Molecular Doping for Hole Transporting Materials in Hybrid Perovskite Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
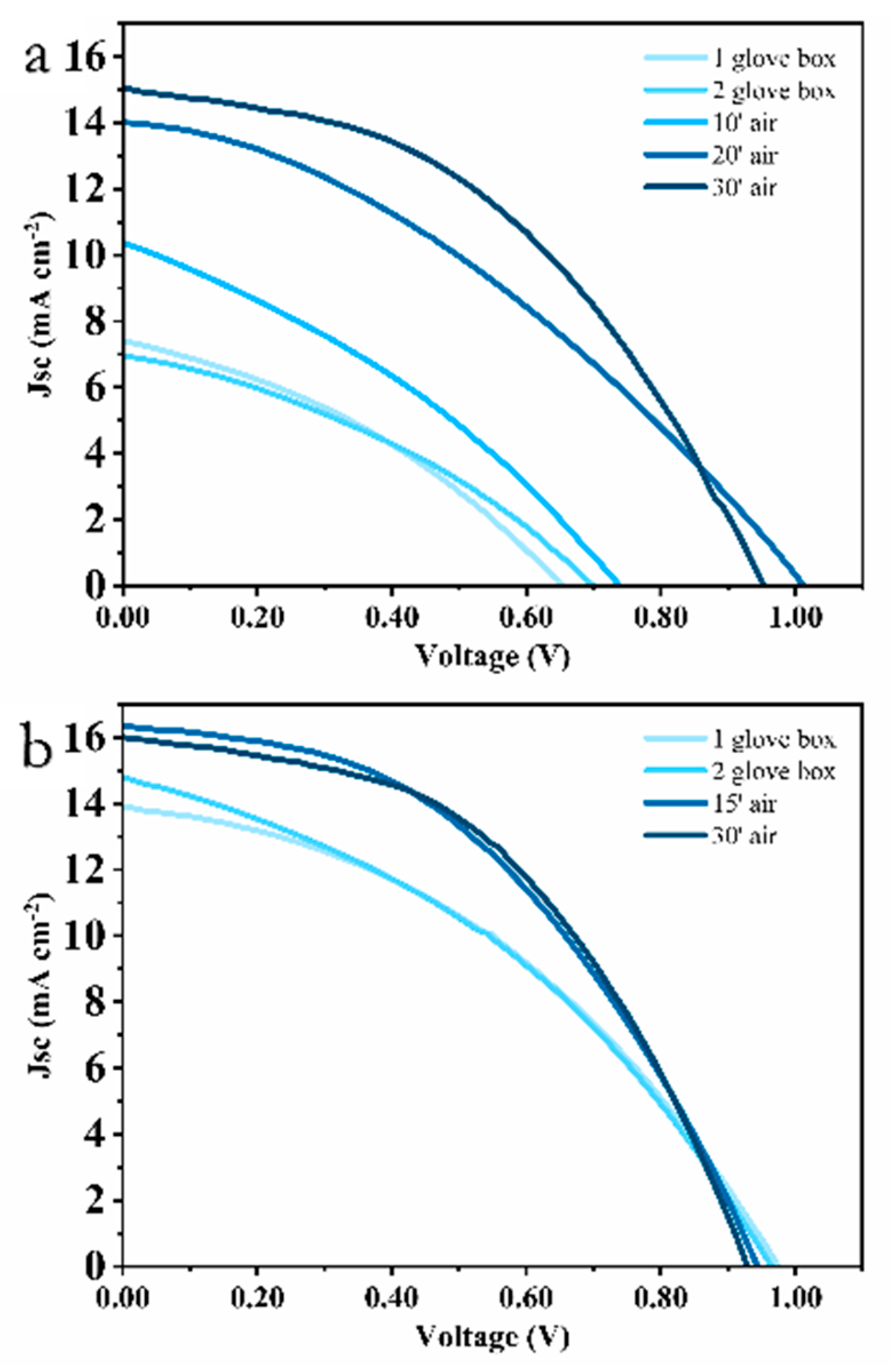
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Colella, S.; Mazzeo, M.; Rizzo, A.; Gigli, G.; Listorti, A. The bright side of perovskites. J. Phys. Chem. Lett. 2016, 7, 4322–4334. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, B.R.; Sargent, E.H. Perovskite photonic sources. Nat. Photonics 2016, 10, 295–302. [Google Scholar] [CrossRef]
- Zhang, W.; Eperon, G.E.; Snaith, H.J. Metal halide perovskites for energy applications. Nat. Energy 2016, 1, 16048. [Google Scholar] [CrossRef]
- Snaith, H.J. Present status and future prospects of perovskite photovoltaics. Nat. Mater. 2018, 17, 372–376. [Google Scholar] [CrossRef]
- Ansari, M.I.H.; Qurashi, A.; Nazeeruddin, M.K. Frontiers, opportunities, and challenges in perovskite solar cells: A critical review. J. Photochem. Photobiol. C Photochem. Rev. 2018, 35, 1–24. [Google Scholar] [CrossRef]
- The national renewable energy laboratory (NREL). Best Research-Cell Efficiency Chart. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 16 December 2019).
