Modulation of DNA Methylation/Demethylation Reactions Induced by Nutraceuticals and Pollutants of Exposome Can Promote a C > T Mutation in the Breast Cancer Predisposing Gene PALB2
Abstract
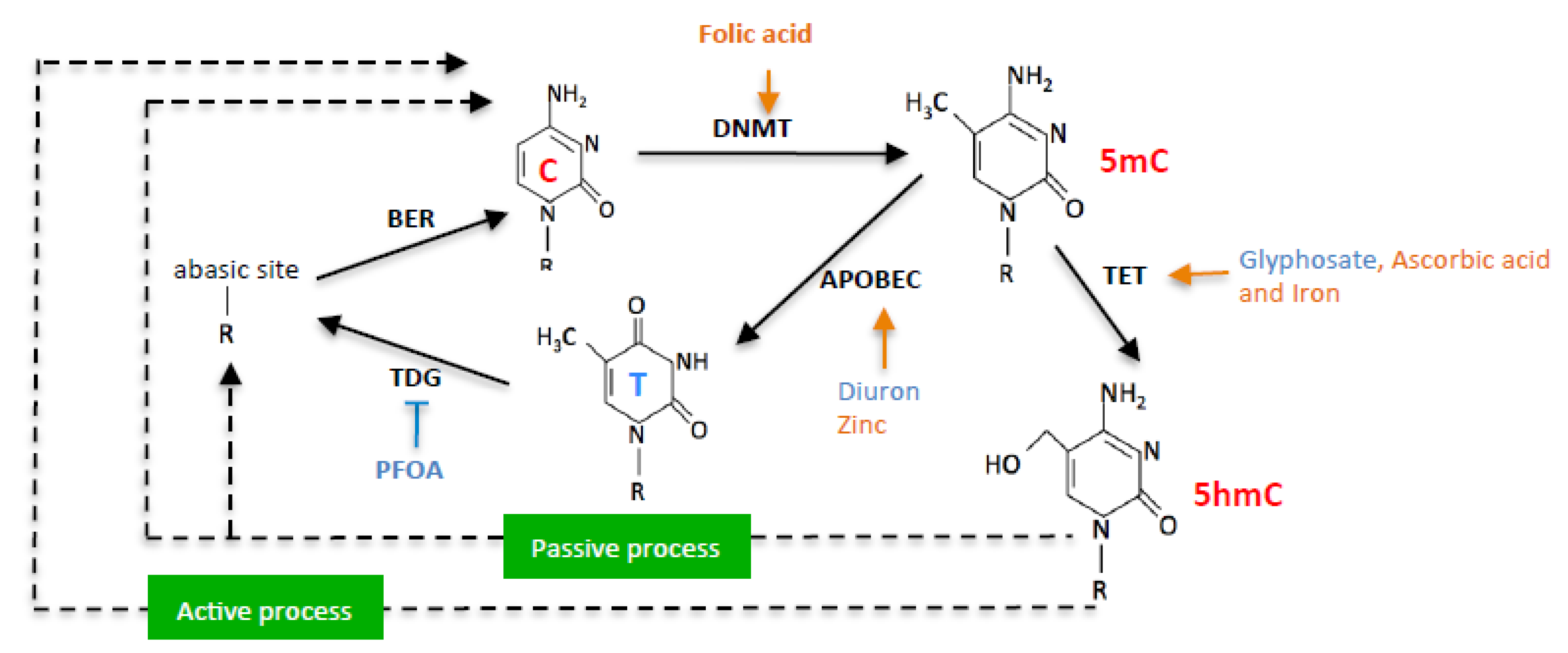
1. Introduction
2. Results
2.1. Folic Acid Supplementation Promotes the Methylation of PALB2 Gene Region Susceptible to Promote the c.1027C > T Mutation
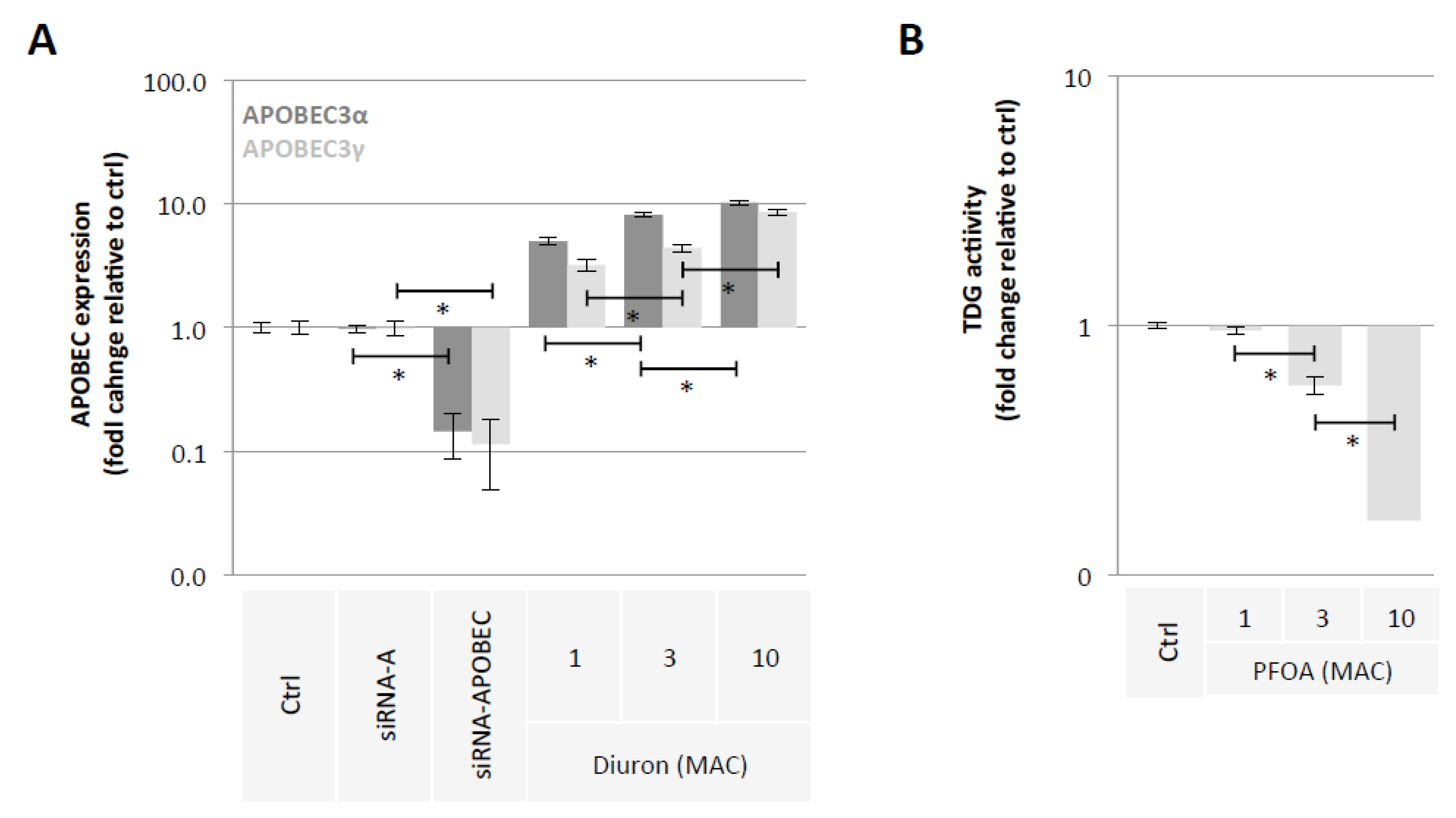
2.2. Diuron and PFOA Supplementation Affect APOBEC Expression and TDG Activity, Respectively
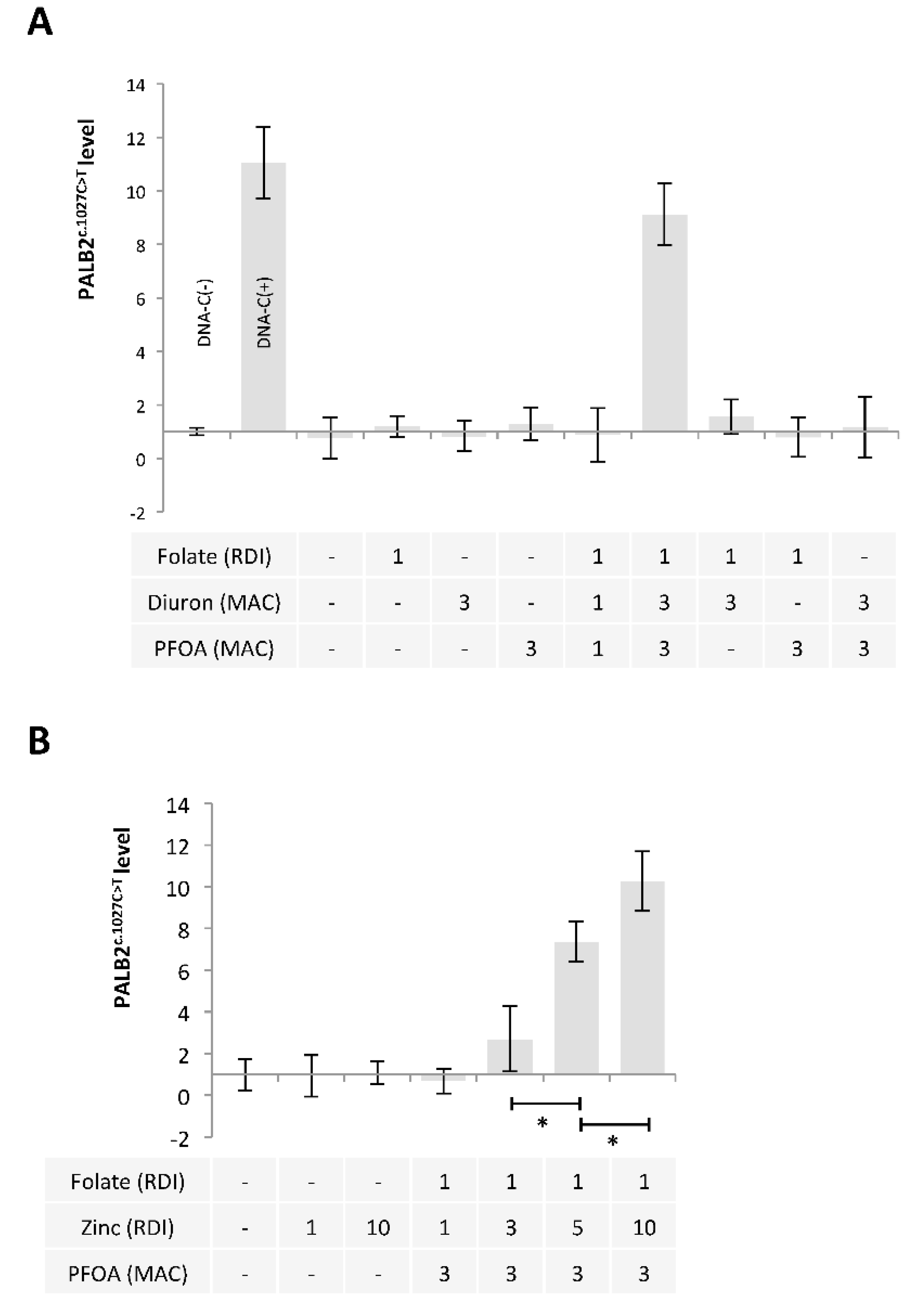
2.3. A Mixture Composed of Folate, Diuron or Zinc, and PFOA has the Ability to Promote the c.1027C > T Mutation in PALB2 Gene
2.4. Ascorbic Acid and Iron Have the Ability to Limit the Presence of the c.1027C > T Mutation in PALB2 Gene
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Cell Culture
5.2. DNA Extraction
5.3. Restriction Site Mutation (RSM) Assay
5.4. Methylated DNA Immunoprecipitation (MeDIP)
6. Elisa
6.1. siRNA Transfection
6.2. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Edwards, J.R.; Yarychkivska, O.; Boulard, M.; Bestor, T.H. DNA Methylation and DNA Methyltransferases. Epigenetics Chromatin 2017, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Kohli, R.M.; Zhang, Y. TET Enzymes, TDG and the Dynamics of DNA Demethylation. Nature 2013, 502, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Turpin, M.; Salbert, G. 5-Methylcytosine Turnover: Mechanisms and Therapeutic Implications in Cancer. Front. Mol. Biosci. 2022, 9, 976862. [Google Scholar] [CrossRef] [PubMed]
