An Approach for Modelling Slag Infiltration and Heat Transfer in Continuous Casting Mold for High Mn–High Al Steel
Abstract
:1. Introduction
2. Model Development
2.1. Model Description
2.2. Compositions and Properties of Slag
3. Results and Discussion
3.1. Formation of Slag Films
3.2. Evolution of Slag Infiltration and Heat Flux
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kim, D.J.; Park, J.H. Interfacial reaction between CaO-SiO2-MgO-Al2O3 flux and Fe-xMn-yAl (x = 10 and 20 mass pct, y = 1, 3, and 6 mass pct) steel at 1873 K (1600 °C). Metall. Mater. Trans. B 2012, 43, 875–886. [Google Scholar] [CrossRef]
- Blazek, K.; Yin, H.; Skoczylas, G.; McClymonds, M.; Frazee, M. Development and evaluation of lime alumina-based mold powders for casting high-aluminum TRIP steel grades. Iron Steel Technol. 2011, 8, 232–240. [Google Scholar]
- Kim, M.; Lee, S.; Cho, J.; Park, M.; Lee, H.; Kang, Y. A reaction between high Mn-high Al steel and CaO-SiO2-type molten mold flux: Part I. Composition evolution in molten mold flux. Metall. Mater. Trans. B 2013, 44, 299–308. [Google Scholar] [CrossRef]
- Zhang, Z.; Wen, G.H.; Tang, P. The influence of Al2O3/SiO2 ratio on the viscosity of mold fluxes. ISIJ Int. 2008, 48, 739–746. [Google Scholar] [CrossRef] [Green Version]
- Ryu, H.G.; Zhang, Z.T.; Cho, J.W.; Wen, G.H.; Sridhar, S. Crystallization behaviors of slags through a heat flux simulator. ISIJ Int. 2010, 50, 1142–1150. [Google Scholar] [CrossRef] [Green Version]
