An Innovative Technique for Comprehensive Utilization of High Aluminum Iron Ore via Pre-Reduced-Smelting Separation-Alkaline Leaching Process: Part I: Pre-Reduced-Smelting Separation to Recover Iron
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. High Aluminum Iron Ore
2.1.2. Flux and Additive
2.1.3. Reductant
2.2. Experimental Methods
2.2.1. Pre-Reduction-Smelting Separation Process
2.2.2. The Slag Modification-Alkaline Leaching
2.3. Characterization of Raw Materials and Products
3. Results and Discussion
3.1. Recover Iron from HAIO by Pre-Reduction-Smelting Separation Process
3.1.1. Pre-Reduction of High Alumina Iron Ore Pellets
3.1.2. Smelting Separation Process
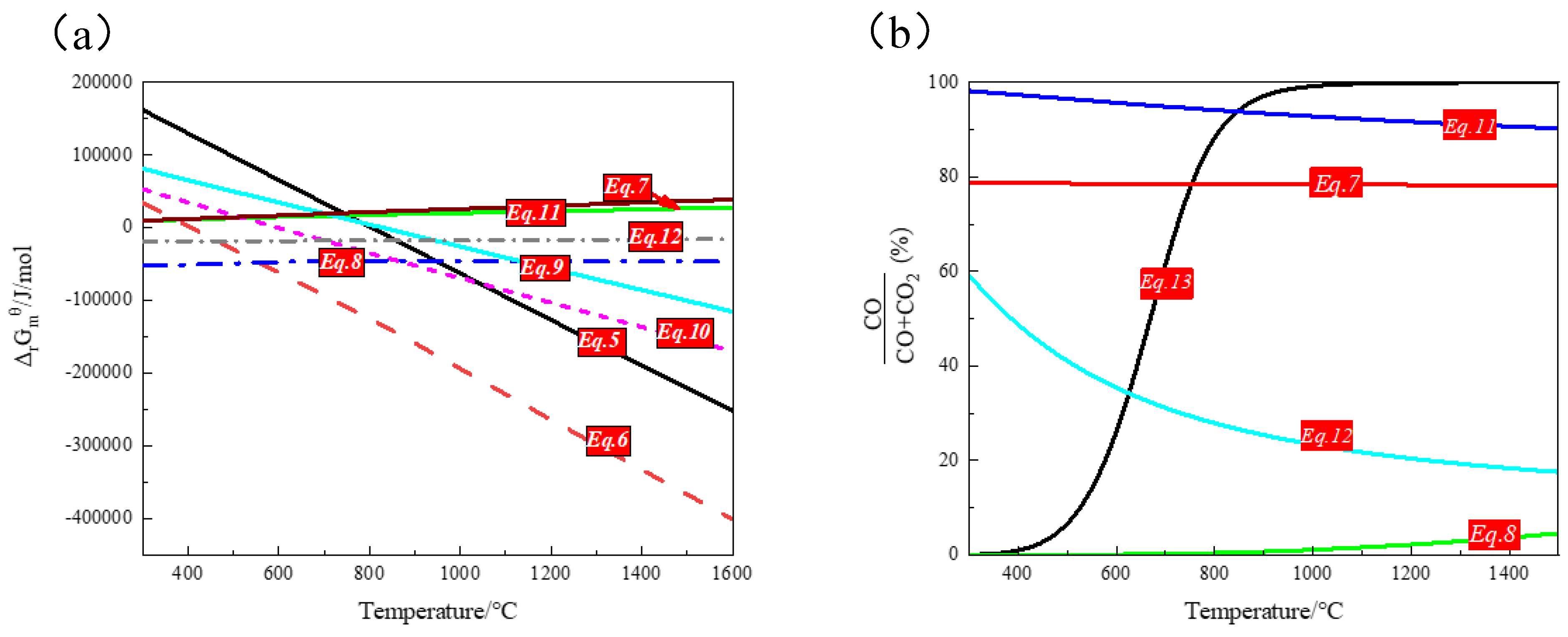
Thermodynamic Analysis
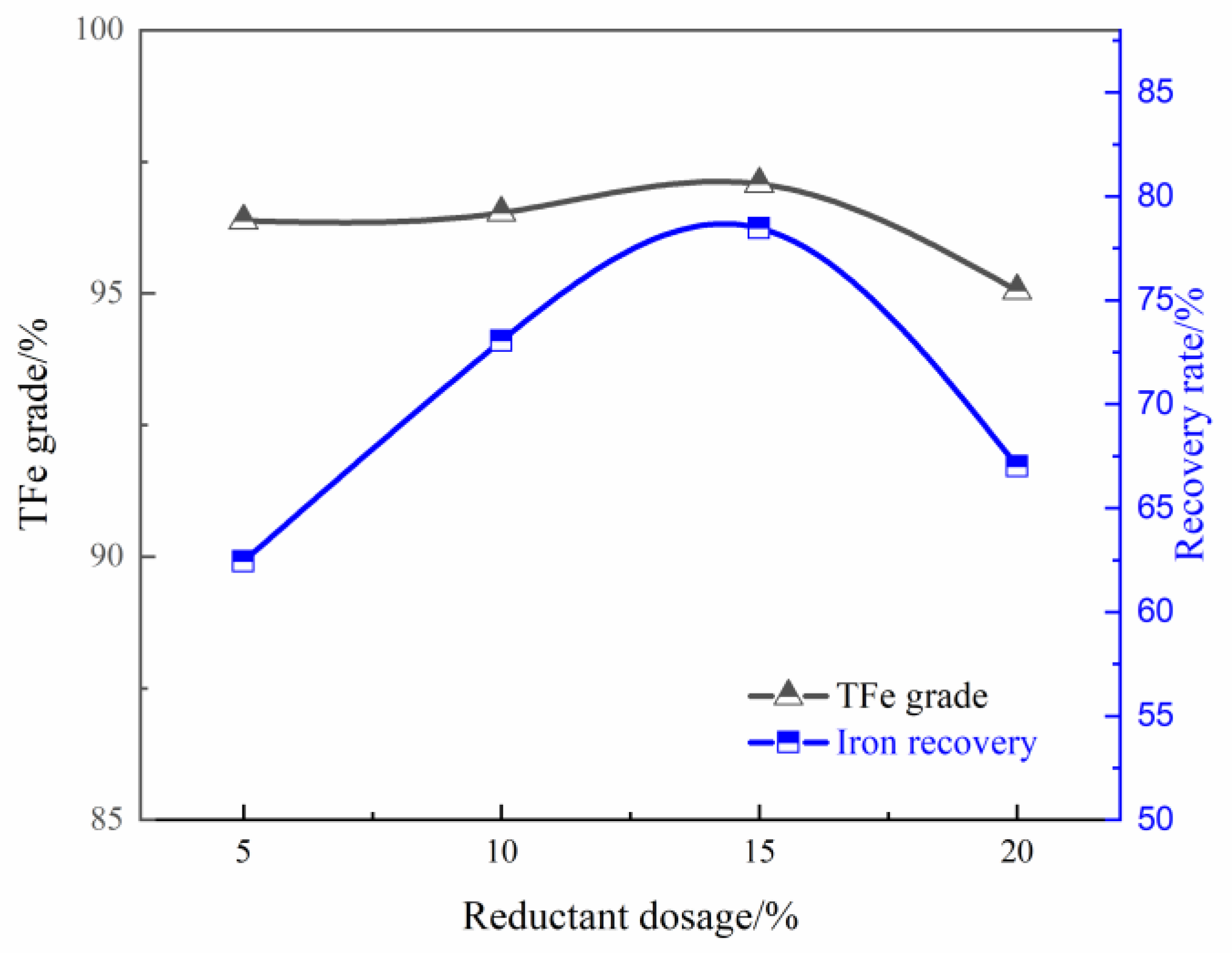
Smelting Separation of the Pre-Reduced Pellets
3.2. Extracting Al2O3 from Smelting Separation Slag by Modifying-Alkaline Leaching
3.3. Fe and Al Balance in the Full Flow Sheet
4. Conclusions
- HAIO, assaying 33.43% Fetotal, 19.09% Al2O3, and 18.36% SiO2, was defined as a refractory iron ore and employed as the raw materials to yield pig iron and extract Al2O3. In addition, the major iron minerals of HAIO are hematite and goethite, while the main aluminum minerals are kaolinite, and all of them are closely associated at superfine size.
- Approximately 94.54% of Fe was recovered in the pig iron when smelting the pre-reduced high aluminum iron pellets at 1625 °C for 30 min with a slag basicity of 0.40, while approximately 68.93% Al2O3 was extracted from the slag by roasting the smelting separation slag at 900 °C for 2 h and alkaline leaching at 95 °C for 2 h with a L/S ratio of 10 mL/g, and the leaching residue is suitable for cement industry.
- The developed process can provide an alternative for effective and green utilization of HAIO.
