadi, Z.; Paulus, W.J.; Edes, I.; Papp, Z.; et al. Circulating ACE2 activity correlates with cardiovascular disease development. J. Renin Angiotensin Aldosterone Syst. 2016, 17, 1470320316668435. [Google Scholar] [CrossRef] [Green Version]
- Moritani, T.; Iwai, M.; Kanno, H.; Nakaoka, H.; Iwanami, J.; Higaki, T.; Ishii, E.; Horiuchi, M. ACE2 deficiency induced perivascular fibrosis and cardiac hypertrophy during postnatal development in mice. J. Am. Soc. Hypertens. 2013, 7, 259–266. [Google Scholar] [CrossRef]
- Iwanami, J.; Mogi, M.; Tsukuda, K.; Wang, X.L.; Nakaoka, H.; Ohshima, K.; Chisaka, T.; Bai, H.Y.; Kanno, H.; Min, L.J.; et al. Role of angiotensin-converting enzyme 2/angiotensin-(1-7)/Mas axis in the hypotensive effect of azilsartan. Hypertens. Res. 2014, 37, 616–620. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, L.; Zhong, J.; Gao, P.; Oudit, G.Y. ACE2/Ang-(1-7) signaling and vascular remodeling. Sci. China Life Sci. 2014, 57, 802–808. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, B.; Wang, B.; Zhang, J.; Wu, J.; Morgan, T. Alteration of cardiac ACE2/Mas expression and cardiac remodelling in rats with aortic constriction. Chin. J. Physiol. 2014, 57, 335–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keidar, S.; Kaplan, M.; Gamliel-Lazarovich, A. ACE2 of the heart: From angiotensin I to angiotensin (1-7). Cardiovasc. Res. 2007, 73, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Vickers, C.; Hales, P.; Kaushik, V.; Dick, L.; Gavin, J.; Tang, J.; Godbout, K.; Parsons, T.; Baronas, E.; Hsieh, F.; et al. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J. Biol. Chem. 2002, 277, 14838–14843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, K.; Ohishi, M.; Katsuya, T.; Ito, N.; Ikushima, M.; Kaibe, M.; Tatara, Y.; Shiota, A.; Sugano, S.; Takeda, S.; et al. Deletion of angiotensin-converting enzyme 2 accelerates pressure overload-induced cardiac dysfunction by increasing local angiotensin II. Hypertension 2006, 47, 718–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crackower, M.A.; Sarao, R.; Oudit, G.Y.; Yagil, C.; Kozieradzki, I.; Scanga, S.E.; Oliveira-dos-Santos, A.J.; da Costa, J.; Zhang, L.; Pei, Y.; et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 2002, 417, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Gurley, S.B.; Allred, A.; Le, T.H.; Griffiths, R.; Mao, L.; Philip, N.; Haystead, T.A.; Donoghue, M.; Breitbart, R.E.; Acton, S.L.; et al. Altered blood pressure responses and normal cardiac phenotype in ACE2-null mice. J. Clin. Investig. 2006, 116, 2218–2225. [Google Scholar] [CrossRef] [Green Version]
- Liang, B.; Li, Y.; Han, Z.; Xue, J.; Zhang, Y.; Jia, S.; Wang, C. ACE2-Ang (1-7) axis is induced in pressure overloaded rat model. Int. J. Clin. Exp. Pathol. 2015, 8, 1443–1450. [Google Scholar]
- Santos, R.A.; Ferreira, A.J.; Nadu, A.P.; Braga, A.N.; de Almeida, A.P.; Campagnole-Santos, M.J.; Baltatu, O.; Iliescu, R.; Reudelhuber, T.L.; Bader, M. Expression of an angiotensin-(1-7)-producing fusion protein produces cardioprotective effects in rats. Physiol. Genom. 2004, 17, 292–299. [Google Scholar] [CrossRef] [Green Version]
- Grobe, J.L.; Mecca, A.P.; Lingis, M.; Shenoy, V.; Bolton, T.A.; Machado, J.M.; Speth, R.C.; Raizada, M.K.; Katovich, M.J. Prevention of angiotensin II-induced cardiac remodeling by angiotensin-(1-7). Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H736–H742. [Google Scholar] [CrossRef] [Green Version]
