Effect of Magnesium Treatment on the Hot Ductility of Ti-Bearing Peritectic Steel
Abstract
1. Introduction
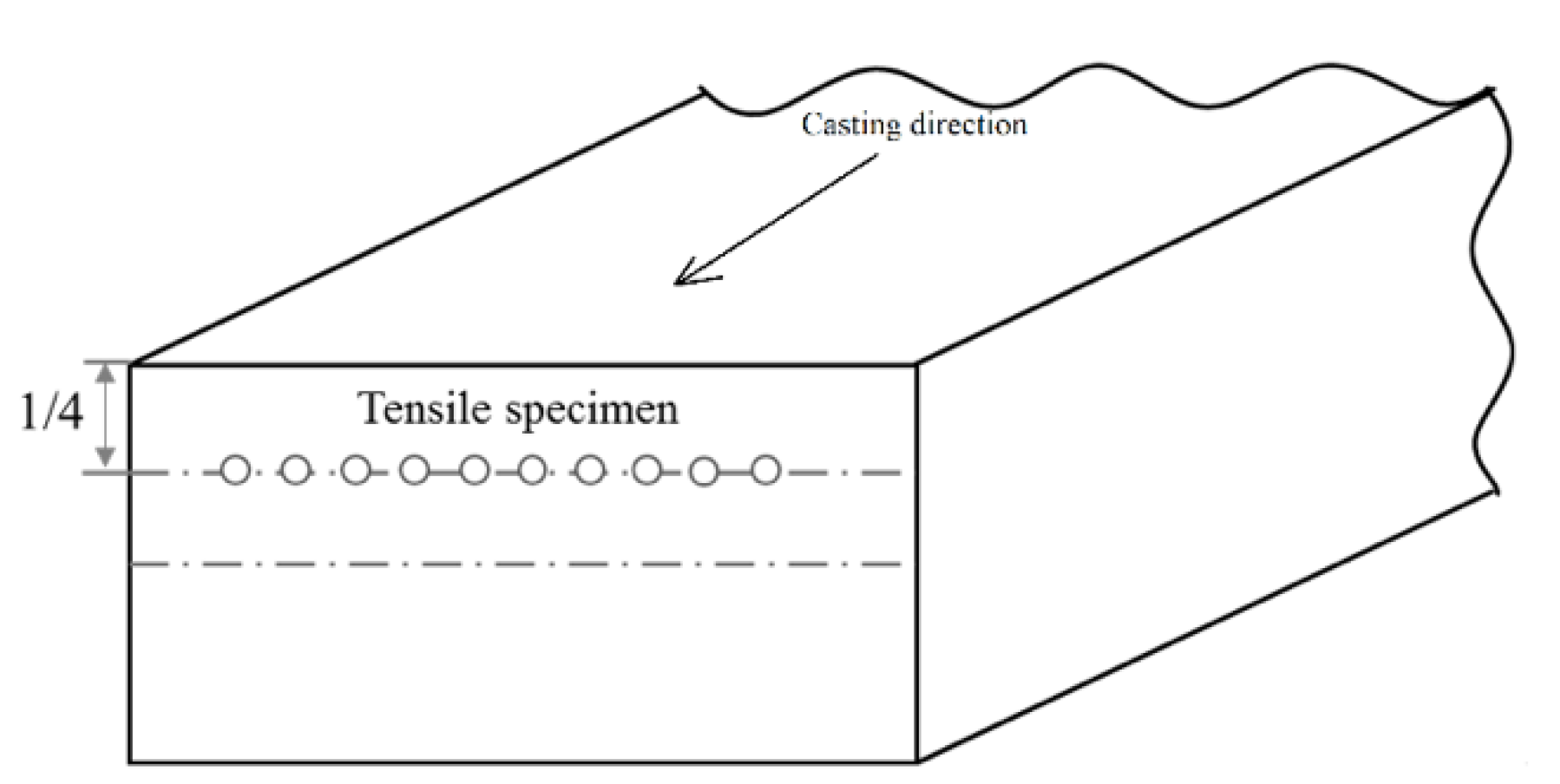
2. Materials and Methods
3. Analysis and Discussion
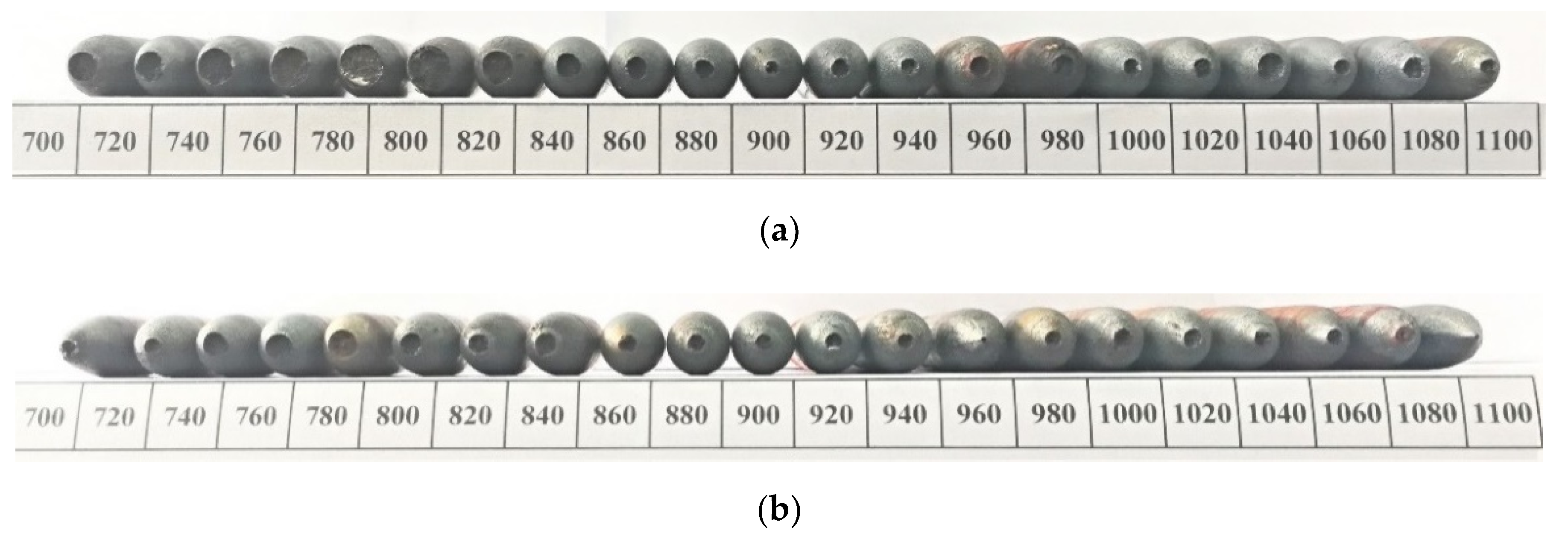
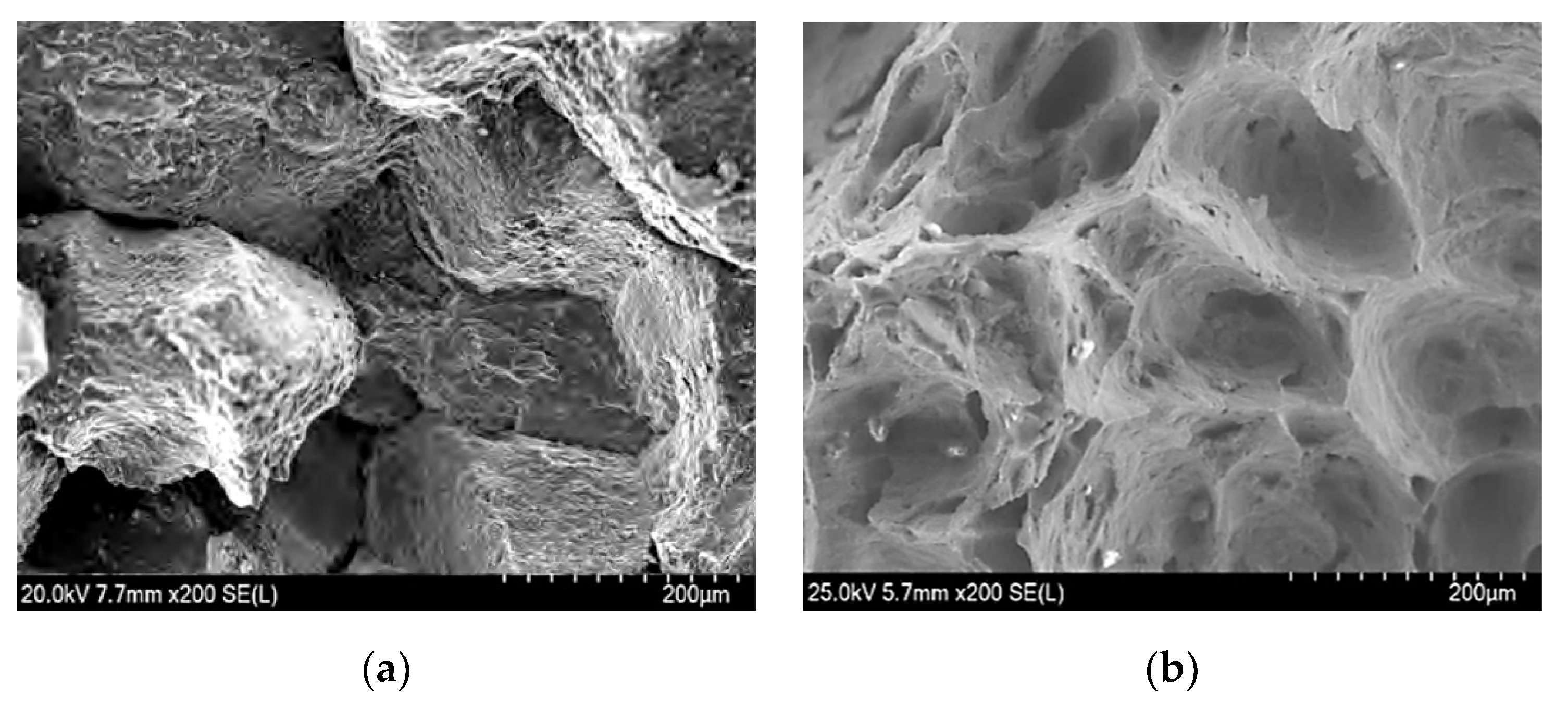
3.1. Analysis of High-Temperature Test Results
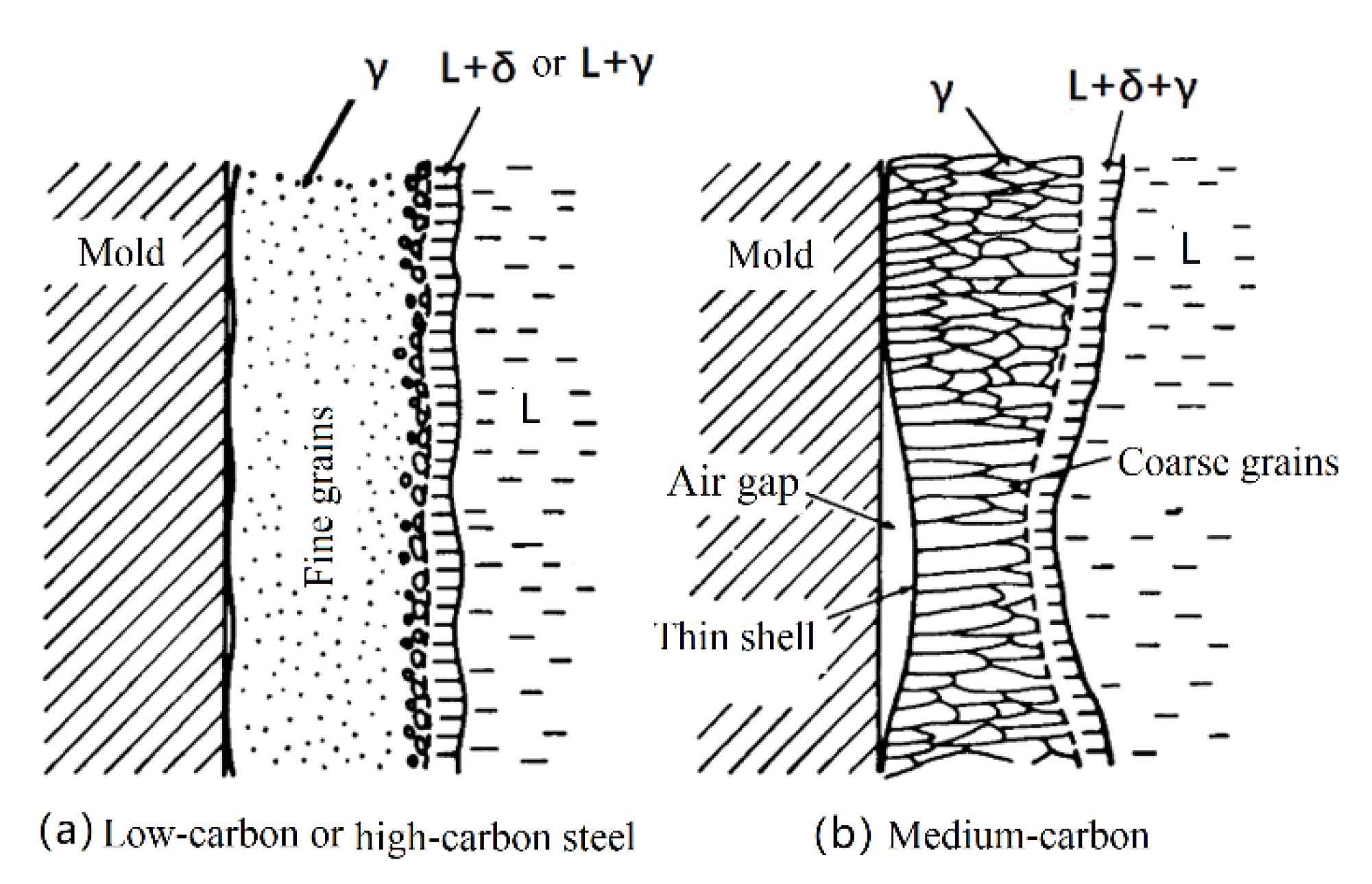
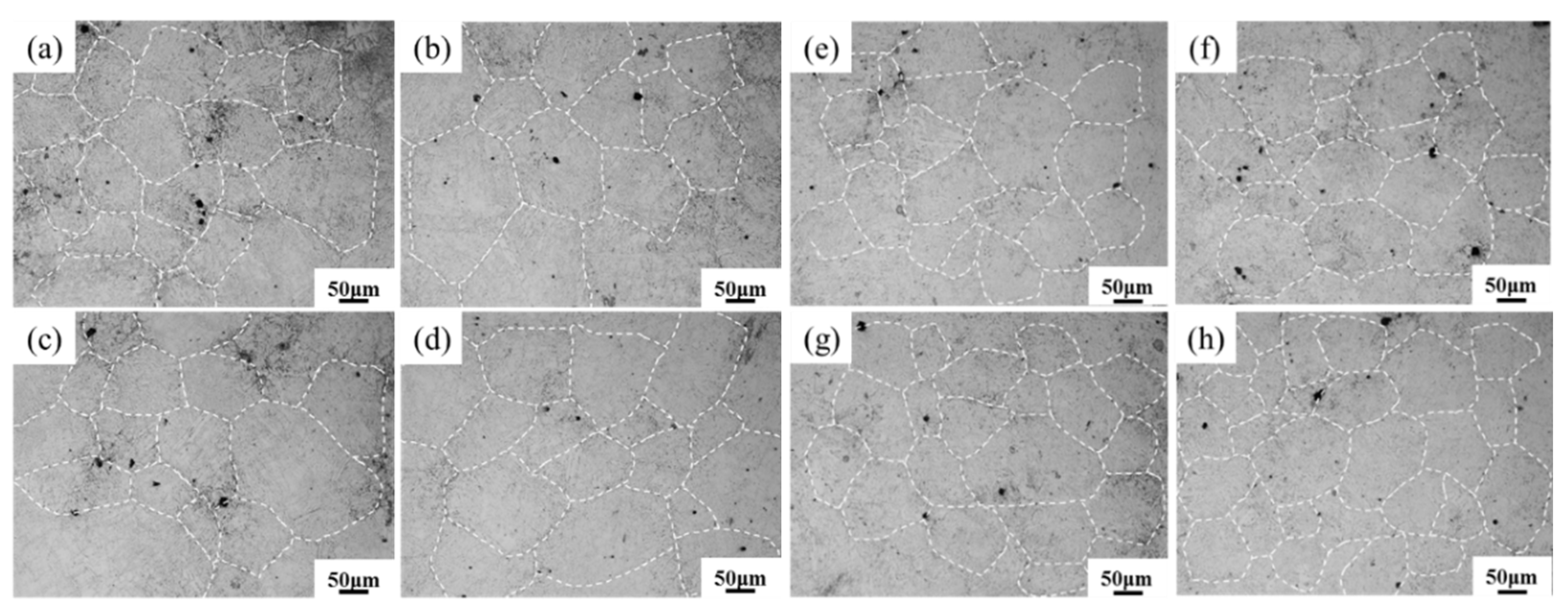
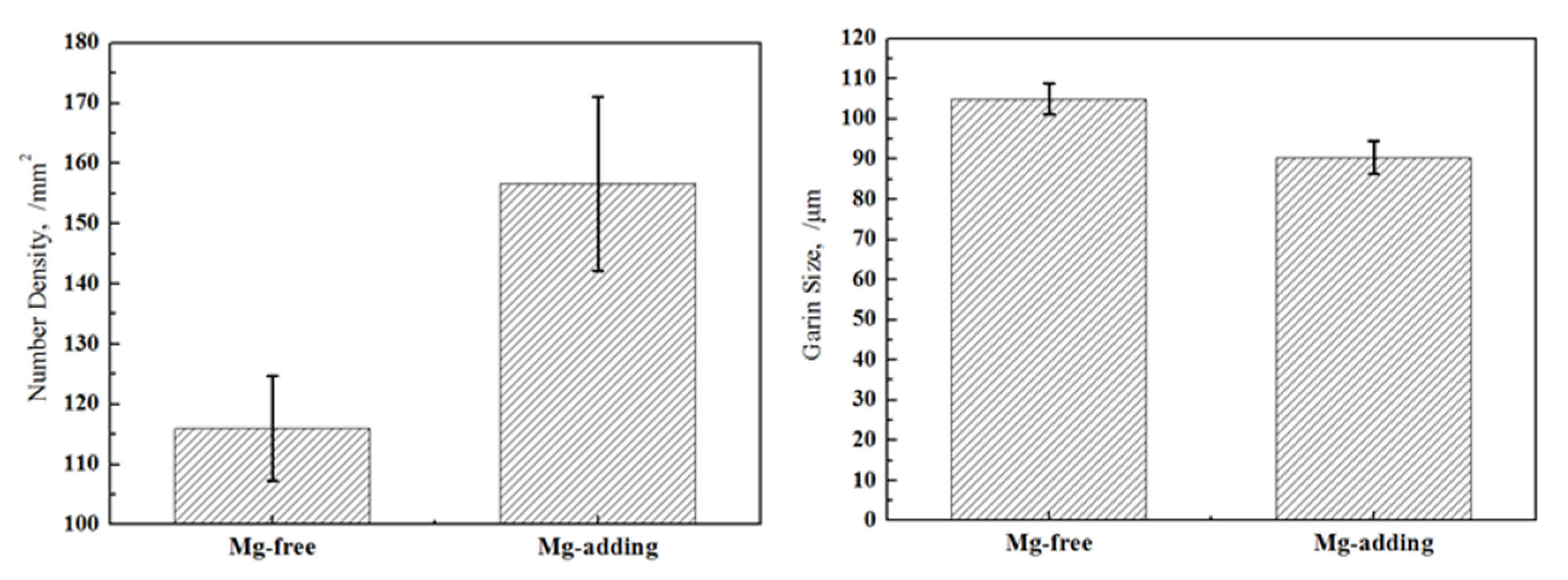
3.2. Solidification Structure Analysis
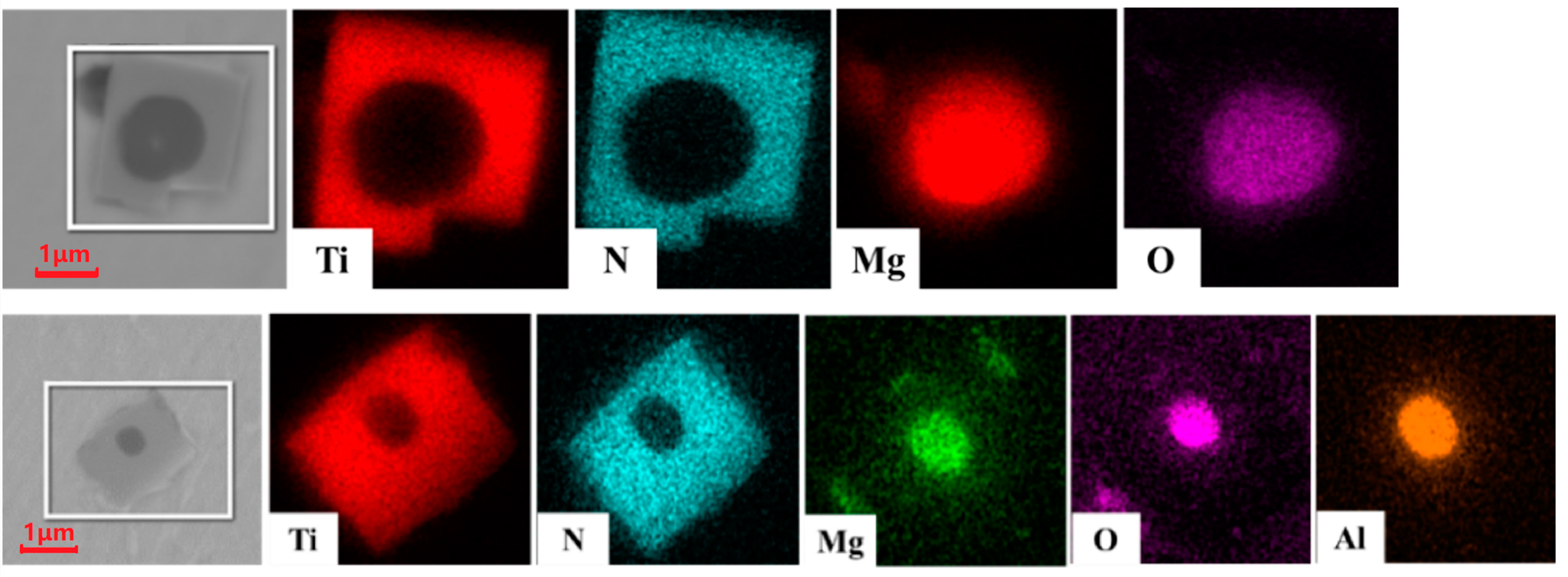
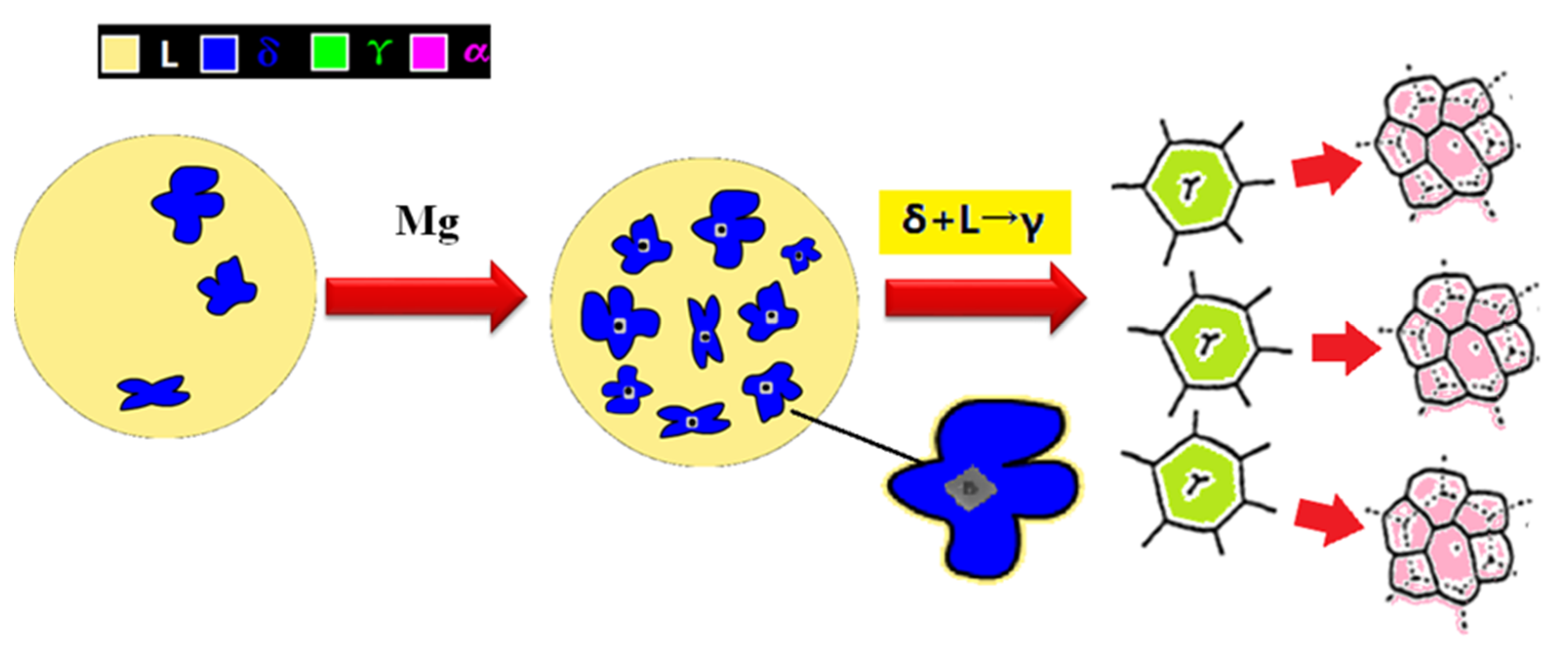
3.3. Appearance Characteristics of Inclusions in Steel
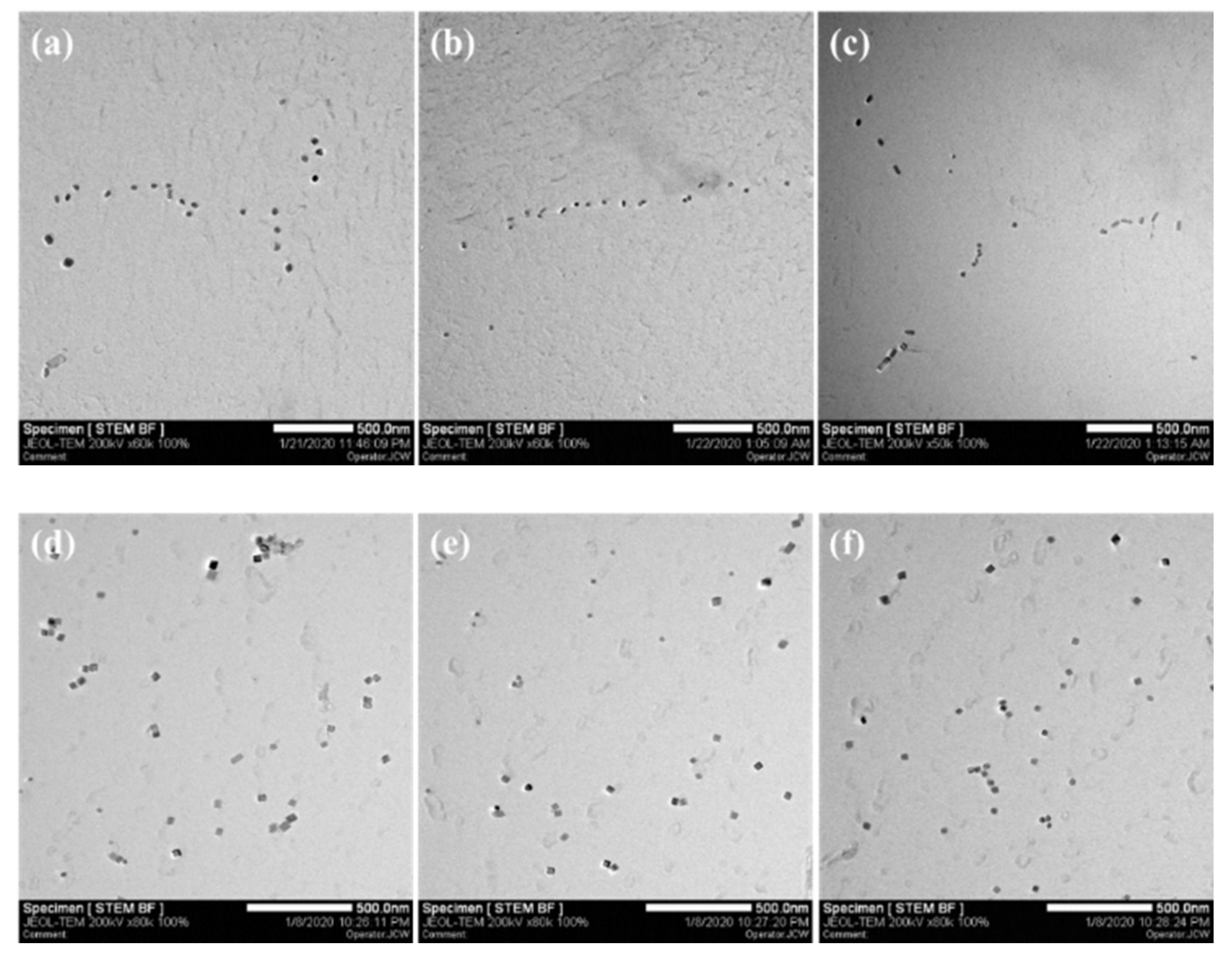
3.4. Precipitation Characteristics of Nano TiN Particles
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Takahashi, T.; Ohsasa, K.; Tanaka, J. Peritectic reaction and delta-gamma transformation mechanism in carbon-steels. Tetsu Hagane 1987, 73, 99–106. [Google Scholar] [CrossRef][Green Version]