- Rehman, W.; McMeekin, D.P.; Patel, J.B.; Milot, R.L.; Johnston, M.B.; Snaith, H.J.; Herz, L.M. Photovoltaic mixed-cation lead mixed-halide perovskites: Links between crystallinity, photo-stability and electronic properties. Energy Environ. Sci. 2017, 10, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; McMeekin, D.P.; Sakai, N.; van Reenen, S.; Wojciechowski, K.; Patel, J.B.; Johnston, M.B.; Snaith, H.J. Efficient and air-stable mixed-cation lead mixed-halide perovskite solar cells with n-doped organic electron extraction layers. Adv. Mater. 2017, 29, 1604186. [Google Scholar] [CrossRef]
- Rong, Y.; Hu, Y.; Mei, A.; Tan, H.; Saidaminov, M.I.; Seok, S.I.; McGehee, M.D.; Sargent, E.H.; Han, H. Challenges for commercializing perovskite solar cells. Science 2018, 361, eaat8235. [Google Scholar] [CrossRef] [Green Version]
- Salhi, B.; Wudil, Y.S.; Hossain, M.K.; Al-Ahmed, A.; Al-Sulaiman, F.A. Review of recent developments and persistent challenges in stability of perovskite solar cells. Renew. Sustain. Energy Rev. 2018, 90, 210–222. [Google Scholar] [CrossRef]
- Sani, F.; Shafie, S.; Lim, H.N.; Musa, A.O. Advancement on lead-free organic-inorganic halide perovskite solar cells: A review. Materials 2018, 11, 1008. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, W.H.; Bailie, C.D.; Unger, E.L.; McGehee, M.D. Enhancing the hole-conductivity of spiro-ometad without oxygen or lithium salts by using Spiro(TFSI)2 in perovskite and dye-sensitized solar cells. J. Am. Chem. Soc. 2014, 136, 10996–11001. [Google Scholar] [CrossRef]
- Li, Z.; Xiao, C.; Yang, Y.; Harvey, S.P.; Kim, D.H.; Christians, J.A.; Yang, M.; Schulz, P.; Nanayakkara, S.U.; Jiang, C.-S.; et al. Extrinsic ion migration in perovskite solar cells. Energy Environ. Sci. 2017, 10, 1234–1242. [Google Scholar] [CrossRef]
- Jung, E.H.; Jeon, N.J.; Park, E.Y.; Moon, C.S.; Shin, T.J.; Yang, T.-Y.; Noh, J.H.; Seo, J. Efficient, stable and scalable perovskite solar cells using poly(3-hexylthiophene). Nature 2019, 567, 511–515. [Google Scholar] [CrossRef]
- Bi, D.; Yang, L.; Boschloo, G.; Hagfeldt, A.; Johansson, E.M.J. Effect of different hole transport materials on recombination in CH3NH3PbI3 perovskite-sensitized mesoscopic solar cells. J. Phys. Chem. Lett. 2013, 4, 1532–1536. [Google Scholar] [CrossRef]
- Domanski, K.; Correa-Baena, J.-P.; Mine, N.; Nazeeruddin, M.K.; Abate, A.; Saliba, M.; Tress, W.; Hagfeldt, A.; Grätzel, M. Not all that glitters is gold: Metal-migration-induced degradation in perovskite solar cells. ACS Nano 2016, 10, 6306–6314. [Google Scholar] [CrossRef]
- Di Giacomo, F.; Razza, S.; Matteocci, F.; D’Epifanio, A.; Licoccia, S.; Brown, T.M.; Di Carlo, A. High efficiency CH3NH3PbI(3−x)Clx perovskite solar cells with poly(3-hexylthiophene) hole transport layer. J. Power Sources 2014, 251, 152–156. [Google Scholar] [CrossRef]
- Zhu, Z.; Bai, Y.; Zhang, T.; Liu, Z.; Long, X.; Wei, Z.; Wang, Z.; Zhang, L.; Wang, J.; Yan, F.; et al. High-performance hole-extraction layer of sol–gel-processed nio nanocrystals for inverted planar perovskite solar cells. Angew. Chem. Int. Ed. 2014, 53, 12571–12575. [Google Scholar]