- Mercure, C.; Yogi, A.; Callera, G.E.; Aranha, A.B.; Bader, M.; Ferreira, A.J.; Santos, R.A.; Walther, T.; Touyz, R.M.; Reudelhuber, T.L. Angiotensin(1-7) blunts hypertensive cardiac remodeling by a direct effect on the heart. Circ. Res. 2008, 103, 1319–1326. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, K.; Koibuchi, N.; Nishimatsu, H.; Higashikuni, Y.; Hirata, Y.; Kugiyama, K.; Nagai, R.; Sata, M. Candesartan ameliorates cardiac dysfunction observed in angiotensin-converting enzyme 2-deficient mice. Hypertens Res. 2008, 31, 1953–1961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simoes e Silva, A.C.; Silveira, K.D.; Ferreira, A.J.; Teixeira, M.M. ACE2, angiotensin-(1-7) and Mas receptor axis in inflammation and fibrosis. Br. J. Pharmacol. 2013, 169, 477–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diez-Freire, C.; Vazquez, J.; Correa de Adjounian, M.F.; Ferrari, M.F.; Yuan, L.; Silver, X.; Torres, R.; Raizada, M.K. ACE2 gene transfer attenuates hypertension-linked pathophysiological changes in the SHR. Physiol. Genom. 2006, 27, 12–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Der Sarkissian, S.; Grobe, J.L.; Yuan, L.; Narielwala, D.R.; Walter, G.A.; Katovich, M.J.; Raizada, M.K. Cardiac overexpression of angiotensin converting enzyme 2 protects the heart from ischemia-induced pathophysiology. Hypertension 2008, 51, 712–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huentelman, M.J.; Grobe, J.L.; Vazquez, J.; Stewart, J.M.; Mecca, A.P.; Katovich, M.J.; Ferrario, C.M.; Raizada, M.K. Protection from angiotensin II-induced cardiac hypertrophy and fibrosis by systemic lentiviral delivery of ACE2 in rats. Exp. Physiol. 2005, 90, 783–790. [Google Scholar] [CrossRef] [Green Version]
- Gava, E.; de Castro, C.H.; Ferreira, A.J.; Colleta, H.; Melo, M.B.; Alenina, N.; Bader, M.; Oliveira, L.A.; Santos, R.A.; Kitten, G.T. Angiotensin-(1-7) receptor Mas is an essential modulator of extracellular matrix protein expression in the heart. Regul. Pept. 2012, 175, 30–42. [Google Scholar] [CrossRef] [Green Version]
- Dias-Peixoto, M.F.; Santos, R.A.; Gomes, E.R.; Alves, M.N.; Almeida, P.W.; Greco, L.; Rosa, M.; Fauler, B.; Bader, M.; Alenina, N.; et al. Molecular mechanisms involved in the angiotensin-(1-7)/Mas signaling pathway in cardiomyocytes. Hypertension 2008, 52, 542–548. [Google Scholar] [CrossRef] [Green Version]
- Santos, R.A.; Castro, C.H.; Gava, E.; Pinheiro, S.V.; Almeida, A.P.; Paula, R.D.; Cruz, J.S.; Ramos, A.S.; Rosa, K.T.; Irigoyen, M.C.; et al. Impairment of in vitro and in vivo heart function in angiotensin-(1-7) receptor MAS knockout mice. Hypertension 2006, 47, 996–1002. [Google Scholar] [CrossRef] [Green Version]
- Tallant, E.A.; Diz, D.I.; Ferrario, C.M. State-of-the-Art lecture. Antiproliferative actions of angiotensin-(1-7) in vascular smooth muscle. Hypertension 1999, 34, 950–957. [Google Scholar] [CrossRef]
- Patel, V.B.; Zhong, J.C.; Grant, M.B.; Oudit, G.Y. Role of the ACE2/Angiotensin 1-7 Axis of the Renin-Angiotensin System in Heart Failure. Circ. Res. 2016, 118, 1313–1326. [Google Scholar] [CrossRef] [Green Version]
- Nemoto, W.; Ogata, Y.; Nakagawasai, O.; Yaoita, F.; Tadano, T.; Tan-No, K. Angiotensin (1-7) prevents angiotensin II-induced nociceptive behaviour via inhibition of p38 MAPK phosphorylation mediated through spinal Mas receptors in mice. Eur. J. Pain. 2014, 18, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Li, G.; Chen, S.; Bihl, J.; Buck, J.; Zhu, Y.; Xia, H.; Lazartigues, E.; Chen, Y.; Olson, J.E. Activation of the ACE2/Ang-(1-7)/Mas pathway reduces oxygen-glucose deprivation-induced tissue swelling, ROS production, and cell death in mouse brain with angiotensin II overproduction. Neuroscience 2014, 273, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Basu, R.; Poglitsch, M.; Bakal, J.A.; Oudit, G.Y. Elevated Angiotensin 1-7/Angiotensin II Ratio Predicts Favorable Outcomes in Patients With Heart Failure. Circ. Heart Fail. 2020, 13, e006939. [Google Scholar] [CrossRef] [PubMed]