- Seo, M.; Shi, C.; Cho, J.; Kim, S. Crystallization behaviors of CaO-SiO2-Al2O3-Na2O-CaF2-(Li2O-B2O3) mold fluxes. Metall. Mater. Trans. B 2014, 45, 1874–1886. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Wang, Q.; He, S.P.; Xu, J.F.; Long, X.; Lu, Y.J. Study on properties of alumina-based mould fluxes for high-Al steel slab casting. Steel Res. Int. 2012, 83, 1194–1202. [Google Scholar] [CrossRef]
- Cho, J.; Blazek, K.; Frazee, M.; Yin, H.B.; Park, J.H.; Moon, S.W. Assessment of CaO-Al2O3 based mold flux system for high aluminum TRIP casting. ISIJ Int. 2013, 53, 62–70. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.; Chen, W.; Yang, Y.; Lippold, C.; McLean, A. Effect of CaO/Al2O3 ratio on viscosity and crystallization behaviour of mould flux for high Al non-magnetic steel. Ironmak. Steelmak. 2015, 42, 698–704. [Google Scholar] [CrossRef]
- Shi, C.; Seo, M.; Cho, J.; Kim, S. Crystallization characteristics of CaO-Al2O3-Based mold flux and their effects on in-mold performance during high-aluminum TRIP steels continuous casting. Metall. Mater. Trans. B 2014, 45, 1081–1097. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Li, H.; Wang, W.; Xiao, D.; Zhang, L.; Yu, J. Effect of Li2O on the behavior of melting, crystallization, and structure for CaO-Al2O3-based mold fluxes. Metall. Mater. Trans. B 2018, 49, 2232–2240. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Ostrovski, O.; Zhang, C.; Cai, D. Effects of B2O3 on crystallization, structure, and heat transfer of CaO-Al2O3-based mold fluxes. Metall. Mater. Trans. B 2019, 50, 291–303. [Google Scholar] [CrossRef]