- Søndergaard, R.; Hösel, M.; Angmo, D.; Larsen-Olsen, T.T.; Krebs, F.C. Roll-to-roll fabrication of polymer solar cells. Mater. Today 2012, 15, 36–49. [Google Scholar] [CrossRef] [Green Version]
- Ratcliff, E.L.; Jenkins, J.L.; Nebesny, K.; Armstrong, N.R. Electrodeposited, “textured” poly(3-hexyl-thiophene) (e-P3HT) films for photovoltaic applications. Chem. Mater. 2008, 20, 5796–5806. [Google Scholar] [CrossRef]
- Abbas, H.A.; Kottokkaran, R.; Ganapathy, B.; Samiee, M.; Zhang, L.; Kitahara, A.; Noack, M.; Dalal, V.L. High efficiency sequentially vapor grown n-i-p CH3NH3PbI3 perovskite solar cells with undoped P3HT as p-type heterojunction layer. APL Mater. 2015, 3, 016105. [Google Scholar] [CrossRef] [Green Version]
- Genco, A.; Mariano, F.; Carallo, S.; Guerra, V.L.P.; Gambino, S.; Simeone, D.; Listorti, A.; Colella, S.; Gigli, G.; Mazzeo, M. Fully vapor-deposited heterostructured light-emitting diode based on organo-metal halide perovskite. Adv. Electron. Mater. 2016, 2, 1500325. [Google Scholar] [CrossRef]
- Jacobs, I.E.; Moulé, A.J. Controlling molecular doping in organic semiconductors. Adv. Mater. 2017, 29, 1703063. [Google Scholar] [CrossRef]
- Larrain, F.A.; Fuentes-Hernandez, C.; Chou, W.-F.; Rodriguez-Toro, V.A.; Huang, T.-Y.; Toney, M.F.; Kippelen, B. Stable solvent for solution-based electrical doping of semiconducting polymer films and its application to organic solar cells. Energy Environ. Sci. 2018, 11, 2216–2224. [Google Scholar] [CrossRef]
- Gatti, T.; Casaluci, S.; Prato, M.; Salerno, M.; Di Stasio, F.; Ansaldo, A.; Menna, E.; Di Carlo, A.; Bonaccorso, F. Boosting perovskite solar cells performance and stability through doping a poly-3(hexylthiophene) hole transporting material with organic functionalized carbon nanostructures. Adv. Funct. Mater. 2016, 26, 7443–7453. [Google Scholar] [CrossRef]
- Loiudice, A.; Rizzo, A.; Biasiucci, M.; Gigli, G. Bulk heterojunction versus diffused bilayer: The role of device geometry in solution p-doped polymer-based solar cells. J. Phys. Chem. Lett. 2012, 3, 1908–1915. [Google Scholar] [CrossRef] [Green Version]
- Gheno, A.; Vedraine, S.; Ratier, B.; Bouclé, J. Π-conjugated materials as the hole-transporting layer in perovskite solar cells. Metals 2016, 6, 21. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Jia, C.; Wan, Z.; Han, F.; Zhao, B.; Wang, R. The novel dopant for hole-transporting material opens a new processing route to efficiently reduce hysteresis and improve stability of planar perovskite solar cells. J. Power Sources 2017, 342, 886–895. [Google Scholar] [CrossRef]
- Liu, D.; Li, Y.; Yuan, J.; Hong, Q.; Shi, G.; Yuan, D.; Wei, J.; Huang, C.; Tang, J.; Fung, M.-K. Improved performance of inverted planar perovskite solar cells with F4-TCNQ doped PEDOT:PSS hole transport layers. J. Mater. Chem. A 2017, 5, 5701–5708. [Google Scholar] [CrossRef]
- Pfeiffer, M.; Beyer, A.; Fritz, T.; Leo, K. Controlled doping of phthalocyanine layers by cosublimation with acceptor molecules: A systematic seebeck and conductivity study. Appl. Phys. Lett. 1998, 73, 3202–3204. [Google Scholar] [CrossRef]
- Colsmann, A.; Junge, J.; Kayser, C.; Lemmer, U. Organic tandem solar cells comprising polymer and small-molecule subcells. Appl. Phys. Lett. 2006, 89, 203506. [Google Scholar] [CrossRef]