- Iwata, M.; Cowling, R.T.; Gurantz, D.; Moore, C.; Zhang, S.; Yuan, J.X.; Greenberg, B.H. Angiotensin-(1-7) binds to specific receptors on cardiac fibroblasts to initiate antifibrotic and antitrophic effects. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H2356–H2363. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Wang, L.; He, W.; Yu, X.; Hu, D.; Bao, M.; Liu, H.; Zhou, J.; Jiang, H. Coronavirus disease 2019 in elderly patients: Characteristics and prognostic factors based on 4-week follow-up. J. Infect. 2020, 80, 639–645. [Google Scholar] [CrossRef]
- Khan, M.S.; Shahid, I.; Anker, S.D.; Solomon, S.D.; Vardeny, O.; Michos, E.D.; Fonarow, G.C.; Butler, J. Cardiovascular implications of COVID-19 versus influenza infection: A review. BMC Med. 2020, 18, 403. [Google Scholar] [CrossRef]
- Chen, T.; Wu, D.; Chen, H.; Yan, W.; Yang, D.; Chen, G.; Ma, K.; Xu, D.; Yu, H.; Wang, H.; et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ 2020, 368, m1091. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.Y.; Ma, Y.T.; Zhang, J.Y.; Xie, X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020, 17, 259–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Zhou, L.; Sun, X.; Yan, Z.; Hu, C.; Wu, J.; Xu, L.; Li, X.; Liu, H.; Yin, P.; et al. Altered Lipid Metabolism in Recovered SARS Patients Twelve Years after Infection. Sci. Rep. 2017, 7, 9110. [Google Scholar] [CrossRef]
- Chan, J.F.; Yuan, S.; Kok, K.H.; To, K.K.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.; Poon, R.W.; et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef] [Green Version]
- Madjid, M.; Safavi-Naeini, P.; Solomon, S.D.; Vardeny, O. Potential Effects of Coronaviruses on the Cardiovascular System: A Review. JAMA Cardiol. 2020, 5, 831–840. [Google Scholar] [CrossRef] [Green Version]
- Poggiali, E.; Bastoni, D.; Ioannilli, E.; Vercelli, A.; Magnacavallo, A. Deep Vein Thrombosis and Pulmonary Embolism: Two Complications of COVID-19 Pneumonia? Eur. J. Case Rep. Intern. Med. 2020, 7, 001646. [Google Scholar] [CrossRef]
- Skeik, N.; Smith, J.E.; Patel, L.; Mirza, A.K.; Manunga, J.M.; Beddow, D. Risk and Management of Venous Thromboembolism in Patients with COVID-19. Ann. Vasc. Surg. 2020, 73, 78–85. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Klok, F.A.; Kruip, M.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020, 191, 145–147. [Google Scholar] [CrossRef]
- Llitjos, J.F.; Leclerc, M.; Chochois, C.; Monsallier, J.M.; Ramakers, M.; Auvray, M.; Merouani, K. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J. Thromb. Haemost. 2020, 18, 1743–1746. [Google Scholar] [CrossRef] [PubMed]
- Nahum, J.; Morichau-Beauchant, T.; Daviaud, F.; Echegut, P.; Fichet, J.; Maillet, J.M.; Thierry, S. Venous Thrombosis Among Critically Ill Patients With Coronavirus Disease 2019 (COVID-19). JAMA Netw. Open 2020, 3, e2010478. [Google Scholar] [CrossRef]
- Cui, S.; Chen, S.; Li, X.; Liu, S.; Wang, F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 1421–1424. [Google Scholar] [CrossRef]
- Lax, S.F.; Skok, K.; Zechner, P.; Kessler, H.H.; Kaufmann, N.; Koelblinger, C.; Vander, K.; Bargfrieder, U.; Trauner, M. Pulmonary Arterial Thrombosis in COVID-19 With Fatal Outcome: Results From a Prospective, Single-Center, Clinicopathologic Case Series. Ann. Intern. Med. 2020, 173, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Bikdeli, B.; Madhavan, M.V.; Jimenez, D.; Chuich, T.; Dreyfus, I.; Driggin, E.; Nigoghossian, C.; Ageno, W.; Madjid, M.; Guo, Y.; et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 2950–2973. [Google Scholar] [CrossRef] [PubMed]