- Yang, J.; Cai, Z.; Zhu, M. Transient thermo-fluid and solidification behaviors in continuous casting mold: Evolution phenomena. ISIJ Int. 2018, 58, 299–308. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Meng, X.; Zhu, M. Transient thermo-fluid and solidification behaviors in continuous casting mold: Oscillation behaviors. ISIJ Int. 2018, 58, 2071–2078. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, M. Evolution of compositions and properties of CaO-SiO2 based mold flux for continuous casting high Mn steel. ISIJ Int. 2016, 56, 2191–2198. [Google Scholar] [CrossRef] [Green Version]
- Mills, K.C.; Fox, A.B.; Li, Z.; Thackray, R.P. Performance and properties of mould fluxes. Ironmak. Steelmak. 2005, 32, 26–34. [Google Scholar] [CrossRef]
- Jonayat, A.; Thomas, B.G. Transient thermo-fluid model of meniscus behavior and slag consumption in steel continuous casting. Metall. Mater. Trans. B 2014, 45, 1842–1864. [Google Scholar] [CrossRef]
- Hasegawa, H.; Ohta, H.; Shibata, H.; Waseda, Y. Recent development in the investigation on thermal conductivity of silicate melts. High Temp. Mater. Proc. 2012, 31, 299–673. [Google Scholar] [CrossRef]
- Hayashi, M.; Ishii, H.; Susa, M.; Fukuyama, H.; Nagata, K. Effect of ionicity of nonbridging oxygen ions on thermal conductivity of molten alkali silicates. Phys. Chem. Glasses 2001, 42, 6–11. [Google Scholar]
- McDavid, R.M.; Thomas, B.G. Flow and thermal behavior of the top surface flux powder layers in continuous casting molds. Metall. Mater. Trans. B 1996, 27, 672–685. [Google Scholar] [CrossRef]
- Fu, X.J.; Wen, G.H.; Liu, Q.; Tang, P.; Li, J.Z.; Li, W. Development and evaluation of CaO-SiO2 based mould fluxes for casting high Aluminum TRIP steel. Steel Res. Int. 2014, 86, 110–120. [Google Scholar] [CrossRef]

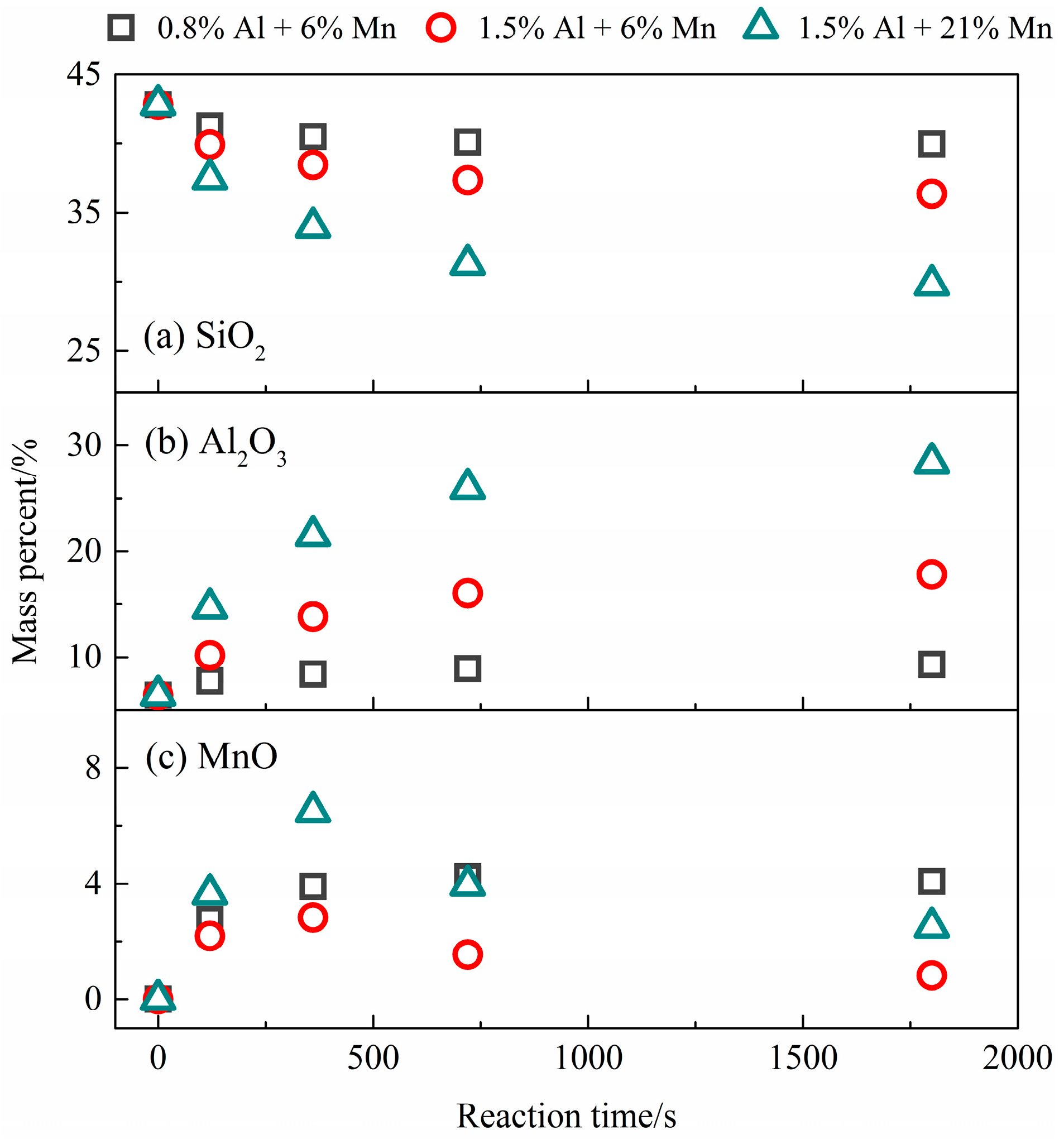
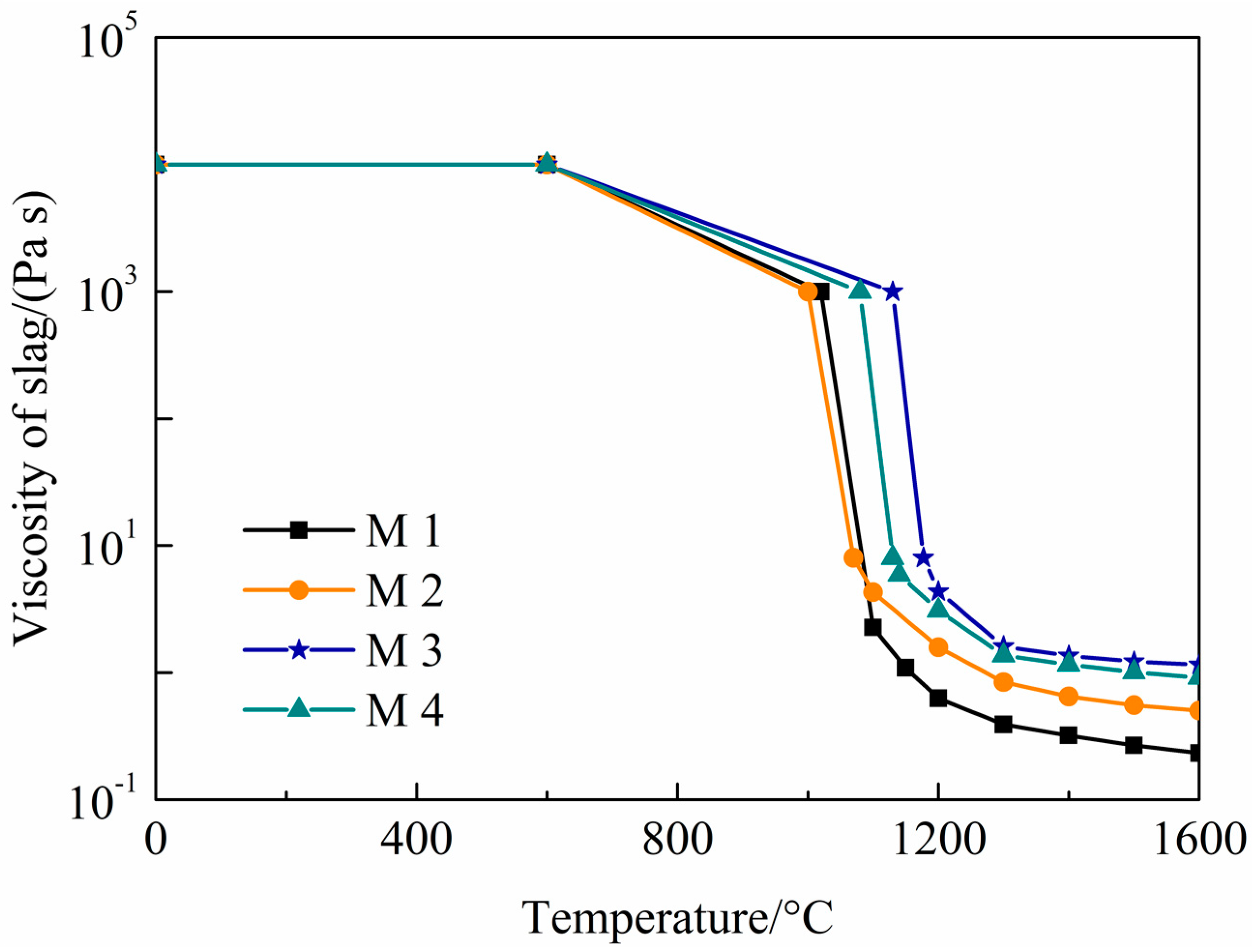