- Maruyama, T.; Matsuura, K.; Kudoh, M.; Itoh, Y. Peritectic transformation and austenite grain formation for hyper-peritectic carbon steel. Tetsu Hagane 1999, 85, 585–591. [Google Scholar] [CrossRef]
- Kudoh, M.; Igarashi, K.; Matsuura, K. Peritectic transformation in low carbon steels containing high phosphorus concentration. ISIJ Int. 2008, 48, 334–339. [Google Scholar] [CrossRef][Green Version]
- Chen, H.; Long, M.; Cao, J. Phase transition of peritectic steel Q345 and its effect on the equilibrium partition coefficients of solutes. Metals 2017, 7, 288. [Google Scholar] [CrossRef]
- Guo, J.; Wen, G.; Tang, P. Analysis of crack susceptibility of peritectic steels based on surface roughness. Steel Res. Int. 2019, 91, 376–382. [Google Scholar] [CrossRef]
- Lopez, E.A.; Trejo, M.H.; Mondragon, J.J.R. Effect of C and Mn variations upon the solidification mode and surface cracking susceptibility of peritectic steels. ISIJ Int. 2009, 49, 851–858. [Google Scholar] [CrossRef][Green Version]
- Korojy, B.; Nassar, H.; Fredriksson, H. Hot crack formation during peritectic reaction in steels. Ironmak. Steelmak. 2010, 37, 63–72. [Google Scholar] [CrossRef]
- Trejo, M.H.; Lopez, E.A.; Mondragon, J.J.R. Effect of solidification path and contraction on the cracking susceptibility of carbon peritectic steels. Met. Mater. Int. 2010, 16, 731–737. [Google Scholar] [CrossRef]
- Saleem, S.; Vynnycky, M.; Fredriksson, H. The Influence of peritectic reaction/transformation on crack susceptibility in the continuous casting of steels. Metall. Mater. Trans. B 2017, 48, 1625–1635. [Google Scholar] [CrossRef]
- Suzuki, M.; Hayashi, H.; Shibata, H. Simulation of transverse crack formation on continuously cast peritectic medium carbon steel slabs. Steel Res. Int. 1999, 70, 412–419. [Google Scholar] [CrossRef]
