Nucleation and Condensation of Magnesium Vapor in Argon Carrier
Abstract
:1. Introduction
2. Experimental Method
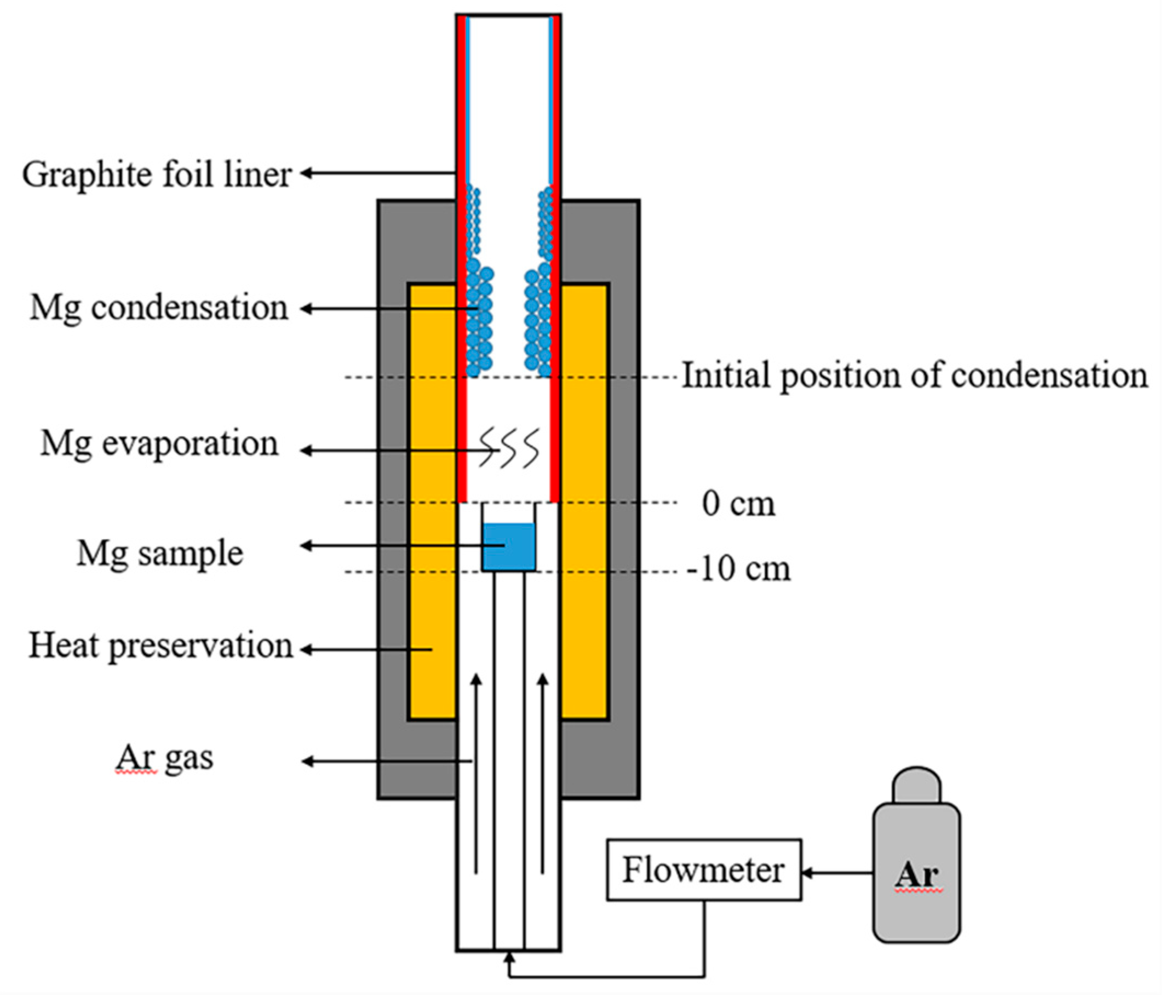
2.1. Experimental Setup
2.2. Experimental Procedure
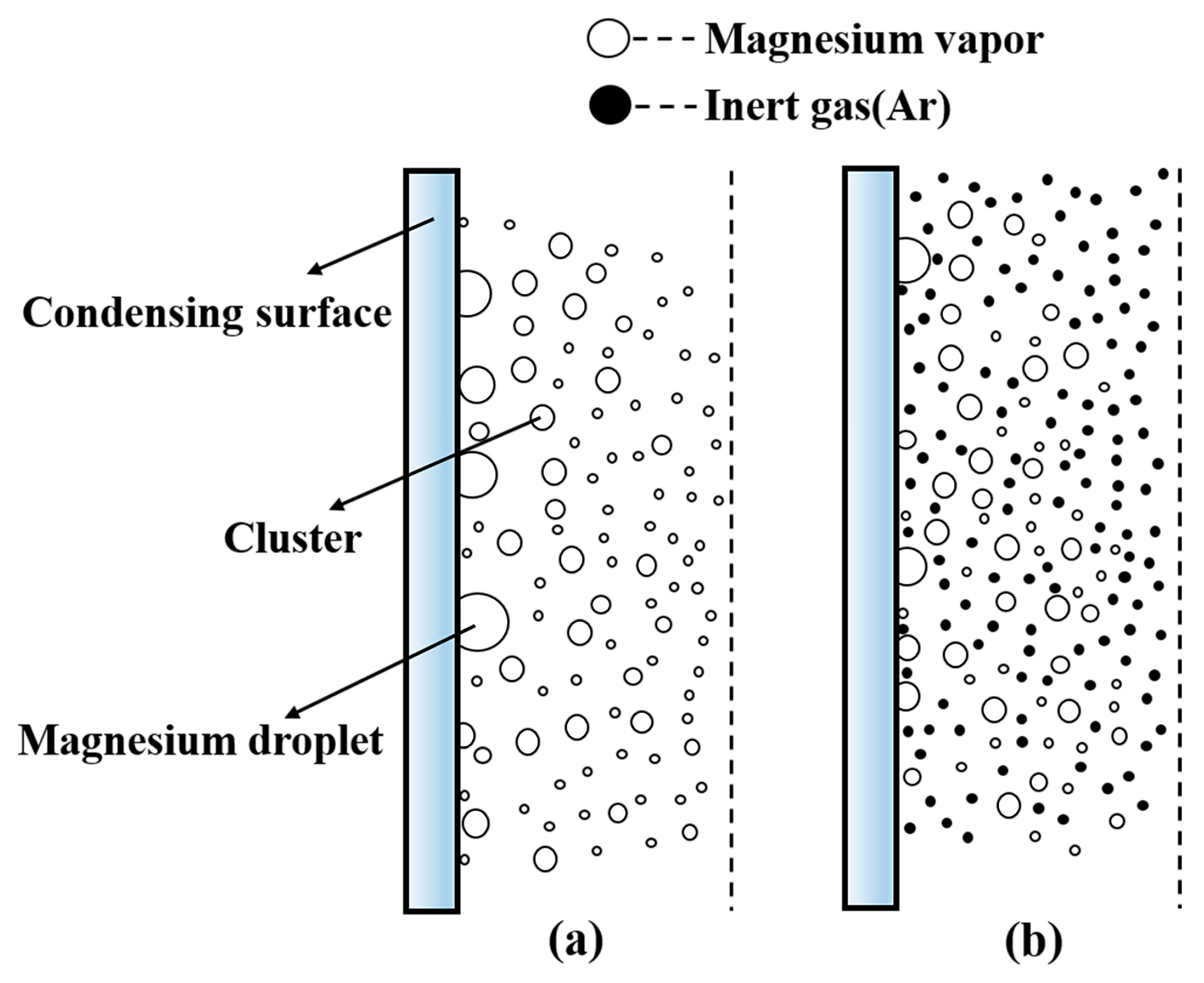
2.3. Physical Model