- Skeik, N.; Mirza, A.; Manunga, J. Management of venous thromboembolism during the COVID-19 pandemic. J. Vasc. Surg. Venous Lymphat. Disord. 2020, 8, 897–898. [Google Scholar] [CrossRef]
- Gao, Y.; Li, T.; Han, M.; Li, X.; Wu, D.; Xu, Y.; Zhu, Y.; Liu, Y.; Wang, X.; Wang, L. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J. Med. Virol. 2020, 92, 791–796. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, M.; Zhang, S.; Xia, P.; Cao, W.; Jiang, W.; Chen, H.; Ding, X.; Zhao, H.; Zhang, H.; et al. Coagulopathy and Antiphospholipid Antibodies in Patients with COVID-19. N. Engl. J. Med. 2020, 382, e38. [Google Scholar] [CrossRef]
- Tang, N.; Bai, H.; Chen, X.; Gong, J.; Li, D.; Sun, Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020, 18, 1094–1099. [Google Scholar] [CrossRef]
- Merkler, A.E.; Parikh, N.S.; Mir, S.; Gupta, A.; Kamel, H.; Lin, E.; Lantos, J.; Schenck, E.J.; Goyal, P.; Bruce, S.S.; et al. Risk of Ischemic Stroke in Patients With Coronavirus Disease 2019 (COVID-19) vs Patients With Influenza. JAMA Neurol. 2020, 77, 1366–1372. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Liu, M.; Lu, L.; Zheng, Y.; Zhang, P. Congenital Heart Disease: Causes, Diagnosis, Symptoms, and Treatments. Cell Biochem. Biophys. 2015, 72, 857–860. [Google Scholar] [CrossRef] [PubMed]
- Bouma, B.J.; Mulder, B.J. Changing Landscape of Congenital Heart Disease. Circ. Res. 2017, 120, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Pierpont, M.E.; Basson, C.T.; Benson, D.W., Jr.; Gelb, B.D.; Giglia, T.M.; Goldmuntz, E.; McGee, G.; Sable, C.A.; Srivastava, D.; Webb, C.L.; et al. Genetic basis for congenital heart defects: Current knowledge: A scientific statement from the American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young: Endorsed by the American Academy of Pediatrics. Circulation 2007, 115, 3015–3038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmerman, M.S.; Smith, A.G.C.; Sable, C.A.; Echko, M.M.; Wilner, L.B.; Olsen, H.E.; Atalay, H.T.; Awasthi, A.; Bhutta, Z.A.; Boucher, J.L.; et al. Global, regional, and national burden of congenital heart disease, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Child Adolesc. Health 2020, 4, 185–200. [Google Scholar] [CrossRef] [Green Version]
- Micheletti, A. Congenital Heart Disease Classification, Epidemiology, Diagnosis, Treatment, and Outcome. In Congenital Heart Disease; Flocco, S., Lillo, A., Dellafiore, F., Goossens, E., Eds.; Springer: Cham, Switzerland, 2019; pp. 1–67. [Google Scholar]
- Thiene, G.; Frescura, C. Anatomical and pathophysiological classification of congenital heart disease. Cardiovasc. Pathol. 2010, 19, 259–274. [Google Scholar] [CrossRef]
- Ombelet, F.; Goossens, E.; Van De Bruaene, A.; Budts, W.; Moons, P. Newly Developed Adult Congenital Heart Disease Anatomic and Physiological Classification: First Predictive Validity Evaluation. J. Am. Heart Assoc. 2020, 9, e014988. [Google Scholar] [CrossRef]
- Franklin, R.C.G.; Beland, M.J.; Colan, S.D.; Walters, H.L.; Aiello, V.D.; Anderson, R.H.; Bailliard, F.; Boris, J.R.; Cohen, M.S.; Gaynor, J.W.; et al. Nomenclature for congenital and paediatric cardiac disease: The International Paediatric and Congenital Cardiac Code (IPCCC) and the Eleventh Iteration of the International Classification of Diseases (ICD-11). Cardiol. Young 2017, 27, 1872–1938. [Google Scholar] [CrossRef]
- Strickland, M.J.; Riehle-Colarusso, T.J.; Jacobs, J.P.; Reller, M.D.; Mahle, W.T.; Botto, L.D.; Tolbert, P.E.; Jacobs, M.L.; Lacour-Gayet, F.G.; Tchervenkov, C.I.; et al. The importance of nomenclature for congenital cardiac disease: Implications for research and evaluation. Cardiol. Young 2008, 18 (Suppl. 2), 92–100. [Google Scholar] [CrossRef] [Green Version]