- Nakato, H.; Ozawa, M.; Kinoshita, K. Factors Affecting the formation of shell and longitudinal cracks in mold during high-speed continuous-casting of slabs. Trans. Iron. Steel Instit. Jpn. 1981, 24, 957–965. [Google Scholar] [CrossRef]
- Weisgerber, B.; Harste, K.; Bleck, W. Phenomenological description of the surface morphology and crack formation of continuously cast peritectic steel slabs. Steel Res. Int. 2004, 75, 686–692. [Google Scholar] [CrossRef]
- Li, S.; Zhang, L.; Yang, X. A Study on control of transverse corner cracks on continuously cast hypo-peritectic steel slabs. Steelmaking 2016, 32, 67–72. [Google Scholar]
- Zhu, Z.; Wang, X.; Wang, W. Transverse surface cracks on hypo-peritectic steel slabs and influence factors. Contin. Cast. 2000, 25, 31–36. [Google Scholar]
- Zeng, Y.; Sun, Y.; Ai, X. Effect of brittle zone of hypo-peritectic steel casting slabs on surface cracks. Iron Steel Vanad Titan. 2013, 34, 79–84. [Google Scholar]
- Mondragon, J.R.; Trejo, M.H.; Roman, M.C. Description of the hypo-peritectic steel solidification under continuous cooling and crack susceptibility. ISIJ Int. 2008, 48, 454–460. [Google Scholar] [CrossRef]
- Fedosov, A.; Skrebtsov, A.; Pashchuk, D. Formation of transverse surface cracks during peritectic steel continuous casting. Metallurgist 2018, 62, 39–48. [Google Scholar] [CrossRef]
- Trejo, M.H.; Ruiz, J.; Castro-Roman, M. Star Cracks in continuously cast peritectic steel slabs. Ironmak. Steelmak. 2010, 37, 452–457. [Google Scholar] [CrossRef]
- Maehara, Y.; Yasumoto, K.; Sugitani, Y. Effect of carbon on hot ductility of as-cast low-alloy steels. Tetsu Hagane 1985, 71, 1534–1541. [Google Scholar] [CrossRef]
- Presoly, P.; Pierer, R.; Bernhard, C. Identification of defect prone peritectic steel grades by analyzing high-temperature phase transformations. Metall. Mater. Trans. A 2013, 44, 5377–5388. [Google Scholar] [CrossRef]