3. Results and Discussion
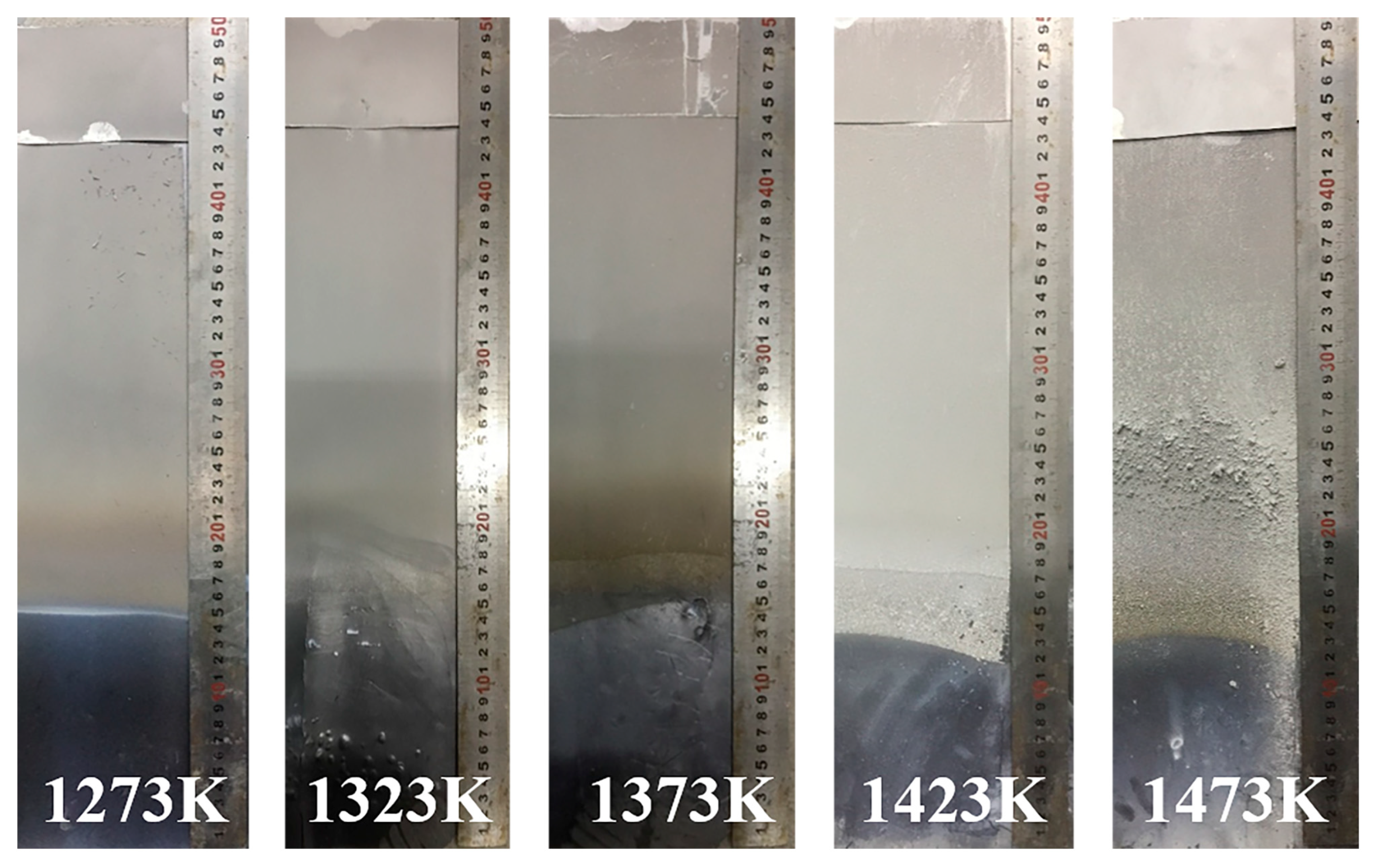
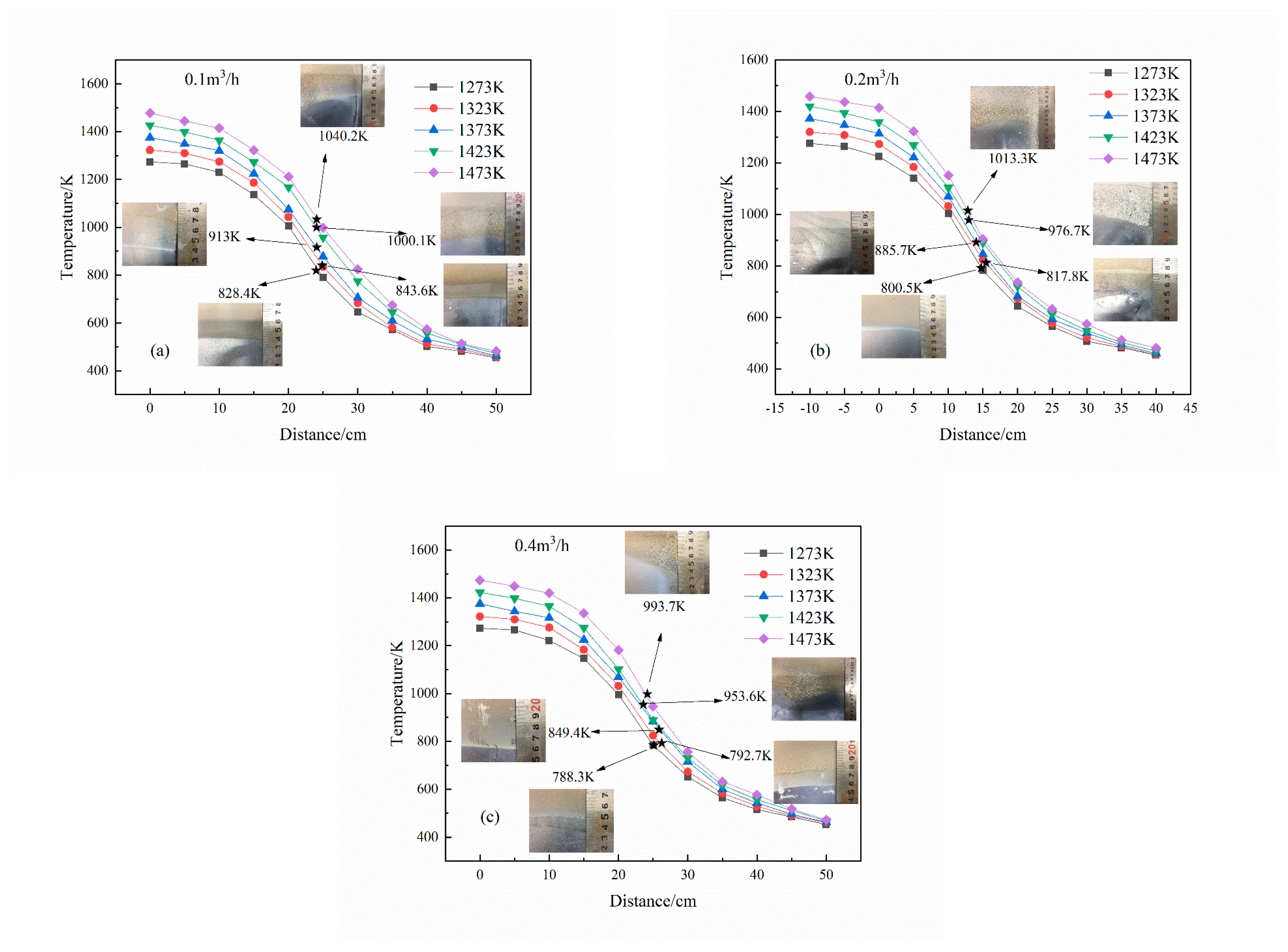
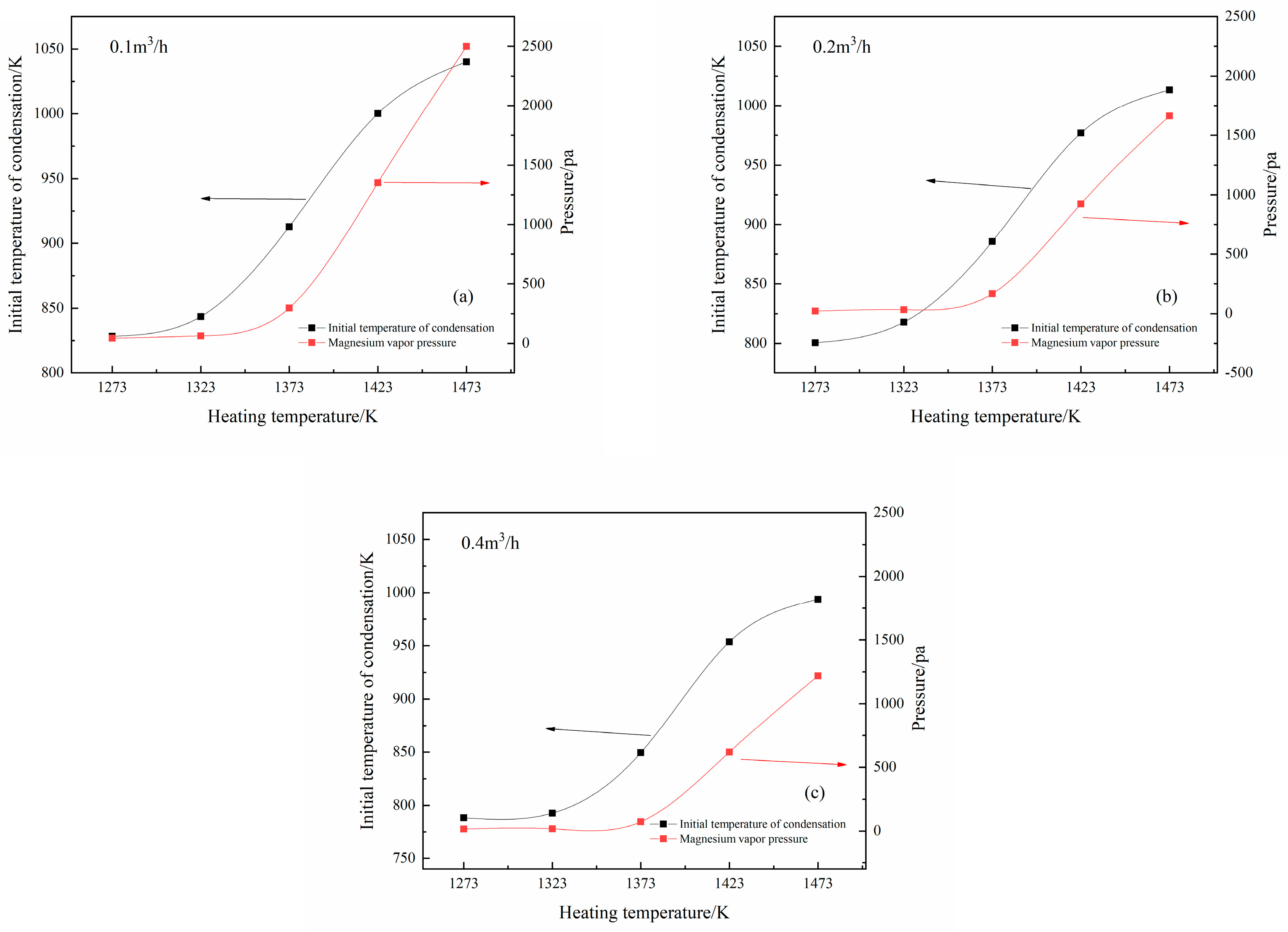
3.1. Condensation of Mg Vapor at Different Temperatures and Gas Flow Rates
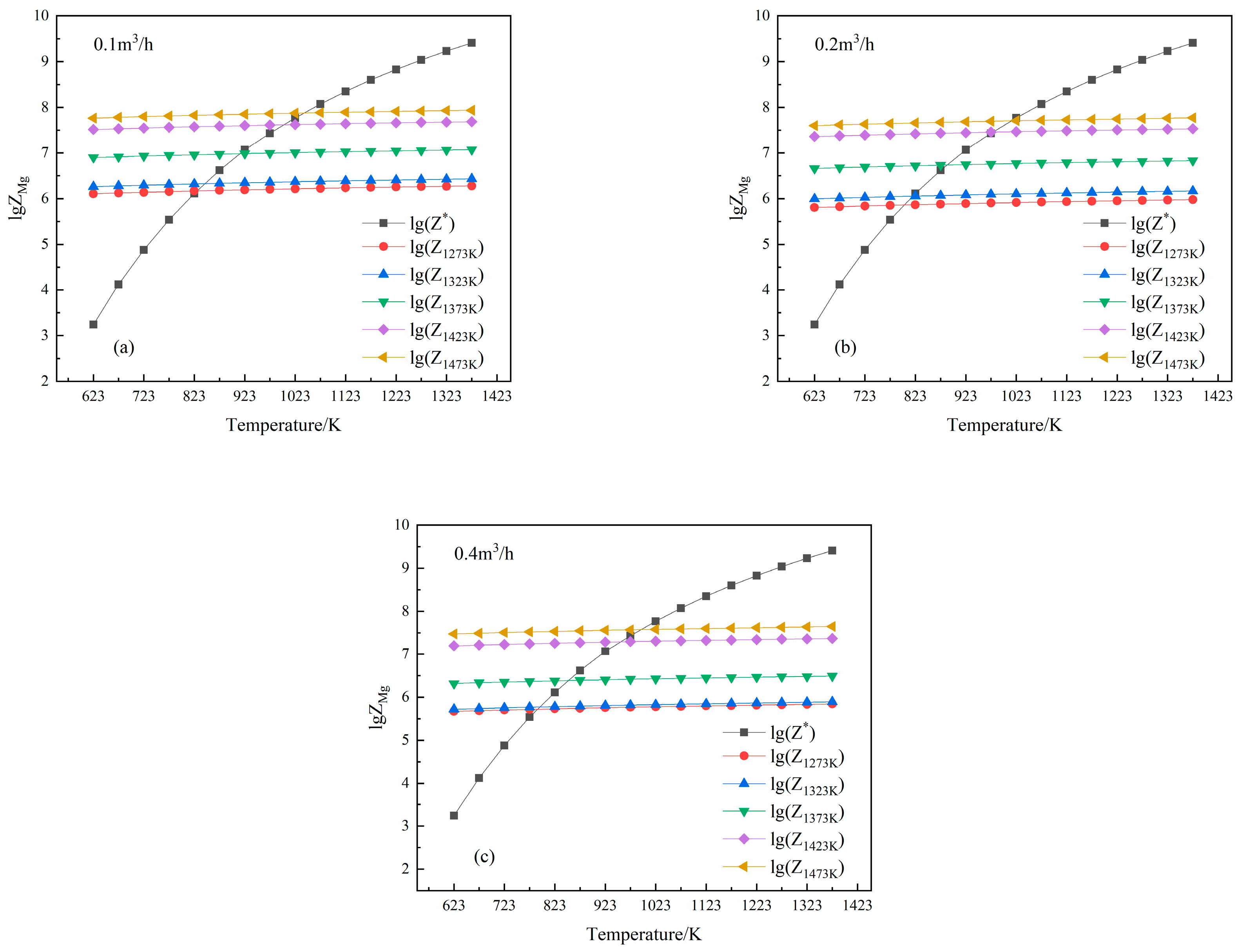