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, K.Y.; Kleit, A.N. Mining rate optimization considering the stockpiling: A theoretical economics and real option model. Resour. Policy 2016, 47, 87–94. [Google Scholar] [CrossRef]
- Pradhan, N.; Das, B.; Gahan, C.S.; Kar, R.N.; Sukla, L.B. Beneficiation of iron ore slime using aspergillus niger and bacillus circulans. Bioresour. Technol. 2006, 97, 1876–1879. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sahoo, H.; Rath, S.S.; Sahu, A.K.; Das, B. Recovery of iron minerals from Indian iron ore slimes using colloidal magnetic coating. Powder Technol. 2015, 269, 38–45. [Google Scholar] [CrossRef]
- Lu, L.; Holmes, R.J.; Manuel, J.R. Effects of alumina on sintering performance of hematite iron ores. ISIJ Int. 2007, 47, 349–358. [Google Scholar] [CrossRef] [Green Version]
- Umadevi, T.; Prakash, S.; Bandopadhyay, U.K.; Mahapatra, P.C.; Prabhu, M.; Ranjan, M. Influence of alumina on iron ore sinter quality and productivity. World Iron Steel 2010, 10, 12–18. [Google Scholar]
- Rocha, L.; Cançado, R.L.; Peres, A.C. Iron ore slimes flotation. Miner. Eng. 2010, 23, 842–845. [Google Scholar] [CrossRef]
- Thella, J.S.; Mukherjee, A.K.; Srikakulapu, N.G. Processing of high alumina iron ore slimes using classification and flotation. Powder Technol. 2012, 217, 418–426. [Google Scholar] [CrossRef]
- Shobhana, D.; Santosh, P.; Ratnakar, S.; Gayna, M.P. Response of process parameters for processing of iron ore slime using column flotation. Int. J. Miner. Process 2015, 140, 58–65. [Google Scholar]
- Mahiuddin, S.; Bondyopadhway, S.; Baruah, J.N. A study on the beneficiation of indian iron-ore fines and slime using chemical additives. Int. J. Miner. Process 1989, 26, 285–296. [Google Scholar] [CrossRef]
- Shu, C.W.; Tang, Y.; Wang, Z.Q.; Zhang, Q. Research on sodium salt roasting-acid leaching technology for high-alumina and high-silicon refractory limonite. Min. Metall. Eng. 2012, 32, 62–65. [Google Scholar]
- Zhu, D.Q.; Chun, T.J.; Pan, J. Recovery of Iron from High-Iron Red Mud by Reduction Roasting with Adding Sodium Salt. J. Iron. Steel Res. Int. 2012, 19, 1–5. [Google Scholar] [CrossRef]
- Chun, T.J.; Zhu, D.Q.; Pan, J. Preparation of metallic iron powder from red mud by sodium salt roasting and magnetic separation. Can. Metall. Q. 2014, 53, 183–189. [Google Scholar] [CrossRef]
- Zhou, X.L.; Zhu, D.Q.; Pan, J.; Luo, Y.H.; Liu, X.Q. Upgrading of High-Aluminum Hematite-Limonite Ore by High Temperature Reduction-Wet Magnetic Separation Process. Metals 2016, 6, 57. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zuo, K.; Yang, G.; Shang, Z.; Zhang, J. Recovery of ferric oxide from bayer red mud by reduction roasting-magnetic separation process. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2016, 31, 404–407. [Google Scholar] [CrossRef]
- Chun, T.J.; Long, H.M.; Li, J.X. Alumina-Iron Separation of High Alumina Iron Ore by Carbothermic Reduction and Magnetic Separation. Sep. Sci. Technol. 2015, 50, 760–766. [Google Scholar] [CrossRef]
- Sellaeg, H.; Kolbeinsen, L.; Safarian, J. Iron separation from bauxite through smelting-reduction process. Light Metals. 2017, 127–135. [Google Scholar]
- He, Y.B.; Tang, B.; Li, Q.; Zou, Z.S. A comprehensive static model of an iron bath smelting reduction process for alumina-rich iron ore. ISIJ Int. 2015, 55, 2135–2144. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.Q.; Zhu, D.Q.; Pan, J.; Zhang, F. Innovative methodology for comprehensive and harmless utilization of waste copper slag via selective reduction-magnetic separation process. J. Clean. Prod. 2018, 187, 910–922. [Google Scholar] [CrossRef]
- Yang, C.C.; Zhu, D.Q.; Pan, J.; Lu, L.M. Simultaneous Recovery of Iron and Phosphorus from a High-Phosphorus Oolitic Iron Ore to Prepare Fe-P Alloy for High-Phosphorus Steel Production. JOM 2017, 69, 1663–1668. [Google Scholar] [CrossRef]
- Li, S.W.; Pan, J.; Zhu, D.Q.; Guo, Z.Q.; Xu, J.W.; Chou, J.L. A novel process to upgrade the copper slag by direct reduction-magnetic separation with the addition of Na2CO3 and CaO. Powder Technol. 2019, 347, 159–169. [Google Scholar] [CrossRef]
- Qin, S.C.; Jiang, K.X.; Zhang, B.S.; Wang, H.B. Study on phase form and content of sulfur and iron in oxygen pressure acidic leaching residue of sphalerites. Chin. J. Inorg. Anal. Chem. 2016, 6, 57–61. [Google Scholar]
- Wang, W.H.; Wang, D.; Li, X.Y. Phase analysis on aluminum metal and aluminum trioxide in aluminium ash. Resour. Environ. Eng. 2009, 23, 33–334. [Google Scholar]
- Iron Ores—Determination of Metallic Iron Content—Sulfosalicylic Acid Spectrophotometric Method; GB/T 24194-2009; Wuhan lron and Steel Company: Wuhan, China, 2010.