- Marinoni, I.; Wiederkeher, A.; Wiedmer, T.; Pantasis, S.; Di Domenico, A.; Frank, R.; Vassella, E.; Schmitt, A.; Perren, A. Hypo-Methylation Mediates Chromosomal Instability in Pancreatic NET. Endocr. Relat. Cancer 2017, 24, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Frigola, J.; Vendrell, E.; Risques, R.-A.; Fraga, M.F.; Morales, C.; Moreno, V.; Esteller, M.; Capellà, G.; Ribas, M.; et al. Chromosomal Instability Correlates with Genome-Wide DNA Demethylation in Human Primary Colorectal Cancers. Cancer Res. 2006, 66, 8462–9468. [Google Scholar] [CrossRef]
- Karpf, A.R.; Matsui, S. Genetic Disruption of Cytosine DNA Methyltransferase Enzymes Induces Chromosomal Instability in Human Cancer Cells. Cancer Res. 2005, 65, 8635–8639. [Google Scholar] [CrossRef]
- You, J.S.; Jones, P.A. Cancer Genetics and Epigenetics: Two Sides of the Same Coin? Cancer Cell 2012, 22, 9–20. [Google Scholar] [CrossRef]
- Han, M.; Jia, L.; Lv, W.; Wang, L.; Cui, W. Epigenetic Enzyme Mutations: Role in Tumorigenesis and Molecular Inhibitors. Front. Oncol. 2019, 9, 194. [Google Scholar] [CrossRef]
- Jarvis, M.C.; Ebrahimi, D.; Temiz, N.A.; Harris, R.S. Mutation Signatures Including APOBEC in Cancer Cell Lines. JNCI Cancer Spectr. 2018, 2, pky002. [Google Scholar] [CrossRef]
- Petljak, M.; Maciejowski, J. Molecular Origins of APOBEC-Associated Mutations in Cancer. DNA Repair 2020, 94, 102905. [Google Scholar] [CrossRef]