- Espinoza, J. Fetal MRI and prenatal diagnosis of congenital heart defects. Lancet 2019, 393, 1574–1576. [Google Scholar] [CrossRef] [Green Version]
- Donofrio, M.T.; Moon-Grady, A.J.; Hornberger, L.K.; Copel, J.A.; Sklansky, M.S.; Abuhamad, A.; Cuneo, B.F.; Huhta, J.C.; Jonas, R.A.; Krishnan, A.; et al. Diagnosis and treatment of fetal cardiac disease: A scientific statement from the American Heart Association. Circulation 2014, 129, 2183–2242. [Google Scholar] [CrossRef] [PubMed]
- van Velzen, C.L.; Clur, S.A.; Rijlaarsdam, M.E.; Pajkrt, E.; Bax, C.J.; Hruda, J.; de Groot, C.J.; Blom, N.A.; Haak, M.C. Prenatal diagnosis of congenital heart defects: Accuracy and discrepancies in a multicenter cohort. Ultrasound Obstet. Gynecol. 2016, 47, 616–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, X.; Weng, Z.; Liu, M.; Chen, X.; Wu, Q.; Ling, W.; Ma, H.; Huang, H.; Lin, Y. Prenatal diagnosis and pregnancy outcomes of 1492 fetuses with congenital heart disease: Role of multidisciplinary-joint consultation in prenatal diagnosis. Sci. Rep. 2020, 10, 7564. [Google Scholar] [CrossRef] [PubMed]
- Lytzen, R.; Vejlstrup, N.; Bjerre, J.; Bjorn Petersen, O.; Leenskjold, S.; Keith Dodd, J.; Stener Jorgensen, F.; Sondergaard, L. The accuracy of prenatal diagnosis of major congenital heart disease is increasing. J. Obstet. Gynaecol. 2020, 40, 308–315. [Google Scholar] [CrossRef]
- Rocha, L.A.; Araujo Junior, E.; Rolo, L.C.; Barros, F.S.; da Silva, K.P.; Leslie, A.T.; Nardozza, L.M.; Moron, A.F. Prenatal detection of congenital heart diseases: One-year survey performing a screening protocol in a single reference center in Brazil. Cardiol. Res. Pract. 2014, 2014, 175635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Letourneau, K.M.; Horne, D.; Soni, R.N.; McDonald, K.R.; Karlicki, F.C.; Fransoo, R.R. Advancing Prenatal Detection of Congenital Heart Disease: A Novel Screening Protocol Improves Early Diagnosis of Complex Congenital Heart Disease. J. Ultrasound Med. 2018, 37, 1073–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharland, G. Changing impact of fetal diagnosis of congenital heart disease. Arch. Dis. Child. Fetal Neonatal Ed. 1997, 77, F1–F3. [Google Scholar] [CrossRef]
- Wang, L.; Nie, H.; Wang, Q.; Zhang, G.; Li, G.; Bai, L.; Hua, T.; Wei, S. Use of magnetic resonance imaging combined with gene analysis for the diagnosis of fetal congenital heart disease. BMC Med. Imaging 2019, 19, 12. [Google Scholar] [CrossRef]
- Wagner, R.; Tse, W.H.; Gosemann, J.H.; Lacher, M.; Keijzer, R. Prenatal maternal biomarkers for the early diagnosis of congenital malformations: A review. Pediatr. Res. 2019, 86, 560–566. [Google Scholar] [CrossRef]
- Gu, M.; Zheng, A.; Tu, W.; Zhao, J.; Li, L.; Li, M.; Han, S.; Hu, X.; Zhu, J.; Pan, Y.; et al. Circulating LncRNAs as Novel, Non-Invasive Biomarkers for Prenatal Detection of Fetal Congenital Heart Defects. Cell. Physiol. Biochem. 2016, 38, 1459–1471. [Google Scholar] [CrossRef]
- Smith, T.; Rajakaruna, C.; Caputo, M.; Emanueli, C. MicroRNAs in congenital heart disease. Ann. Transl. Med. 2015, 3, 333. [Google Scholar] [CrossRef] [PubMed]
- Kardasevic, M.; Jovanovic, I.; Samardzic, J.P. Modern Strategy for Identification of Congenital Heart Defects in the Neonatal Period. Med. Arch. 2016, 70, 384–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benson, L.N.; Freedom, R.M. The Clinical Diagnostic Approach in Congenital Heart Disease. In Neonatal Heart Disease; Springer: London, UK, 1992; pp. 165–176. [Google Scholar]