- Yang, Y.X.; Qi, X. X-ray Diffraction Analysis; Shanghai Jiaotong University Press: Shanghai, China, 1989; pp. 270–278. [Google Scholar]
- Zhang, F.; Zhu, D.Q.; Pan, J.; Guo, Z.Q. Effect of basicity on the structure characteristics of chromium-nickel bearing iron ore pellets. Powder Technol. 2019, 342, 409–417. [Google Scholar] [CrossRef]
- Gu, X.; Dong, W.; Chen, Y.; Li, Y. Investigation on the glass-anorthite insulating composites with low dielectric constant and low sintering temperature. Rare Metal Mater. Eng. 2007, 36, 464–467. [Google Scholar]
- Liu, Q.; Pan, Z.; Li, Q.; Ruan, Y. Preparation of anorthite lightweight thermal insulating brick and the formation process of anorthite. Bull. Chin. Ceram. Soc. 2010, 29, 1269–1274. [Google Scholar]
- Baev, S.V.; Uryavin, G.A.; Leont’ev, L.N. Heat exchange and reduction in high-alumina charges in the blast furnace. Metallurgist 1975, 19, 351–354. [Google Scholar] [CrossRef]
- He, Y.B. Study on Slag-Iron Bath Smelting Reduction Process for Alumina-Rich Iron Ore Processing; Northeastern University: Shenyang, China, 2016; pp. 24–34. [Google Scholar]
- Gao, J.J.; Wang, X.Y.; Qi, Y.H.; Wang, F. Technical analysis on ironmaking process of rotary kiln pre-reduction and smelting by coal and oxygen. J. Iron Steel Res. 2018, 30, 91–96. [Google Scholar]

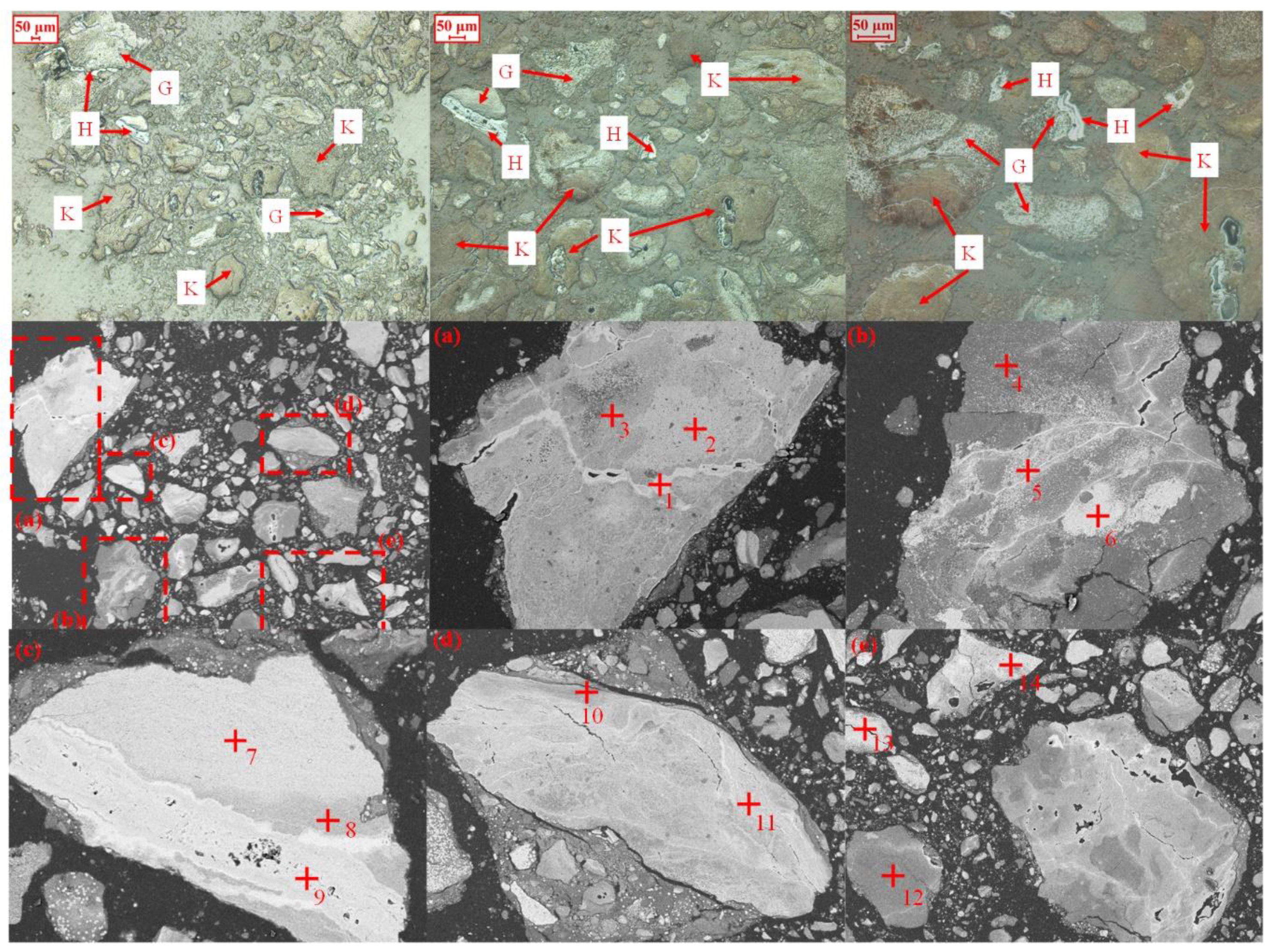












| Element | TFe | Al2O3 | SiO2 | K2O | Na2O | MgO | CaO | P | S | LOI |
|---|---|---|---|---|---|---|---|---|---|---|
| Content | 33.43 | 19.09 | 18.36 | 0.12 | 0.033 | 0.063 | 0.039 | 0.20 | 0.028 | 12.34 |
| Minerals | Iron Carbonate | Iron Sulfide | Magnetite | Hematite/Goethite | Fayalite | TFe |
|---|---|---|---|---|---|---|
| Fe Content | 0.14 | 0.020 | 0.050 | 37.46 | 0.47 | 38.14 |
| Fraction | 0.36 | 0.06 | 0.14 | 98.21 | 1.23 | 100 |
| Minerals | Diasporite | Gibbsite | Kaolinite | TAl2O3 |