- Aziz, E.F.; Vollmer, A.; Eisebitt, S.; Eberhardt, W.; Pingel, P.; Neher, D.; Koch, N. Localized charge transfer in a molecularly doped conducting polymer. Adv. Mater. 2007, 19, 3257–3260. [Google Scholar] [CrossRef]
- Yim, K.-H.; Whiting, G.L.; Murphy, C.E.; Halls, J.J.M.; Burroughes, J.H.; Friend, R.H.; Kim, J.-S. Controlling electrical properties of conjugated polymers via a solution-based p-type doping. Adv. Mater. 2008, 20, 3319–3324. [Google Scholar] [CrossRef]
- Lee, J.H.; Yoon, S.; Ko, M.S.; Lee, N.; Hwang, I.; Lee, M.J. Improved performance of organic photovoltaic devices by doping F4-TCNQ onto solution-processed graphene as a hole transport layer. Org. Electron. 2016, 30, 302–311. [Google Scholar] [CrossRef]
- Hwang, S.; Potscavage, W.J., Jr.; Nakamichi, R.; Adachi, C. Processing and doping of thick polymer active layers for flexible organic thermoelectric modules. Org. Electron. 2016, 31, 31–40. [Google Scholar] [CrossRef]
- Zhang, Y.; Elawad, M.; Yu, Z.; Jiang, X.; Lai, J.; Sun, L. Enhanced performance of perovskite solar cells with P3HT hole-transporting materials via molecular p-type doping. RSC Adv. 2016, 6, 108888–108895. [Google Scholar] [CrossRef]
- Hawash, Z.; Ono, L.K.; Qi, Y. Recent advances in spiro-meotad hole transport material and its applications in organic–inorganic halide perovskite solar cells. Adv. Mater. Interfaces 2018, 5, 1700623. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Hu, Z.; Xu, J.; Zhang, K.; Zhang, J.; Zhang, J.; Zhu, Y. Efficient and stable planar perovskite solar cells with a non-hygroscopic small molecule oxidant doped hole transport layer. Electrochim. Acta 2016, 196, 328–336. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Johnston, M.B.; Snaith, H.J. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 2013, 501, 395–398. [Google Scholar] [CrossRef]
- Trifiletti, V.; Manfredi, N.; Listorti, A.; Altamura, D.; Giannini, C.; Colella, S.; Gigli, G.; Rizzo, A. Engineering TiO2/perovskite planar heterojunction for hysteresis-less solar cells. Adv. Mater. Interfaces 2016, 3, 1600493. [Google Scholar] [CrossRef]
- Trifiletti, V.; Cannavale, A.; Listorti, A.; Rizzo, A.; Colella, S. Sequential deposition of hybrid halide perovskite starting both from lead iodide and lead chloride on the most widely employed substrates. Thin Solid Films 2018, 657, 110–117. [Google Scholar] [CrossRef]
- Guerra, V.L.P.; Altamura, D.; Trifiletti, V.; Colella, S.; Listorti, A.; Giannuzzi, R.; Pellegrino, G.; Condorelli, G.G.; Giannini, C.; Gigli, G.; et al. Implications of TiO2 surface functionalization on polycrystalline mixed halide perovskite films and photovoltaic devices. J. Mater. Chem. A 2015, 3, 20811–20818. [Google Scholar] [CrossRef]
- Duong, D.T.; Phan, H.; Hanifi, D.; Jo, P.S.; Nguyen, T.-Q.; Salleo, A. Direct observation of doping sites in temperature-controlled, p-doped P3HT thin films by conducting atomic force microscopy. Adv. Mater. 2014, 26, 6069–6073. [Google Scholar] [CrossRef]
- The physics of the solar cell. In Handbook of Photovoltaic Science and Engineering; Wiley: Chichester, West Sussex, UK, 2011; pp. 82–129.