- Wren, C.; Richmond, S.; Donaldson, L. Presentation of congenital heart disease in infancy: Implications for routine examination. Arch. Dis. Child. Fetal Neonatal Ed. 1999, 80, F49–F53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, A.C. Managing Uncertainty in Prenatal Diagnosis of Congenital Heart Disease. JAMA Netw. Open 2020, 3, e204353. [Google Scholar] [CrossRef]
- Harris, K.W.; Brelsford, K.M.; Kavanaugh-McHugh, A.; Clayton, E.W. Uncertainty of Prenatally Diagnosed Congenital Heart Disease: A Qualitative Study. JAMA Netw. Open 2020, 3, e204082. [Google Scholar] [CrossRef]
- Biber, S.; Andonian, C.; Beckmann, J.; Ewert, P.; Freilinger, S.; Nagdyman, N.; Kaemmerer, H.; Oberhoffer, R.; Pieper, L.; Neidenbach, R.C. Current research status on the psychological situation of parents of children with congenital heart disease. Cardiovasc. Diagn. Ther. 2019, 9, S369–S376. [Google Scholar] [CrossRef]
- David Vainberg, L.; Vardi, A.; Jacoby, R. The Experiences of Parents of Children Undergoing Surgery for Congenital Heart Defects: A Holistic Model of Care. Front. Psychol. 2019, 10, 2666. [Google Scholar] [CrossRef]
- Kolaitis, G.A.; Meentken, M.G.; Utens, E. Mental Health Problems in Parents of Children with Congenital Heart Disease. Front. Pediatr. 2017, 5, 102. [Google Scholar] [CrossRef]
- Sanapo, L.; Moon-Grady, A.J.; Donofrio, M.T. Perinatal and Delivery Management of Infants with Congenital Heart Disease. Clin. Perinatol. 2016, 43, 55–71. [Google Scholar] [CrossRef]
- Penny, D.J.; Shekerdemian, L.S. Management of the neonate with symptomatic congenital heart disease. Arch. Dis. Child. Fetal Neonatal Ed. 2001, 84, F141–F145. [Google Scholar] [CrossRef] [Green Version]
- Akkinapally, S.; Hundalani, S.G.; Kulkarni, M.; Fernandes, C.J.; Cabrera, A.G.; Shivanna, B.; Pammi, M. Prostaglandin E1 for maintaining ductal patency in neonates with ductal-dependent cardiac lesions. Cochrane Database Syst. Rev. 2018, 2, CD011417. [Google Scholar] [CrossRef] [PubMed]
- Cinteza, E.; Carminati, M. Balloon atrial septostomy—Almost half a century after. Maedica 2013, 8, 280–284. [Google Scholar] [PubMed]
- Matter, M.; Almarsafawy, H.; Hafez, M.; Attia, G.; Elkhier, M.M.A. Balloon atrial septostomy: The oldest cardiac interventional procedure in Mansoura. Egypt. Heart J. 2011, 63, 125–129. [Google Scholar] [CrossRef] [Green Version]
- Holst, K.A.; Said, S.M.; Nelson, T.J.; Cannon, B.C.; Dearani, J.A. Current Interventional and Surgical Management of Congenital Heart Disease: Specific Focus on Valvular Disease and Cardiac Arrhythmias. Circ. Res. 2017, 120, 1027–1044. [Google Scholar] [CrossRef] [Green Version]
- McGovern, E.; Sands, A.J. Perinatal management of major congenital heart disease. Ulster Med. J. 2014, 83, 135–139. [Google Scholar] [PubMed]
- Granbom, E.; Fernlund, E.; Sunnegardh, J.; Lundell, B.; Naumburg, E. Respiratory Tract Infection and Risk of Hospitalization in Children with Congenital Heart Defects During Season and Off-Season: A Swedish National Study. Pediatr. Cardiol. 2016, 37, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- de Souza, R.P.; Ribeiro, A.L.R.; de Menezes, S.A.F.; Machado, L.F.A. Incidence of respiratory syncytial virus infection in children with congenital heart disease undergoing immunoprophylaxis with palivizumab in Para state, north region of Brazil. BMC Pediatr. 2019, 19, 299. [Google Scholar] [CrossRef] [Green Version]
- Ghimire, L.V.; Chou, F.S.; Moon-Grady, A.J. Impact of congenital heart disease on outcomes among pediatric patients hospitalized for influenza infection. BMC Pediatr. 2020, 20, 450. [Google Scholar] [CrossRef]
