Phase Transformation of a Ti-15Mo-5Zr-3Al Brazed Joint Using Clad Ti-15Cu-15Ni Filler
Abstract
:1. Introduction
2. Materials and Experimental Procedures
3. Results and Discussion
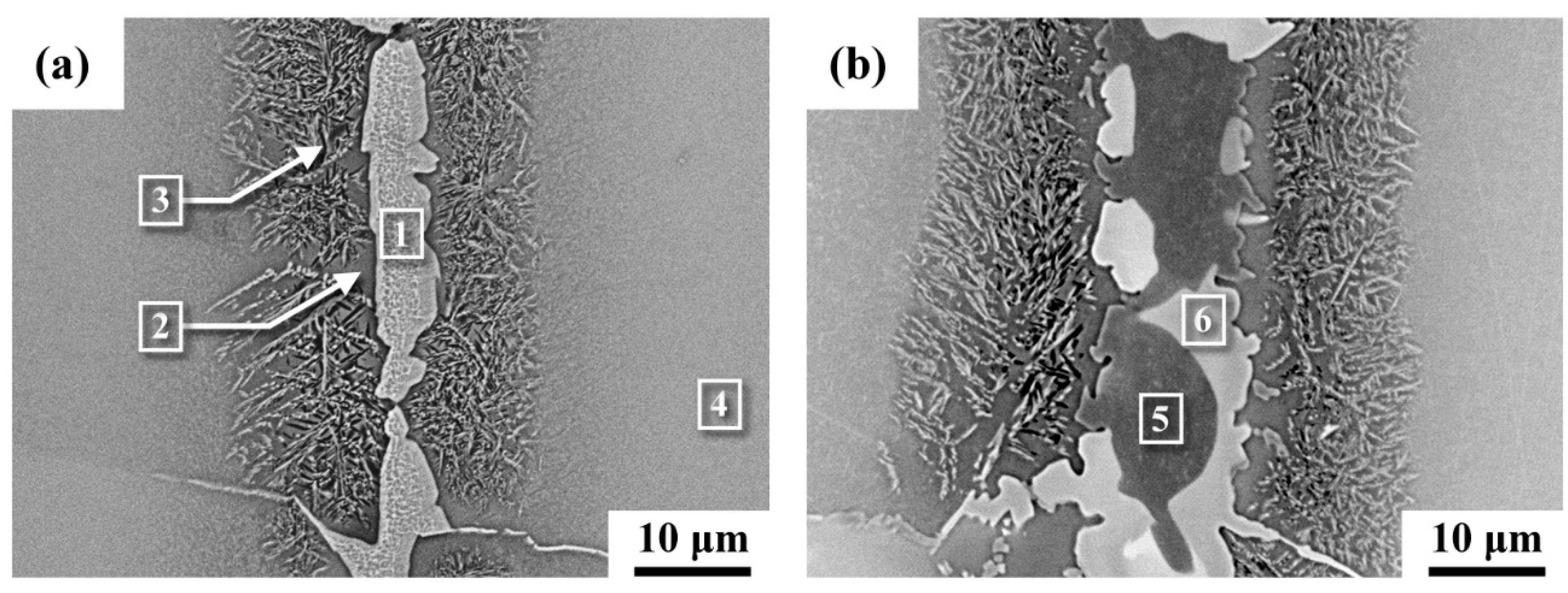
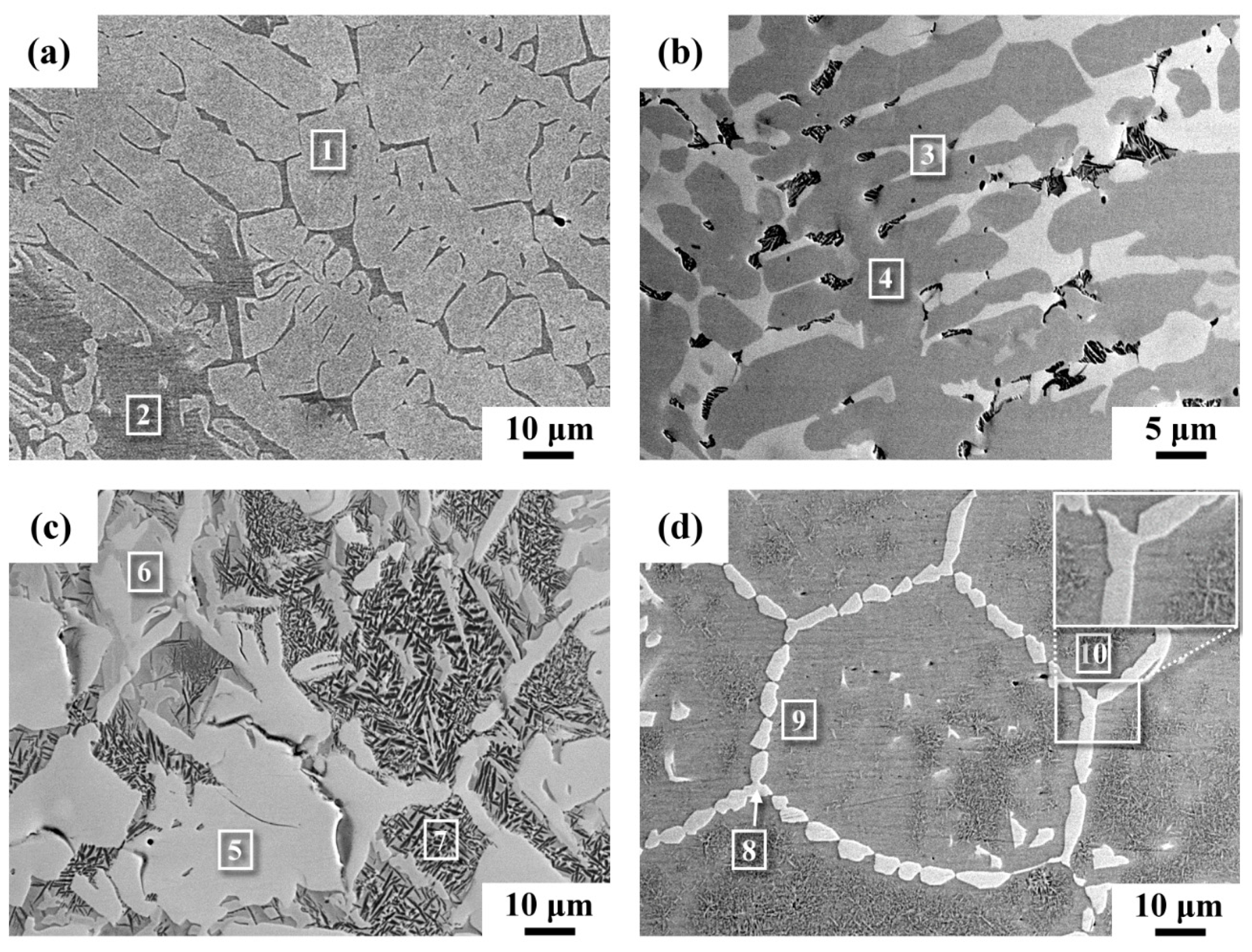
3.1. Microstructural Observations and Chemical Composition Determinations
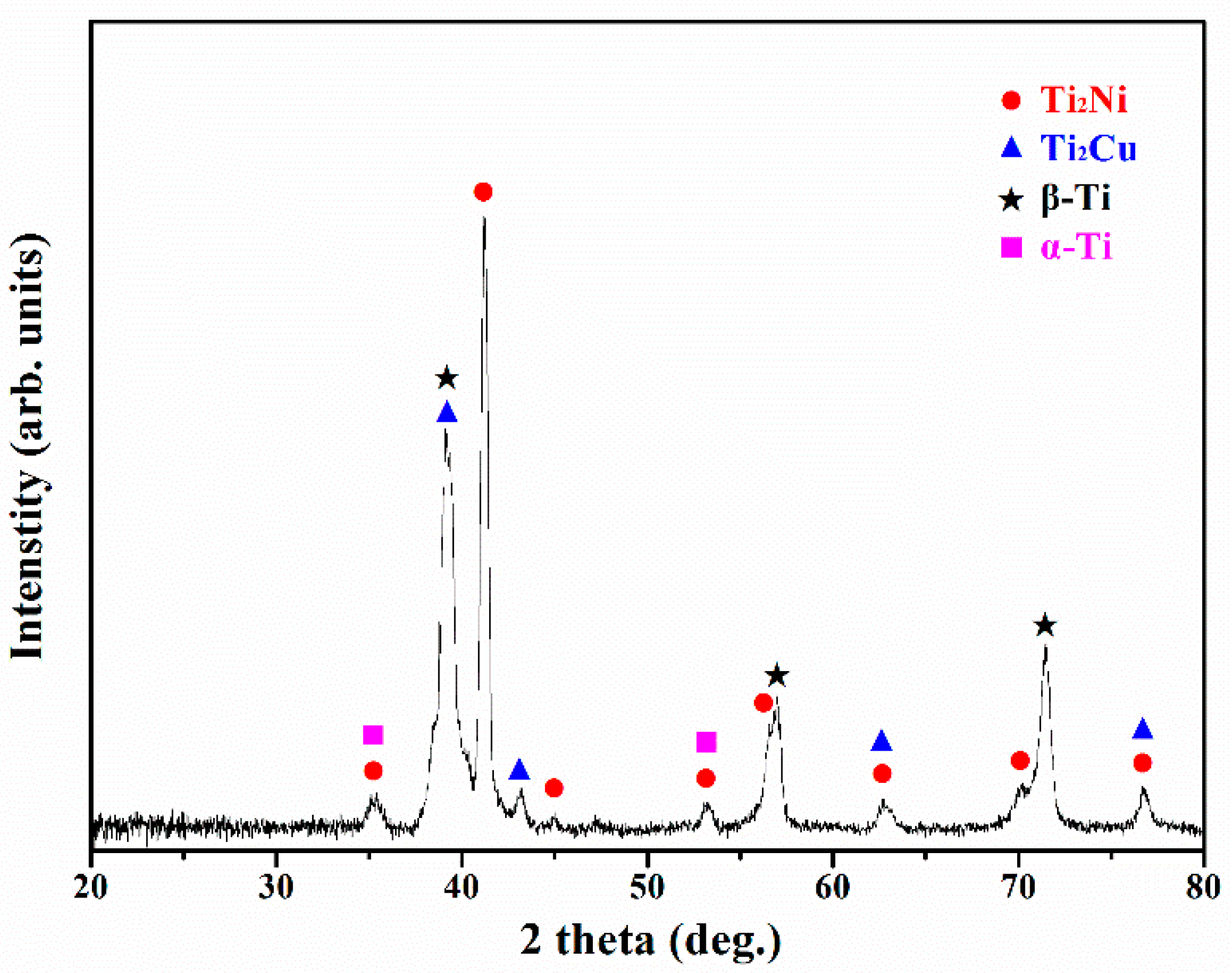
3.2. EBSD Phase Identifications
3.3. Shear Strength Measurements and Fracture Feature Observations
3.4. Formation and Dissolution of Ti2Cu/Ti2Ni Intermetallics in the Brazed Joint
4. Conclusions
- (1)
- The two major phases found in the brazed zone of the sample brazed at 1243 K for 180 s were Ti2Ni and Ti2Cu intermetallic compounds. The XRD pattern revealed that Ti2Ni mixed with Ti2Cu, β-Ti and scarce α-Ti formed in the brazed zone. Central blocky intermetallics in the brazed zone of the B3 sample were replaced by grain boundary intermetallics when the brazing time was extended to 600 s. The results indicated that there were no intermetallic phases present in the sample brazed at 1243 K for 1800 s.
- (2)
- The specimens brazed at 1243 K for 180 s had the lowest shear strength of 310 MPa among the tested samples and predominantly showed cleavage fracture after shear tests. Increasing the brazing time to 600 s changed the failure mechanism of the joint into grain boundary separations of Ti2Ni. The sample brazed at 1243 K for 1800 s had the highest shear strength of 427 MPa and exhibited ductile dimple fracture.