|---|---|---|---|---|
| Al Content | 3.03 | 2.59 | 16.16 | 21.78 |
| Fraction | 13.93 | 11.89 | 74.18 | 100 |
| Area No. | Elemental Compositions/(atomic conc, %) | Mineral Phase | |||
|---|---|---|---|---|---|
| Fe | Al | Si | O | ||
| 1 | 33.49 | 6.57 | 4.89 | 55.13 | hematite |
| 2 | 18.19 | 12.51 | 8.66 | 60.64 | goethite |
| 3 | 5.34 | 18.53 | 18.19 | 57.94 | kaolinite |
| 4 | 6.82 | 18.34 | 18.34 | 56.50 | kaolinite |
| 5 | 18.82 | 14.66 | 12.18 | 54.34 | hematite/kaolinite |
| 6 | 30.77 | 8.34 | 6.69 | 54.20 | hematite |
| 7 | 29.29 | 8.47 | 6.50 | 55.74 | hematite |
| 8 | 20.21 | 12.58 | 6.42 | 60.79 | goethite |
| 9 | 38.55 | 3.18 | 57.89 | 0.38 | hematite |
| 10 | 4.66 | 19.31 | 19.70 | 56.32 | kaolinite |
| 11 | 18.19 | 12.51 | 8.66 | 60.64 | goethite |
| 12 | 5.34 | 18.53 | 18.19 | 57.94 | kaolinite |
| 13 | 20.21 | 12.58 | 6.42 | 60.79 | goethite |
| 14 | 32.96 | 7.73 | 52.36 | 6.95 | hematite |
| Proximate Analysis * | Main Chemical Compositions of ash | ||||||
|---|---|---|---|---|---|---|---|
| FCad | Mad | Ad | Vdaf | Fe2O3 | SiO2 | Al2O3 | CaO |
| 52.12 | 12.98 | 4.49 | 30.41 | 16.86 | 40.19 | 15.15 | 26.55 |
| Basicity | Iron | Hercynite | Fayalite | Quartz | Gehlenite | Albite |
|---|---|---|---|---|---|---|
| 0.0020 | 12 | 58 | 12 | 18 | - | - |
| 0.20 | 15 | 45 | 10 | 14 | 8 | 8 |
| 0.40 | 18 | 31 | 9 | 7 | 23 | 12 |
| 0.60 | 13 | 27 | 10 | 8 | 35 | 7 |
| 0.80 | 13 | 19 | 8 | 6 | 47 | 7 |
| Basicity | Iron | Hercynite | Mullite | Corundum | Anorthite |
|---|---|---|---|---|---|
| 0.0020 | 3 | 5 | 77 | 15 | - |
| 0.20 | 6 | 5 | 56 | 33 | - |
| 0.40 | - | - | - | 40 | 60 |
| 0.60 | - | - | - | - | - |
| 0.80 | - | - | - | - | - |
| Point | Fe | Al2O3 | SiO2 | CaO |
|---|---|---|---|---|
| 1 | 100 | - | - | - |
| 2 | - | 51.35 | 48.04 | 0.94 |
| 3 | 100 | - | - | -- |
| 4 | - | 100 | - | - |