- Alexandrov, L.B.; Kim, J.; Haradhvala, N.J.; Huang, M.N.; Tian Ng, A.W.; Wu, Y.; Boot, A.; Covington, K.R.; Gordenin, D.A.; Bergstrom, E.N.; et al. The Repertoire of Mutational Signatures in Human Cancer. Nature 2020, 578, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Poulos, R.C.; Wong, Y.T.; Ryan, R.; Pang, H.; Wong, J.W.H. Analysis of 7,815 Cancer Exomes Reveals Associations between Mutational Processes and Somatic Driver Mutations. PLoS Genet. 2018, 14, e1007779. [Google Scholar] [CrossRef] [PubMed]
- Casadei, S.; Norquist, B.M.; Walsh, T.; Stray, S.; Mandell, J.B.; Lee, M.K.; Stamatoyannopoulos, J.A.; King, M.-C. Contribution of Inherited Mutations in the BRCA2-Interacting Protein PALB2 to Familial Breast Cancer. Cancer Res. 2011, 71, 2222–2229. [Google Scholar] [CrossRef] [PubMed]
- Vagena, A.; Papamentzelopoulou, M.; Kalfakakou, D.; Kollia, P.; Papadimitriou, C.; Psyrri, A.; Apostolou, P.; Fountzilas, G.; Konstantopoulou, I.; Yannoukakos, D.; et al. PALB2 c.2257C>T Truncating Variant Is a Greek Founder and Is Associated with High Breast Cancer Risk. J. Hum. Genet. 2019, 64, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Kwong, A.; Shin, V.Y.; Ho, C.Y.S.; Khalid, A.; Au, C.H.; Chan, K.K.L.; Ngan, H.Y.S.; Chan, T.-L.; Ma, E.S.K. Germline PALB2 Mutation in High-Risk Chinese Breast and/or Ovarian Cancer Patients. Cancers 2021, 13, 4195. [Google Scholar] [CrossRef] [PubMed]
- Catucci, I.; Peterlongo, P.; Ciceri, S.; Colombo, M.; Pasquini, G.; Barile, M.; Bonanni, B.; Verderio, P.; Pizzamiglio, S.; Foglia, C.; et al. PALB2 Sequencing in Italian Familial Breast Cancer Cases Reveals a High-Risk Mutation Recurrent in the Province of Bergamo. Genet. Med. 2014, 16, 688–694. [Google Scholar] [CrossRef][Green Version]