- Motaung, D.E.; Malgas, G.F.; Arendse, C.J.; Mavundla, S.E.; Oliphant, C.J.; Knoesen, D. The influence of thermal annealing on the morphology and structural properties of a conjugated polymer in blends with an organic acceptor material. J. Mater. Sci. 2009, 44, 3192–3197. [Google Scholar] [CrossRef]
- Han, X.; Wu, Z.; Sun, B. Enhanced performance of inverted organic solar cell by a solution-based fluorinated acceptor doped P3HT:PCBM layer. Org. Electron. 2013, 14, 1116–1121. [Google Scholar] [CrossRef]
- Pingel, P.; Zhu, L.; Park, K.S.; Vogel, J.-O.; Janietz, S.; Kim, E.-G.; Rabe, J.P.; Brédas, J.-L.; Koch, N. Charge-transfer localization in molecularly doped thiophene-based donor polymers. J. Phys. Chem. Lett. 2010, 1, 2037–2041. [Google Scholar] [CrossRef]
- Deschler, F.; Riedel, D.; Deák, A.; Ecker, B.; von Hauff, E.; Da Como, E. Imaging of morphological changes and phase segregation in doped polymeric semiconductors. Synth. Met. 2015, 199, 381–387. [Google Scholar] [CrossRef] [Green Version]
- Calado, P.; Telford, A.M.; Bryant, D.; Li, X.; Nelson, J.; O’Regan, B.C.; Barnes, P.R.F. Evidence for ion migration in hybrid perovskite solar cells with minimal hysteresis. Nat. Commun. 2016, 7, 13831. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, M.; Eperon, G.E.; Leijtens, T.C.; McMeekin, D.; Saliba, M.; Zhang, W.; de Bastiani, M.; Petrozza, A.; Herz, L.M.; et al. Charge selective contacts, mobile ions and anomalous hysteresis in organic-inorganic perovskite solar cells. Mater. Horizons 2015, 2, 315–322. [Google Scholar] [CrossRef]
- Kim, B.J.; Kim, M.-c.; Lee, D.G.; Lee, G.; Bang, G.J.; Jeon, J.B.; Choi, M.; Jung, H.S. Interface design of hybrid electron extraction layer for relieving hysteresis and retarding charge recombination in perovskite solar cells. Adv. Mater. Interfaces 2018, 5, 1800993. [Google Scholar] [CrossRef]
- Ravishankar, S.; Gharibzadeh, S.; Roldán-Carmona, C.; Grancini, G.; Lee, Y.; Ralaiarisoa, M.; Asiri, A.M.; Koch, N.; Bisquert, J.; Nazeeruddin, M.K. Influence of charge transport layers on open-circuit voltage and hysteresis in perovskite solar cells. Joule 2018, 2, 788–798. [Google Scholar] [CrossRef] [Green Version]
- Salzmann, I.; Heimel, G.; Oehzelt, M.; Winkler, S.; Koch, N. Molecular electrical doping of organic semiconductors: Fundamental mechanisms and emerging dopant design rules. Acc. Chem. Res. 2016, 49, 370–378. [Google Scholar] [CrossRef] [Green Version]
- Méndez, H.; Heimel, G.; Winkler, S.; Frisch, J.; Opitz, A.; Sauer, K.; Wegner, B.; Oehzelt, M.; Röthel, C.; Duhm, S.; et al. Charge-transfer crystallites as molecular electrical dopants. Nat. Commun. 2015, 6, 8560. [Google Scholar] [CrossRef] [Green Version]
- Lei, X.; Zhang, F.; Song, T.; Sun, B. P-type doping effect on the performance of organic-inorganic hybrid solar cells. Appl. Phys. Lett. 2011, 99, 233305. [Google Scholar] [CrossRef]