- (3)
- During brazing at 1243 K, the dissolution of brittle Ti2Cu/Ti2Ni compounds in the brazed zone resulted from the rapid diffusion of Ni and Cu into the Ti-15-5-3 substrate. With proper combinations of brazing time and temperature, the harmful phases in the brazed zone can be eliminated thoroughly.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boyer, R.; Welsch, G.; Collings, E.W. Materials Properties Handbook: Titanium Alloys; ASM International: Materials Park, OH, USA, 1993; pp. 949–956. [Google Scholar]
- Smith, W.F. Structure and Properties of Engineering Alloys; McGraw-Hill Book, Co.: New York, NY, USA, 1993; pp. 433–484. [Google Scholar]
- Mouritz, A.P. Introduction to Aerospace Materials: Titanium Alloys for Aerospace Structures and Engines; Woodhead Publishing: Cambridge, UK, 2012; pp. 202–223. [Google Scholar]
- Inagaki, I.; Takechi, T.; Shirai, Y.; Ariyasu, N. Application and features of titanium for the aerospace industry. Nippon. Steel Sumitomo Met. Tech. Rep. 2014, 106, 22–27. [Google Scholar]
- Donachie, M.J., Jr. Titanium: A Technical Guide; ASM international: Materials Park, OH, USA, 2000; pp. 5–11. [Google Scholar]
- Fujii, H.; Takahashi, K.; Yamashita, Y. Application of titanium and its alloys for automobile parts. Nippon. Steel Tech. Rep. 2003, 88, 70–75. [Google Scholar]
- Boyer, R.R. An overview on the use of titanium in the aerospace industry. Mater. Sci. Eng. A 1996, 213, 103–114. [Google Scholar] [CrossRef]
- Ghaderi, A.; Hodgson, P.D.; Barnett, M.R. Microstructure and texture development in Ti-5Al-5Mo-5V-3Cr alloy during cold rolling and annealing. Key Eng. Mater. 2013, 551, 210–216. [Google Scholar] [CrossRef]
- Tokaji, K.; Bian, J.C.; Ogawa, T.; Nakajima, M. The microstructure dependence of fatigue behaviour in Ti-15Mo-5Zr-3Al alloy. Mater. Sci. Eng. A 1996, 213, 86–92. [Google Scholar] [CrossRef]
- Tokaji, K.; Shiota, H.; Bian, J.C. Fatigue crack propagation in β Ti–15Mo–5Zr–3Al alloy. Mater. Sci. Eng. A 1998, 243, 155–162. [Google Scholar] [CrossRef]
- Ikeda, M.; Komatsu, S.Y.; Sugimoto, T.; Hasegawa, M. Effect of two phase warm rolling on aging behavior and mechanical properties of Ti–15Mo–5Zr–3Al alloy. Mater. Sci. Eng. A 1998, 243, 140–145. [Google Scholar] [CrossRef]
- Inamura, T.; Hosoda, H.; Wakashima, K.; Miyazaki, S. Anisotropy and temperature dependence of Young’s modulus in textured TiNbAl biomedical shape memory alloy. Mater. Trans. 2005, 46, 1597–1603. [Google Scholar] [CrossRef] [Green Version]
- Niinomi, M. Mechanical biocompatibilities of titanium alloys for biomedical applications. J. Mech. Behav. Biomed. Mat. 2008, 1, 30–42. [Google Scholar] [CrossRef]
- Wang, K. The use of titanium for medical applications in the USA. Mater. Sci. Eng. A 1996, 213, 134–137. [Google Scholar] [CrossRef]
- Brozek, C.; Sun, F.; Vermaut, P.; Millet, Y.; Lenain, A.; Embury, D.; Jacques, P.J.; Prima, F. A β-titanium alloy with extra high strain-hardening rate: Design and mechanical properties. Scr. Mater. 2016, 114, 60–64. [Google Scholar] [CrossRef]