- Antoniou, A.C.; Casadei, S.; Heikkinen, T.; Barrowdale, D.; Pylkäs, K.; Roberts, J.; Lee, A.; Subramanian, D.; De Leeneer, K.; Fostira, F.; et al. Breast-Cancer Risk in Families with Mutations in PALB2. N. Engl. J. Med. 2014, 371, 497–506. [Google Scholar] [CrossRef]
- Breast Cancer Association Consortium; Dorling, L.; Carvalho, S.; Allen, J.; González-Neira, A.; Luccarini, C.; Wahlström, C.; Pooley, K.A.; Parsons, M.T.; Fortuno, C.; et al. Breast Cancer Risk Genes—Association Analysis in More than 113,000 Women. N. Engl. J. Med. 2021, 384, 428–439. [Google Scholar] [CrossRef]
- Moretta, J.; Berthet, P.; Bonadona, V.; Caron, O.; Cohen-Haguenauer, O.; Colas, C.; Corsini, C.; Cusin, V.; De Pauw, A.; Delnatte, C.; et al. The French Genetic and Cancer Consortium guidelines for multigene panel analysis in hereditary breast and ovarian cancer predisposition. Bull. Cancer 2018, 105, 907–917. [Google Scholar] [CrossRef]
- Wild, C.P. Complementing the Genome with an “Exposome”: The Outstanding Challenge of Environmental Exposure Measurement in Molecular Epidemiology. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1847–1850. [Google Scholar] [CrossRef]
- Duforestel, M.; Nadaradjane, A.; Bougras-Cartron, G.; Briand, J.; Olivier, C.; Frenel, J.-S.; Vallette, F.M.; Lelièvre, S.A.; Cartron, P.-F. Glyphosate Primes Mammary Cells for Tumorigenesis by Reprogramming the Epigenome in a TET3-Dependent Manner. Front. Genet. 2019, 10, 885. [Google Scholar] [CrossRef] [PubMed]