- Sarkar, R.; Sengupta, A.; Kumar, V. Effects of alloying elements on the ferrite potential of peritectic and ultra-low carbon steels. ISIJ Int. 2015, 55, 781–790. [Google Scholar] [CrossRef][Green Version]
- Gao, Z.; Zhang, X.; Yao, S. An experimental study on formation mechanism of cracks on peritectic steel casting slabs. J. Iron Steel Res. 2009, 21, 8–11. [Google Scholar]
- Ma, Q. Continuous casting process design and transverse cracks control of low-carbon peritectic steel. Steelmaking 2000, 16, 28–30. [Google Scholar]
- Cao, L. Transverse surface cracks on continuously cast peritectic steel slabs and selection of performance of mould flux. Iron Steel 2015, 50, 38–42. [Google Scholar]
- Zhu, L. Theoretical Research and Application of a Highly Alkaline Mould Flux for Peritectic Steel; Chongqing University: Chongqing, China, 2018. [Google Scholar]
- Ito, Y.; Kato, T.; Yamanaka, A.; Watanabe, T. Improvement of Hot Ductility in Continuously Cast Strand by Ferrite Precipitation Control. Tetsu Hagane 2003, 89, 19–26. [Google Scholar]
- Kimura, K.; Fukumoto, S.; Shigesato, G.-I.; Takahashi, A. Effect of Mg Addition on Equiaxed Grain Formation in Ferritic Stainless Steel. ISIJ Int. 2013, 53, 2167–2175. [Google Scholar] [CrossRef]
- Fujimura, H.; Tsuge, S.; Komizo, Y.; Nishizawa, T. Effect of Oxide Composition on Solidification Structure of Ti added Ferritic Stainless Steel. Tetsu Hagane 2001, 87, 707–712. [Google Scholar] [CrossRef]






| Element | C | Si | Mn | P | S | Al | Ti | Mg | O | N |
|---|---|---|---|---|---|---|---|---|---|---|
| Before Mg treatment | 0.13 | 0.02 | 0.34 | 0.01 | 0.009 | 0.0226 | 0.020 | 0 | 0.0032 | 0.0041 |