3.2. Effects of Partial Pressure on Mg Vapor Condensation Nucleation
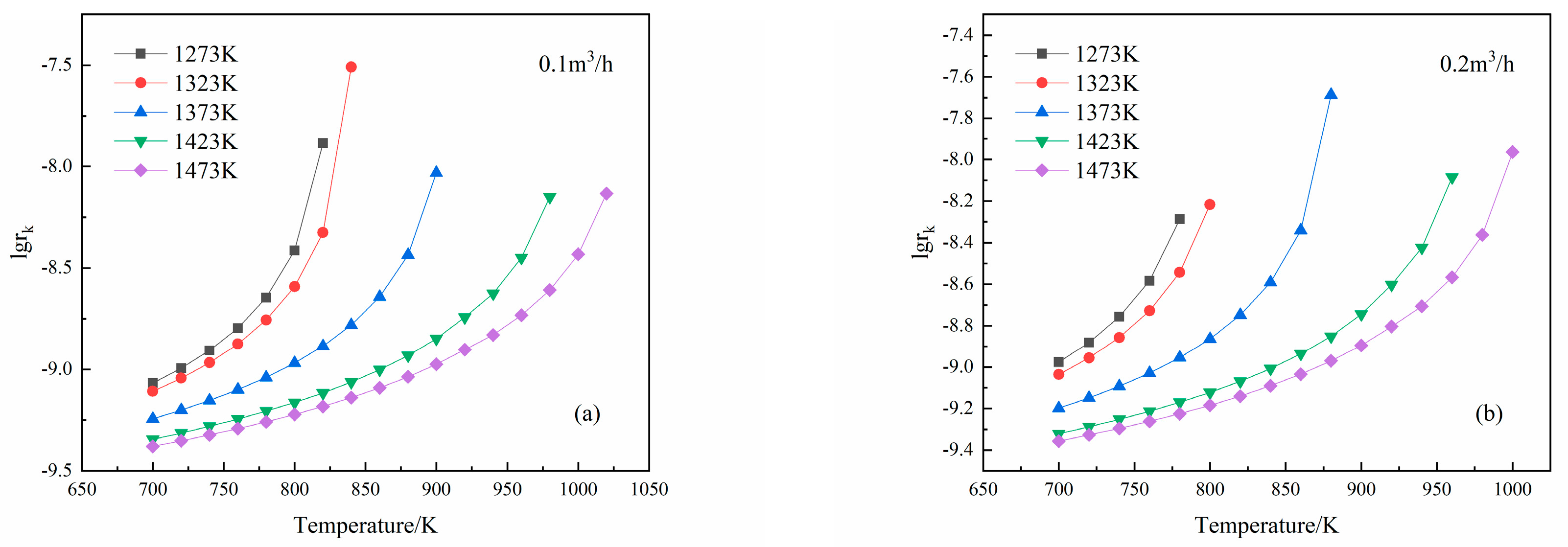
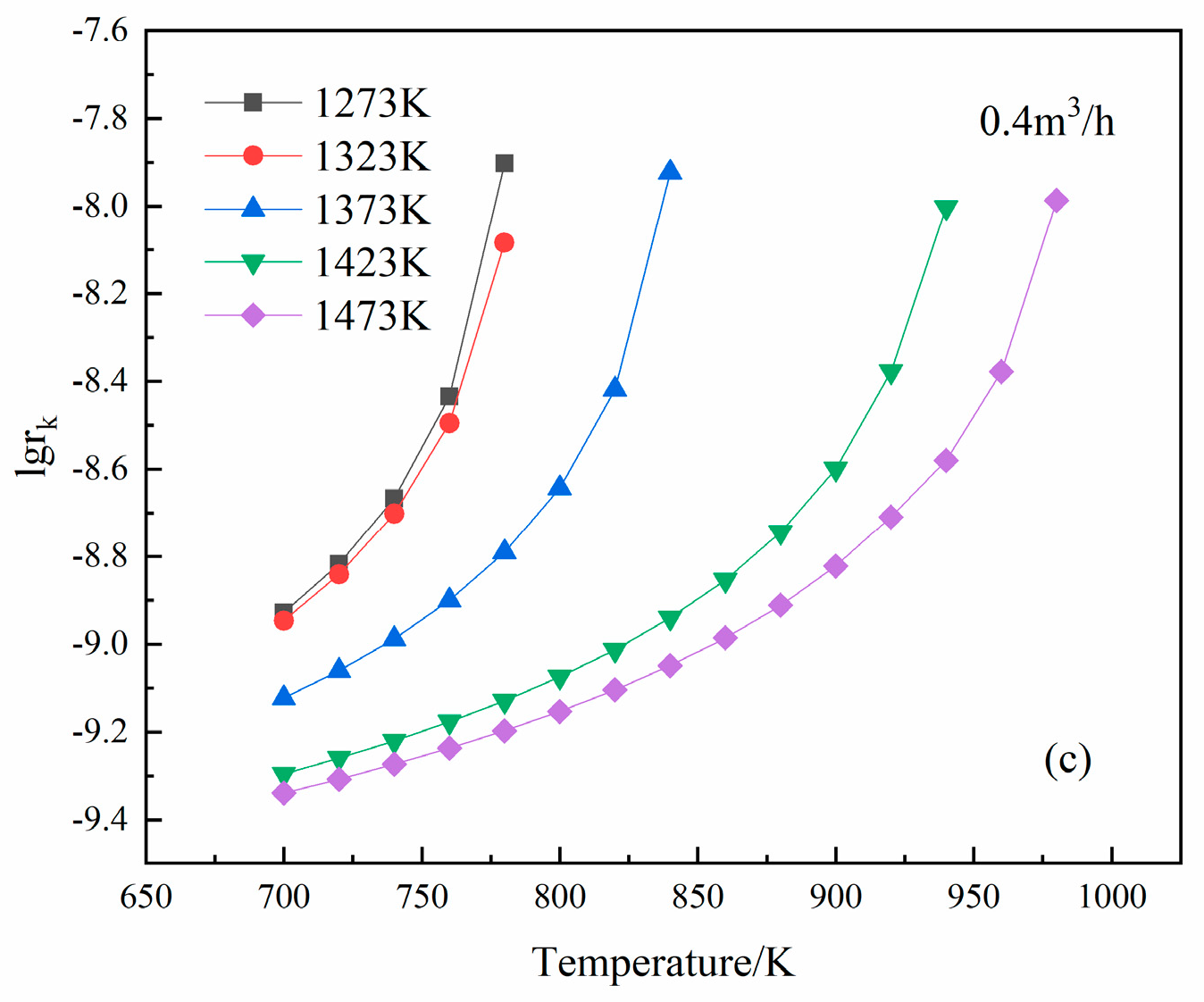
3.3. Effects of Temperature on Mg Vapor Nucleation
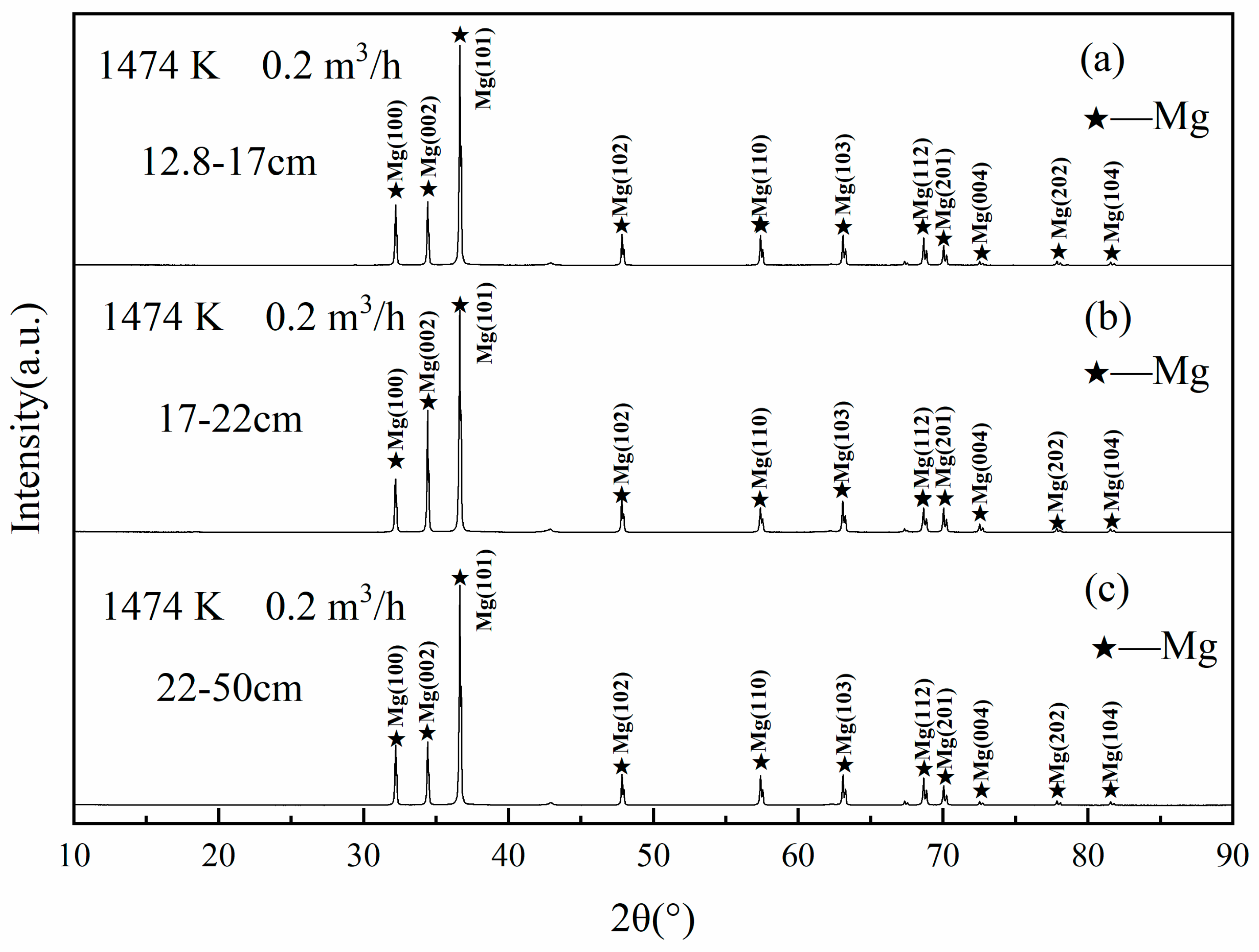