| 5 | - | 32.28 | 53.72 | 14 |
| 6 | - | 38.36 | 44.06 | 17.58 |
| 7 | - | 100 | - | - |
| 8 | - | 48.11 | 38.55 | 13.34 |
| 9 | - | 36.98 | 39.54 | 23.48 |
| 10 | - | 36.76 | 38.08 | 25.16 |
| Sample | Fe | Al2O3 | SiO2 | CaO | MgO | K2O | Na2O | P | S |
|---|---|---|---|---|---|---|---|---|---|
| Pig iron | 97.08 | 0.13 | 0.10 | 0.025 | 0.0074 | 0.0029 | 0.0045 | 0.28 | 0.14 |
| Alkaline leaching residue | 3.4 | 9.77 | 20.93 | 31.53 | 0.38 | 0.11 | 3.73 | 0.84 | 0.015 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Pan, J.; Zhu, D.; Guo, Z.; Shi, Y.; Chou, J.; Xu, J. An Innovative Technique for Comprehensive Utilization of High Aluminum Iron Ore via Pre-Reduced-Smelting Separation-Alkaline Leaching Process: Part I: Pre-Reduced-Smelting Separation to Recover Iron. Metals 2020, 10, 57. https://doi.org/10.3390/met10010057
Li S, Pan J, Zhu D, Guo Z, Shi Y, Chou J, Xu J. An Innovative Technique for Comprehensive Utilization of High Aluminum Iron Ore via Pre-Reduced-Smelting Separation-Alkaline Leaching Process: Part I: Pre-Reduced-Smelting Separation to Recover Iron. Metals. 2020; 10(1):57. https://doi.org/10.3390/met10010057
Chicago/Turabian StyleLi, Siwei, Jian Pan, Deqing Zhu, Zhengqi Guo, Yue Shi, Jianlei Chou, and Jiwei Xu. 2020. "An Innovative Technique for Comprehensive Utilization of High Aluminum Iron Ore via Pre-Reduced-Smelting Separation-Alkaline Leaching Process: Part I: Pre-Reduced-Smelting Separation to Recover Iron" Metals 10, no. 1: 57. https://doi.org/10.3390/met10010057
APA StyleLi, S., Pan, J., Zhu, D., Guo, Z., Shi, Y., Chou, J., & Xu, J. (2020). An Innovative Technique for Comprehensive Utilization of High Aluminum Iron Ore via Pre-Reduced-Smelting Separation-Alkaline Leaching Process: Part I: Pre-Reduced-Smelting Separation to Recover Iron. Metals, 10(1), 57. https://doi.org/10.3390/met10010057