- Diller, G.P.; Enders, D.; Lammers, A.E.; Orwat, S.; Schmidt, R.; Radke, R.M.; Gerss, J.; De Torres Alba, F.; Kaleschke, G.; Bauer, U.M.; et al. Mortality and morbidity in patients with congenital heart disease hospitalised for viral pneumonia. Heart 2020, 13, 1069–1076. [Google Scholar] [CrossRef]
- Woolford, S.J.; D’Angelo, S.; Curtis, E.M.; Parsons, C.M.; Ward, K.A.; Dennison, E.M.; Patel, H.P.; Cooper, C.; Harvey, N.C. COVID-19 and associations with frailty and multimorbidity: A prospective analysis of UK Biobank participants. Aging Clin. Exp. Res. 2020, 32, 1897–1905. [Google Scholar] [CrossRef]
- Walmsley, D. Routine pediatric immunization, special cases in pediatrics: Prematurity, chronic disease, congenital heart disease: Recent advancements/changes in pediatric vaccines. Prim. Care 2011, 38, 595–609. [Google Scholar] [CrossRef]
- Woodward, C.S. Keeping children with congenital heart disease healthy. J. Pediatr. Health Care 2011, 25, 373–378. [Google Scholar] [CrossRef]
- Alsaied, T.; Aboulhosn, J.A.; Cotts, T.B.; Daniels, C.J.; Etheridge, S.P.; Feltes, T.F.; Gurvitz, M.Z.; Lewin, M.B.; Oster, M.E.; Saidi, A. Coronavirus Disease 2019 (COVID-19) Pandemic Implications in Pediatric and Adult Congenital Heart Disease. J. Am. Heart Assoc. 2020, 9, e017224. [Google Scholar] [CrossRef]
- Lewis, M.J.; Anderson, B.R.; Fremed, M.; Argenio, M.; Krishnan, U.; Weller, R.; Levasseur, S.; Sommer, R.; Lytrivi, I.D.; Bacha, E.A.; et al. Impact of Coronavirus Disease 2019 (COVID-19) on Patients With Congenital Heart Disease Across the Lifespan: The Experience of an Academic Congenital Heart Disease Center in New York City. J. Am. Heart Assoc. 2020, 9, e017580. [Google Scholar] [CrossRef]
- Magoon, R. COVID-19 and congenital heart disease: Cardiopulmonary interactions for the worse! Paediatr. Anaesth. 2020, 30, 1160–1161. [Google Scholar] [CrossRef]
- Iacobazzi, D.; Baquedano, M.; Madeddu, P.; Caputo, M. COVID-19, State of the Adult and Pediatric Heart: From Myocardial Injury to Cardiac Effect of Potential Therapeutic Intervention. Front. Cardiovasc. Med. 2020, 7, 140. [Google Scholar] [CrossRef]
- Simpson, M.; Collins, C.; Nash, D.B.; Panesar, L.E.; Oster, M.E. Coronavirus Disease 2019 Infection in Children with Pre-Existing Heart Disease. J. Pediatr. 2020, 227, 302–307.e2. [Google Scholar] [CrossRef]
- Stephens, E.H.; Dearani, J.A.; Guleserian, K.J.; Overman, D.M.; Tweddell, J.S.; Backer, C.L.; Romano, J.C.; Bacha, E. COVID-19: Crisis management in congenital heart surgery. J. Thorac. Cardiovasc. Surg. 2020, 160, 522–528. [Google Scholar] [CrossRef]
- Giordano, R.; Cantinotti, M. Congenital heart disease in the era of COVID-19 pandemic. Gen. Thorac. Cardiovasc. Surg. 2020, 69, 172–174. [Google Scholar] [CrossRef]
- Hemphill, N.M.; Kuan, M.T.Y.; Harris, K.C. Reduced Physical Activity During COVID-19 Pandemic in Children With Congenital Heart Disease. Can. J. Cardiol. 2020, 36, 1130–1134. [Google Scholar] [CrossRef]
- Chang, K.P. Vaccination for Disease Prevention and Control: The Necessity of Renewed Emphasis and New Approaches. J. Immunol. Immunotech. 2014, 1. [Google Scholar] [CrossRef] [Green Version]
- Mahase, E. COVID-19: Pfizer and BioNTech submit vaccine for US authorisation. BMJ 2020, 371, m4552. [Google Scholar] [CrossRef]
- Callaway, E. COVID vaccine excitement builds as Moderna reports third positive result. Nature 2020, 587, 337–338. [Google Scholar] [CrossRef]
- Mullard, A. How COVID vaccines are being divvied up around the world. Nature 2020, 30. [Google Scholar] [CrossRef]
- Ismail, S.J.; Zhao, L.; Tunis, M.C.; Deeks, S.L.; Quach, C.; National Advisory Committee on Immunization. Key populations for early COVID-19 immunization: Preliminary guidance for policy. CMAJ 2020, 192, E1620–E1632. [Google Scholar] [CrossRef]