- Rashid, F.; Ramakrishnan, A.; Fields, C.; Irudayaraj, J. Acute PFOA Exposure Promotes Epigenomic Alterations in Mouse Kidney Tissues. Toxicol. Rep. 2020, 7, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Briand, J.; Nadaradjane, A.; Bougras-Cartron, G.; Olivier, C.; Vallette, F.M.; Cartron, P.-F. Diuron Exposure and Akt Overexpression Promote Glioma Formation through DNA Hypomethylation. Clin. Epigenetics 2019, 11, 159. [Google Scholar] [CrossRef] [PubMed]
- DeFelice, S.L. The Nutraceutical Revolution: Its Impact on Food Industry R&D. Trends Food Sci. Technol. 1995, 6, 59–61. [Google Scholar] [CrossRef]
- Crider, K.S.; Yang, T.P.; Berry, R.J.; Bailey, L.B. Folate and DNA Methylation: A Review of Molecular Mechanisms and the Evidence for Folate’s Role. Adv. Nutr. 2012, 3, 21–38. [Google Scholar] [CrossRef]
- Hervouet, E.; Debien, E.; Campion, L.; Charbord, J.; Menanteau, J.; Vallette, F.M.; Cartron, P.-F. Folate Supplementation Limits the Aggressiveness of Glioma via the Remethylation of DNA Repeats Element and Genes Governing Apoptosis and Proliferation. Clin. Cancer Res. 2009, 15, 3519–3529. [Google Scholar] [CrossRef]
- Cartron, P.-F.; Hervouet, E.; Debien, E.; Olivier, C.; Pouliquen, D.; Menanteau, J.; Loussouarn, D.; Martin, S.A.; Campone, M.; Vallette, F.M. Folate Supplementation Limits the Tumourigenesis in Rodent Models of Gliomagenesis. Eur. J. Cancer 2012, 48, 2431–2441. [Google Scholar] [CrossRef]
- Parker, M.J.; Weigele, P.R.; Saleh, L. Insights into the Biochemistry, Evolution, and Biotechnological Applications of the Ten-Eleven Translocation (TET) Enzymes. Biochemistry 2019, 58, 450–467. [Google Scholar] [CrossRef]