- Hintz, H.; Peisert, H.; Egelhaaf, H.J.; Chassé, T. Reversible and irreversible light-induced p-doping of P3HT by oxygen studied by photoelectron spectroscopy (XPS/UPS). J. Phys. Chem. C 2011, 115, 13373–13376. [Google Scholar] [CrossRef]
- Duong, D.T.; Wang, C.; Antono, E.; Toney, M.F.; Salleo, A. The chemical and structural origin of efficient p-type doping in P3HT. Org. Electron. 2013, 14, 1330–1336. [Google Scholar] [CrossRef]
- Jacobs, I.E.; Aasen, E.W.; Oliveira, J.L.; Fonseca, T.N.; Roehling, J.D.; Li, J.; Zhang, G.; Augustine, M.P.; Mascal, M.; Moulé, A.J. Comparison of solution-mixed and sequentially processed P3HT:PCBM films: Effect of doping-induced aggregation on film morphology. J. Mater. Chem. C 2016, 4, 3454–3466. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Roehling, J.D.; Li, Y.; Guo, H.; Moulé, A.J.; Grey, J.K. The effect of 2,3,5,6-tetrafluoro-7,7,8,8-tetracyanoquinodimethane charge transfer dopants on the conformation and aggregation of poly(3-hexylthiophene). J. Mater. Chem. C 2013, 1, 5638–5646. [Google Scholar] [CrossRef] [Green Version]
- Pingel, P.; Neher, D. Comprehensive picture of p-type doping of P3HT with the molecular acceptor F4TCNQ. Phys. Rev. B 2013, 87, 115209. [Google Scholar] [CrossRef]







| HTM | F4-TCNQ Doping (wt %) | Jsc (mA cm−2) | Voc (V) | FF | PCE (%) | HI |
|---|---|---|---|---|---|---|
| Spiro-OMeTAD | 0.0 | 16.31 | 0.91 | 0.45 | 6.71 | 0.01 |
| 0.1 | 24.51 | 1.01 | 0.47 | 11.75 | 0.00 | |
| 0.5 | 14.96 | 1.01 | 0.59 | 9.07 | 0.01 | |
| 1.0 | 16.73 | 0.97 | 0.48 | 7.85 | 0.02 | |
| 5.0 | 16.31 | 0.91 | 0.45 | 6.71 | 0.02 | |
| P3HT | 0.0 | 16.73 | 0.95 | 0.45 | 7.14 | 0.15 |
| 0.1 | 15.97 | 0.85 | 0.59 | 8.01 | 0.02 | |
| 0.3 | 16.85 | 0.85 | 0.58 | 8.30 | 0.00 | |
| 0.5 | 19.44 | 0.86 | 0.58 | 9.70 | 0.13 | |
| 0.7 | 16.28 | 0.84 | 0.46 | 6.15 | 0.15 | |
| 1.0 | 14.47 | 0.86 | 0.48 | 5.97 | 0.20 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trifiletti, V.; Degousée, T.; Manfredi, N.; Fenwick, O.; Colella, S.; Rizzo, A. Molecular Doping for Hole Transporting Materials in Hybrid Perovskite Solar Cells. Metals 2020, 10, 14. https://doi.org/10.3390/met10010014
Trifiletti V, Degousée T, Manfredi N, Fenwick O, Colella S, Rizzo A. Molecular Doping for Hole Transporting Materials in Hybrid Perovskite Solar Cells. Metals. 2020; 10(1):14. https://doi.org/10.3390/met10010014
Chicago/Turabian StyleTrifiletti, Vanira, Thibault Degousée, Norberto Manfredi, Oliver Fenwick, Silvia Colella, and Aurora Rizzo. 2020. "Molecular Doping for Hole Transporting Materials in Hybrid Perovskite Solar Cells" Metals 10, no. 1: 14. https://doi.org/10.3390/met10010014
APA StyleTrifiletti, V., Degousée, T., Manfredi, N., Fenwick, O., Colella, S., & Rizzo, A. (2020). Molecular Doping for Hole Transporting Materials in Hybrid Perovskite Solar Cells. Metals, 10(1), 14. https://doi.org/10.3390/met10010014