- Ishimoto, T.; Hagihara, K.; Hisamoto, K.; Sun, S.H.; Nakano, T. Crystallographic texture control of beta-type Ti–15Mo–5Zr–3Al alloy by selective laser melting for the development of novel implants with a biocompatible low Young’s modulus. Scr. Mater. 2017, 132, 34–38. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef] [PubMed]
- Balachandar, K.; Sarma, V.S.; Pant, B.; Phanikumar, G. Microstructure and mechanical properties of gas-tungsten-arc–welded Ti-15-3 beta titanium alloy. Metall. Mater. Trans. A 2009, 40, 2685–2693. [Google Scholar] [CrossRef]
- Babu, N.K.; Raman, S.G.S.; Mythili, R.; Saroja, S. Correlation of microstructure with mechanical properties of TIG weldments of Ti–6Al–4V made with and without current pulsing. Mater Charact. 2007, 58, 581–587. [Google Scholar] [CrossRef]
- Xu, P.Q.; Li, L.J.; Zhang, C.B. Microstructure characterization of laser welded Ti-6Al-4V fusion zones. Mater. Charact. 2014, 87, 179–185. [Google Scholar] [CrossRef]
- Gouret, N.; Dour, G.; Miguet, B.; Ollivier, E.; Fortunier, R. Assessment of the origin of porosity in electron-beam-welded TA6V plates. Metall. Mater. Trans. A 2004, 35, 879–889. [Google Scholar] [CrossRef]
- Messler, R.W., Jr. Joining of Materials and Structures: From Pragmatic Process to Enabling Technology; Elsevier Butterworth-Heinemann: New York, NY, USA, 2004; pp. 349–355. [Google Scholar]
- Jacobson, D.M.; Humpston, G. Principles of Brazing; ASM International: Materials Park, OH, USA, 2005; pp. 1–45. [Google Scholar]
- Gussone, J.; Kasperovich, G.; Haubrich, J.; Requena, G. Interfacial Reactions and Fracture Behavior of Ti Alloy-Ag28Cu Brazing Joints: Influence of Titanium Alloy Composition. Metals 2018, 8, 830. [Google Scholar] [CrossRef] [Green Version]
- Bian, H.; Song, X.G.; Hu, S.P.; Lei, Y.Z.; Jiao, Y.D.; Duan, S.T.; Feng, J.C.; Long, W.M. Microstructure evolution and mechanical properties of titanium/alumina brazed joints for medical implants. Metals 2019, 9, 664. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Wu, P.; Wang, W.; Zhu, D.D.; Tan, C.W.; Su, Y.S.; Shi, X.Y.; Cao, W. Brazing Ti-48Al-2Nb-2Cr Alloys with Cu-Based Amorphous Alloy Filler. Appl. Sci. 2018, 8, 920. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, A.; Rabinkin, A. State of the art of titanium-based brazing filler metals. Weld. J. 2003, 82, 36–43. [Google Scholar]
- Yeh, T.Y.; Shiue, R.K.; Chang, C.S. Microstructural observation of brazed Ti-15-3 alloy using the clad Ti-20Zr-20Cu-20Ni foil. ISIJ Int. 2013, 53, 726–728. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.B.; Kao, C.S.; Tsay, L.W.; Shiue, R.K. The Application of 40Ti-35Ni-25Nb filler foil in brazing commercially pure titanium. Metals 2018, 8, 154. [Google Scholar] [CrossRef] [Green Version]
- Kao, C.S.; Tsay, L.W.; Wang, S.B.; Shiue, R.K. Vacuum brazing Ti-15-3 with a TiNiNb braze alloy. Metals 2019, 9, 1085. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.T.; Shiue, R.K. Infrared brazing of Ti-6Al-4V using the Ti-15Cu-15Ni braze alloy. J. Mater. Sci. 2006, 41, 2145–2150. [Google Scholar] [CrossRef]
- Ren, H.S.; Xiong, H.P.; Chen, B.; Pang, S.J.; Wu, X.; Cheng, Y.Y.; Chen, B.Q. Transient liquid phase diffusion bonding of Ti–24Al–15Nb–1Mo alloy to TiAl intermetallics. Mater. Sci. Eng. A 2016, 651, 45–54. [Google Scholar] [CrossRef]