- Yin, R.; Mao, S.-Q.; Zhao, B.; Chong, Z.; Yang, Y.; Zhao, C.; Zhang, D.; Huang, H.; Gao, J.; Li, Z.; et al. Ascorbic Acid Enhances Tet-Mediated 5-Methylcytosine Oxidation and Promotes DNA Demethylation in Mammals. J. Am. Chem. Soc. 2013, 135, 10396–10403. [Google Scholar] [CrossRef]
- Mikeska, T.; Alsop, K.; Australian Ovarian Cancer Study Group; Mitchell, G.; Bowtell, D.D.; Dobrovic, A. No Evidence for PALB2 Methylation in High-Grade Serous Ovarian Cancer. J. Ovarian Res. 2013, 6, 26. [Google Scholar] [CrossRef]
- Lobo, J.; Constâncio, V.; Guimarães-Teixeira, C.; Leite-Silva, P.; Miranda-Gonçalves, V.; Sequeira, J.P.; Pistoni, L.; Guimarães, R.; Cantante, M.; Braga, I.; et al. Promoter Methylation of DNA Homologous Recombination Genes Is Predictive of the Responsiveness to PARP Inhibitor Treatment in Testicular Germ Cell Tumors. Mol. Oncol. 2021, 15, 846–865. [Google Scholar] [CrossRef] [PubMed]
- Potapova, A.; Hoffman, A.M.; Godwin, A.K.; Al-Saleem, T.; Cairns, P. Promoter Hypermethylation of the PALB2 Susceptibility Gene in Inherited and Sporadic Breast and Ovarian Cancer. Cancer Res. 2008, 68, 998–1002. [Google Scholar] [CrossRef] [PubMed]
- Ducy, M.; Sesma-Sanz, L.; Guitton-Sert, L.; Lashgari, A.; Gao, Y.; Brahiti, N.; Rodrigue, A.; Margaillan, G.; Caron, M.-C.; Côté, J.; et al. The Tumor Suppressor PALB2: Inside Out. Trends Biochem. Sci. 2019, 44, 226–240. [Google Scholar] [CrossRef] [PubMed]
- European Union RÈGLEMENT (UE) No 1169/2011 DU PARLEMENT EUROPÉEN ET DU CONSEIL (ANNEXE XIII). 2011. Available online: https://eur-lex.europa.eu/eli/reg/2011/1169/oj/fra (accessed on 16 August 2022).