- Wu, W.; He, J.; Shao, X. Incidence and mortality trend of congenital heart disease at the global, regional, and national level, 1990–2017. Medicine 2020, 99, e20593. [Google Scholar] [CrossRef]
- Araujo, A.; Almeida, E.R.B.; Lima, L.; Sandes-Freitas, T.V.; Pinto, A.G.A. Fall in organ donations and transplants in Ceara in the COVID-19 pandemic: A descriptive study, April–June 2020. Epidemiol. Serv. Saude 2020, 30, e2020754. [Google Scholar] [CrossRef]
- Schoelz, J.M.; Riddle, N.C. Chapter Twenty-Seven—CRISPR/Cas9 technologies in epigenetics research. In Epigenetics Methods; Tollefsbol, T., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 18, pp. 537–567. [Google Scholar]
- Sen, R.; Pezoa, S.A.; Carpio Shull, L.; Hernandez-Lagunas, L.; Niswander, L.A.; Artinger, K.B. Kat2a and Kat2b Acetyltransferase Activity Regulates Craniofacial Cartilage and Bone Differentiation in Zebrafish and Mice. J. Dev. Biol. 2018, 6, 27. [Google Scholar] [CrossRef] [Green Version]
- King, A. A CRISPR edit for heart disease. Nature 2018, 555, S23–S25. [Google Scholar] [CrossRef] [Green Version]
- Rauch, J.N.; Valois, E.; Ponce-Rojas, J.C.; Aralis, Z.; Lach, R.S.; Zappa, F.; Audouard, M.; Solley, S.C.; Vaidya, C.; Costello, M.; et al. Comparison of Severe Acute Respiratory Syndrome Coronavirus 2 Screening Using Reverse Transcriptase-Quantitative Polymerase Chain Reaction or CRISPR-Based Assays in Asymptomatic College Students. JAMA Netw. Open 2021, 4, e2037129. [Google Scholar] [CrossRef]
- Broughton, J.P.; Deng, X.; Yu, G.; Fasching, C.L.; Servellita, V.; Singh, J.; Miao, X.; Streithorst, J.A.; Granados, A.; Sotomayor-Gonzalez, A.; et al. CRISPR-Cas12-based detection of SARS-CoV-2. Nat. Biotechnol. 2020, 38, 870–874. [Google Scholar] [CrossRef] [Green Version]
- Shademan, B.; Nourazarian, A.; Hajazimian, S.; Isazadeh, A.; Biray Avci, C.; Oskouee, M.A. CRISPR Technology in Gene-Editing-Based Detection and Treatment of SARS-CoV-2. Front. Mol. Biosci. 2021, 8, 772788. [Google Scholar] [CrossRef]
- Ullah, M.F.; Ali, Y.; Khan, M.R.; Khan, I.U.; Yan, B.; Ijaz Khan, M.; Malik, M.Y. A review of COVID-19: Treatment strategies and CRISPR/Cas9 gene editing technology approaches to the coronavirus disease. Saudi J. Biol. Sci. 2022, 29, 860–871. [Google Scholar] [CrossRef]

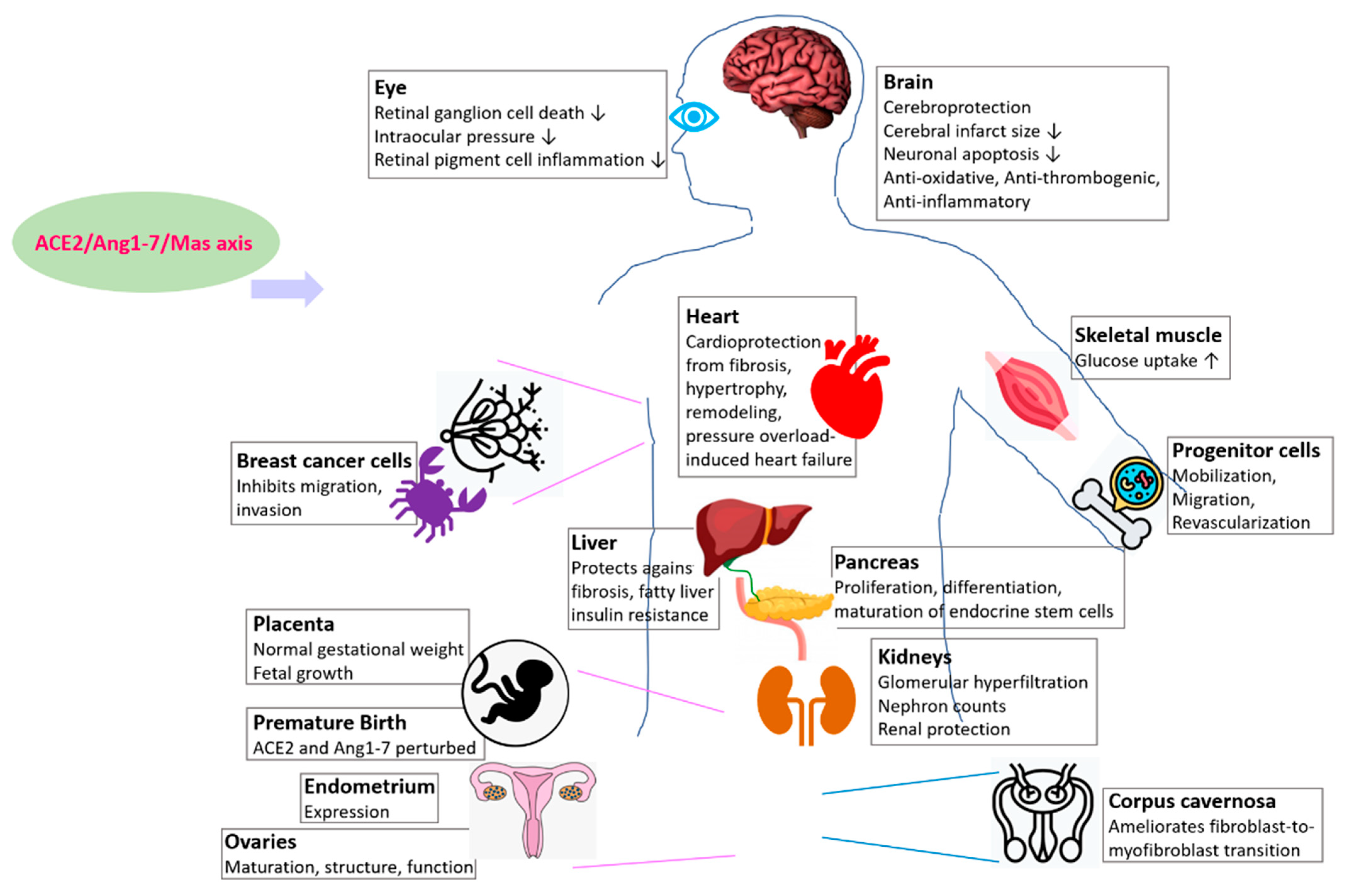



| Organ | Model Organism of Study | References |
|---|---|---|
| Kidney | Mouse | [73] |
| Liver | Mouse, rat | [74] |
| Pancreas | Mouse embryonic explant culture, peripheral blood CD34+ cells | [47,48,49,50] |
| Brain | Mouse | [51,52,53] |
| Eye | Rat, mouse, human | [54,55,56,57] |
| Placenta | Mouse | [58] |
| Skeletal muscle | Rat | [66,67] |
| Ovary | Human | [68,69] |
| Endometrium | Human | [70] |
| Corpus cavernosa | Rat | [71] |
| Breast (cancer) | Human | [72] |
| Hematopoietic and vasculogenic progenitor cells | Human, rodent | [73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarkar, S.; Sen, R. Insights into Cardiovascular Defects and Cardiac Epigenome in the Context of COVID-19. Epigenomes 2022, 6, 13. https://doi.org/10.3390/epigenomes6020013
Sarkar S, Sen R. Insights into Cardiovascular Defects and Cardiac Epigenome in the Context of COVID-19. Epigenomes. 2022; 6(2):13. https://doi.org/10.3390/epigenomes6020013
Chicago/Turabian StyleSarkar, Shreya, and Rwik Sen. 2022. "Insights into Cardiovascular Defects and Cardiac Epigenome in the Context of COVID-19" Epigenomes 6, no. 2: 13. https://doi.org/10.3390/epigenomes6020013
APA StyleSarkar, S., & Sen, R. (2022). Insights into Cardiovascular Defects and Cardiac Epigenome in the Context of COVID-19. Epigenomes, 6(2), 13. https://doi.org/10.3390/epigenomes6020013