- Zhu, W.J.; Duarte, L.I.; Leinenbach, C. Experimental study and thermodynamic assessment of the Cu–Ni–Ti system. Comput. Coupling Phase Diagr. Thermochem. 2014, 47, 9–22. [Google Scholar] [CrossRef]
- Villars, P.; Prince, A.; Okamoto, H. Handbook of Ternary Alloy Phase Diagrams; ASM International: Materials Park, OH, USA, 1995. [Google Scholar]
- Steubenann, S.G.; Mausli, P.A.; Moncler, S.S.; Semlitsch, M.; Pohler, O.; Hinter-mann, H.R.; Perren, S.M. Titanium’92 Science and Technology; The Minerals, Metals and Materials Society: Warrendale, MI, USA, 1993; pp. 2689–2696. [Google Scholar]
- Hibbeler, R.C. Mechanics of Materials; Pearson Education Inc.: Upper Saddle River, NJ, USA, 2003; pp. 526–527. [Google Scholar]
- Massalski, T.B. Binary alloy Phase Diagrams; ASM International: Materials Park, OH, USA, 1990; pp. 2874–2876. [Google Scholar]






| Location | Element | Phase/Structure | |||||
|---|---|---|---|---|---|---|---|
| Al | Mo | Ni | Zr | Cu | Ti | ||
| 1 | 0.4 | 0.1 | 26.4 | 2.1 | 4.9 | 66.1 | Ti2Ni |
| 2 | 3.1 | 3.9 | 4.0 | 1.6 | 3.5 | 83.9 | β-Ti |
| 3 | 2.2 | 3.0 | 6.2 | 1.5 | 7.2 | 79.9 | Eutectoid |
| 4 | 4.3 | 7.5 | 3.2 | 2.3 | 1.5 | 81.2 | β-Ti |
| 5 | 0.3 | 0.2 | 11.9 | 0.7 | 19.1 | 67.8 | Ti2Cu |
| 6 | 0.6 | 0.1 | 26.2 | 0.8 | 6.4 | 65.9 | Ti2Ni |
| Location | Element | Phase/Structure | |||||
|---|---|---|---|---|---|---|---|
| Al | Mo | Ni | Zr | Cu | Ti | ||
| 1 | 1.1 | 0.1 | 27.0 | 2.0 | 4.1 | 65.7 | Ti2Ni |
| 2 | 3.6 | 4.2 | 4.1 | 1.5 | 4.2 | 82.4 | β-Ti |
| 3 | 0.5 | 0.1 | 27.2 | 0.4 | 4.3 | 67.5 | Ti2Ni |
| 4 | 0.1 | 0.1 | 13.5 | 0.3 | 18.4 | 67.6 | Ti2Cu |
| 5 | 0.4 | 0.1 | 26.4 | 0.5 | 5.0 | 67.6 | Ti2Ni |
| 6 | 0.3 | 0.1 | 12.0 | 0.6 | 19.8 | 67.2 | Ti2Cu |
| 7 | 3.2 | 2.6 | 4.1 | 1.0 | 3.7 | 85.4 | Eutectoid |
| 8 | 1.0 | 0.5 | 24.8 | 1.8 | 5.1 | 66.8 | Ti2Ni |
| 9 | 3.5 | 4.3 | 4.2 | 1.7 | 4.4 | 81.9 | β-Ti |
| 10 | 3.2 | 3.6 | 4.8 | 1.8 | 4.8 | 81.8 | Eutectoid |
| Specimen | Brazing Temperature | Brazing Time | Average Shear Strength |
|---|---|---|---|
| B3 | 1243 K | 180 s | 310 ± 40 MPa |
| B10 | 600 s | 381 ± 21 MPa | |
| B30 | 1800 s | 427 ± 20 MPa |
| Location | Element | |||||
|---|---|---|---|---|---|---|
| Al | Mo | Ni | Zr | Cu | Ti | |
| 1 | 0.4 | 0.2 | 25.0 | 1.3 | 5.1 | 68.0 |
| 2 | 1.0 | 0.7 | 21.9 | 0.9 | 5.0 | 70.5 |
| 3 | 0.8 | 0.8 | 23.0 | 1.0 | 4.6 | 69.8 |
| 4 | 5.1 | 7.3 | 0.0 | 1.5 | 0.1 | 86.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, G.-L.; Chen, T.-C.; Shiue, R.-K.; Tsay, L.-W. Phase Transformation of a Ti-15Mo-5Zr-3Al Brazed Joint Using Clad Ti-15Cu-15Ni Filler. Metals 2020, 10, 83. https://doi.org/10.3390/met10010083
Yue G-L, Chen T-C, Shiue R-K, Tsay L-W. Phase Transformation of a Ti-15Mo-5Zr-3Al Brazed Joint Using Clad Ti-15Cu-15Ni Filler. Metals. 2020; 10(1):83. https://doi.org/10.3390/met10010083
Chicago/Turabian StyleYue, Gui-Lin, Tai-Cheng Chen, Ren-Kae Shiue, and Leu-Wen Tsay. 2020. "Phase Transformation of a Ti-15Mo-5Zr-3Al Brazed Joint Using Clad Ti-15Cu-15Ni Filler" Metals 10, no. 1: 83. https://doi.org/10.3390/met10010083