- Briand, J.; Joalland, M.-P.; Nadaradjane, A.; Bougras-Cartron, G.; Olivier, C.; Vallette, F.M.; Perruche, S.; Cartron, P.-F. Diuron Modulates the DNA Methylation Status of the ILT7 and TRAIL/TNFSF10 Genes and Decreases the Killing Activity of Plasmacytoid Dendritic Cells. Environ. Sci. Eur. 2019, 31, 35. [Google Scholar] [CrossRef]
- Ahmad, S.; Wen, Y.; Irudayaraj, J.M.K. PFOA Induces Alteration in DNA Methylation Regulators and SARS-CoV-2 Targets Ace2 and Tmprss2 in Mouse Lung Tissues. Toxicol. Rep. 2021, 8, 1892–1898. [Google Scholar] [CrossRef]
- Health Canada Diuron. Available online: https://publications.gc.ca/Collection/H48-10-1-45-1989E.pdf (accessed on 16 August 2022).
- Health Canada PFOA. Available online: https://www.canada.ca/fr/sante-canada/programmes/consultation-acide-perfluorooctanoique-apfo-eau-potable/document.html (accessed on 16 August 2022).
- Rebhandl, S.; Huemer, M.; Greil, R.; Geisberger, R. AID/APOBEC Deaminases and Cancer. Oncoscience 2015, 2, 320–333. [Google Scholar] [CrossRef]
- Health Canada Glyphosate. Available online: https://www.canada.ca/content/dam/canada/health-canada/migration/healthy-canadians/publications/healthy-living-vie-saine/water-glyphosate-eau/alt/water-glyphosate-eau-eng.pdf (accessed on 16 August 2022).
- Ghazarian, A.A.; Simonds, N.I.; Lai, G.Y.; Mechanic, L.E. Opportunities for Gene and Environment Research in Cancer: An Updated Review of NCI’s Extramural Grant Portfolio. Cancer Epidemiol. Biomark. Prev. 2021, 30, 576–583. [Google Scholar] [CrossRef]
- Patel, C.J.; Kerr, J.; Thomas, D.C.; Mukherjee, B.; Ritz, B.; Chatterjee, N.; Jankowska, M.; Madan, J.; Karagas, M.R.; McAllister, K.A.; et al. Opportunities and Challenges for Environmental Exposure Assessment in Population-Based Studies. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1370–1380. [Google Scholar] [CrossRef]
- Gaudet, F.; Hodgson, J.G.; Eden, A.; Jackson-Grusby, L.; Dausman, J.; Gray, J.W.; Leonhardt, H.; Jaenisch, R. Induction of Tumors in Mice by Genomic Hypomethylation. Science 2003, 300, 489–492. [Google Scholar] [CrossRef]
- Hervouet, E.; Lalier, L.; Debien, E.; Cheray, M.; Geairon, A.; Rogniaux, H.; Loussouarn, D.; Martin, S.A.; Vallette, F.M.; Cartron, P.-F. Disruption of Dnmt1/PCNA/UHRF1 Interactions Promotes Tumorigenesis from Human and Mice Glial Cells. PLoS ONE 2010, 5, e11333. [Google Scholar] [CrossRef]
- Pacaud, R.; Brocard, E.; Lalier, L.; Hervouet, E.; Vallette, F.M.; Cartron, P.-F. The DNMT1/PCNA/UHRF1 Disruption Induces Tumorigenesis Characterized by Similar Genetic and Epigenetic Signatures. Sci. Rep. 2014, 4, 4230. [Google Scholar] [CrossRef] [PubMed]
- Sciandrello, G.; Caradonna, F.; Mauro, M.; Barbata, G. Arsenic-Induced DNA Hypomethylation Affects Chromosomal Instability in Mammalian Cells. Carcinogenesis 2004, 25, 413–417. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shimizu, T.; Marusawa, H.; Matsumoto, Y.; Inuzuka, T.; Ikeda, A.; Fujii, Y.; Minamiguchi, S.; Miyamoto, S.; Kou, T.; Sakai, Y.; et al. Accumulation of Somatic Mutations in TP53 in Gastric Epithelium with Helicobacter Pylori Infection. Gastroenterology 2014, 147, 407–417.e3. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Smith, L.E.; Feng, Z.; Tang, M.; Lee, C.S.; Pfeifer, G.P. Methylated CpG Dinucleotides Are the Preferential Targets for G-to-T Transversion Mutations Induced by Benzo[a]Pyrene Diol Epoxide in Mammalian Cells: Similarities with the P53 Mutation Spectrum in Smoking-Associated Lung Cancers. Cancer Res. 2001, 61, 7110–7117. [Google Scholar]