| Parameter | Value |
|---|---|
| Superheat temperature/°C | 25 |
| Casting speed/(m/min) | 1.2 |
| Water temperature/°C | 30 |
| Amplitude/mm | 2.5 |
| Frequency/(min−1) | 152 |
| Samples | CaO | SiO2 | Al2O3 | MgO | Na2O | F | MnO |
|---|---|---|---|---|---|---|---|
| M1 | 33.15% | 42.82% | 6.41% | 2.24% | 8.86% | 6.51% | 0% |
| M2 | 31.83% | 39.96% | 9.32% | 2.15% | 8.51% | 6.25% | 4.08% |
| M3 | 31.33% | 36.36% | 17.82% | 2.11% | 8.38% | 6.15% | 0.83% |
| M4 | 29.75% | 29.78% | 28.39% | 2.01% | 7.95% | 5.84% | 2.50% |
| Properties for High Mn–High Al Steel | ||||
| Liquidus Temperature/°C | 1421 | |||
| Solidus Temperature/°C | 1378 | |||
| Latent heat/(kJ/kg) | 315 | |||
| Slag Properties | ||||
| M1 | M2 | M3 | M4 | |
| Solidification temperature/°C | 1117 | 1195 | 1222 | 1199 |
| Viscosity at 1300 °C/(Pa s) | 0.391 | 0.842 | 1.362 | 1.608 |
| Slag/steel interfacial tension/(N/m) | 1.33 | 1.35 | 1.34 | 1.36 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Chen, D.; Long, M.; Duan, H. An Approach for Modelling Slag Infiltration and Heat Transfer in Continuous Casting Mold for High Mn–High Al Steel. Metals 2020, 10, 51. https://doi.org/10.3390/met10010051
Yang J, Chen D, Long M, Duan H. An Approach for Modelling Slag Infiltration and Heat Transfer in Continuous Casting Mold for High Mn–High Al Steel. Metals. 2020; 10(1):51. https://doi.org/10.3390/met10010051
Chicago/Turabian StyleYang, Jie, Dengfu Chen, Mujun Long, and Huamei Duan. 2020. "An Approach for Modelling Slag Infiltration and Heat Transfer in Continuous Casting Mold for High Mn–High Al Steel" Metals 10, no. 1: 51. https://doi.org/10.3390/met10010051