| After Mg treatment | 0.12 | 0.03 | 0.36 | 0.01 | 0.007 | 0.0235 | 0.018 | 0.0015 | 0.0035 | 0.0039 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, T.; Wang, D.; Wang, H.; Hou, D.; Tian, J. Effect of Magnesium Treatment on the Hot Ductility of Ti-Bearing Peritectic Steel. Metals 2020, 10, 1282. https://doi.org/10.3390/met10101282
Qu T, Wang D, Wang H, Hou D, Tian J. Effect of Magnesium Treatment on the Hot Ductility of Ti-Bearing Peritectic Steel. Metals. 2020; 10(10):1282. https://doi.org/10.3390/met10101282
Chicago/Turabian StyleQu, Tianpeng, Deyong Wang, Huihua Wang, Dong Hou, and Jun Tian. 2020. "Effect of Magnesium Treatment on the Hot Ductility of Ti-Bearing Peritectic Steel" Metals 10, no. 10: 1282. https://doi.org/10.3390/met10101282
APA StyleQu, T., Wang, D., Wang, H., Hou, D., & Tian, J. (2020). Effect of Magnesium Treatment on the Hot Ductility of Ti-Bearing Peritectic Steel. Metals, 10(10), 1282. https://doi.org/10.3390/met10101282