- Bernstein Molho, R.; Zalmanoviz, S.; Laitman, Y.; Friedman, E. De Novo Pathogenic Germline Variant in PALB2 in a Patient with Pancreatic Cancer. Fam. Cancer 2020, 19, 193–196. [Google Scholar] [CrossRef]
- Mancini, F.R.; Cano-Sancho, G.; Gambaretti, J.; Marchand, P.; Boutron-Ruault, M.-C.; Severi, G.; Arveux, P.; Antignac, J.-P.; Kvaskoff, M. Perfluorinated Alkylated Substances Serum Concentration and Breast Cancer Risk: Evidence from a Nested Case-Control Study in the French E3N Cohort. Int. J. Cancer 2020, 146, 917–928. [Google Scholar] [CrossRef]
- Timmermann, C.A.G.; Rossing, L.I.; Grøntved, A.; Ried-Larsen, M.; Dalgård, C.; Andersen, L.B.; Grandjean, P.; Nielsen, F.; Svendsen, K.D.; Scheike, T.; et al. Adiposity and Glycemic Control in Children Exposed to Perfluorinated Compounds. J. Clin. Endocrinol. Metab. 2014, 99, E608–E614. [Google Scholar] [CrossRef]



| Mixtures inducing the PALB2c.1027C>T mutation | Mixtures having a protective effect against the induction of the PALB2 c.1027C>T mutation |
| FolateRDI/Diuron3MAC/PFOA3MAC | FolateRDI/Diuron3MAC/PFOA3MAC/Ascorbic Acid3RDI/Iron3RDI |
| FolateRDI/Zn5RDI/PFOA3MAC | FolateRDI/Zn5RDI/PFOA3MAC/Ascorbic Acid3RDI/Iron3RDI |
| FolateRDI/Diuron10MAC/PFOA10MAC Ascorbic Acid3RDI/Iron3RDI | |
| FolateRDI/Zn10RDI/PFOA10MAC/Ascorbic Acid3RDI/Iron3RDI |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Courant, F.; Bougras-Cartron, G.; Abadie, C.; Frenel, J.-S.; Cartron, P.-F. Modulation of DNA Methylation/Demethylation Reactions Induced by Nutraceuticals and Pollutants of Exposome Can Promote a C > T Mutation in the Breast Cancer Predisposing Gene PALB2. Epigenomes 2022, 6, 32. https://doi.org/10.3390/epigenomes6040032
Courant F, Bougras-Cartron G, Abadie C, Frenel J-S, Cartron P-F. Modulation of DNA Methylation/Demethylation Reactions Induced by Nutraceuticals and Pollutants of Exposome Can Promote a C > T Mutation in the Breast Cancer Predisposing Gene PALB2. Epigenomes. 2022; 6(4):32. https://doi.org/10.3390/epigenomes6040032
Chicago/Turabian StyleCourant, Florestan, Gwenola Bougras-Cartron, Caroline Abadie, Jean-Sébastien Frenel, and Pierre-François Cartron. 2022. "Modulation of DNA Methylation/Demethylation Reactions Induced by Nutraceuticals and Pollutants of Exposome Can Promote a C > T Mutation in the Breast Cancer Predisposing Gene PALB2" Epigenomes 6, no. 4: 32. https://doi.org/10.3390/epigenomes6040032
APA StyleCourant, F., Bougras-Cartron, G., Abadie, C., Frenel, J.-S., & Cartron, P.-F. (2022). Modulation of DNA Methylation/Demethylation Reactions Induced by Nutraceuticals and Pollutants of Exposome Can Promote a C > T Mutation in the Breast Cancer Predisposing Gene PALB2. Epigenomes, 6(4), 32. https://doi.org/10.3390/epigenomes6040032