3.4. Condensation Phenomenon and SEM Analysis at Ar Flow Rate of 0.2 m3/h and Heat Source Temperature of 1473 K
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Joost, W.J.; Krajewski, P.E. Towards magnesium alloys for high-volume automotive applications. Scr. Mater. 2016, 128, 107–112. [Google Scholar] [CrossRef]
- Karakulak, E. A review: Past, present and future of grain refining of magnesium castings. J. Magnes. Alloys 2019, 7, 355–369. [Google Scholar] [CrossRef]
- Fu, D.; Ji, Z.; Guo, J.; Han, J.; Dou, Z.; Liu, Y.; Zhang, T.; Guan, L. Diffusion and phase transformations during the reaction between ferrosilicon and CaO·MgO under vacuum. J. Mater. Res. Technol. 2020, 9, 4379–4385. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, B.; Yang, C.; Yang, B.; Qu, T.; Liu, H.; Dai, Y.; Liu, D. Analysis of magnesia carbothermic reduction process in vacuum. Metall. Mater. Trans. B 2014, 45, 1936–1941. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, B.; Yang, B.; Yang, C.; Qu, T.; Liu, D.; Dai, Y. Magnesium production by carbothermic reduction in vacuum. J. Magnes. Alloys 2015, 3, 149–154. [Google Scholar] [CrossRef] [Green Version]
- Halmann, M.; Frei, A.; Steinfeld, A. Magnesium production by the pidgeon process involving dolomite calcination and MgO silicothermic reduction: Thermodynamic and environmental analyses. Ind. Eng. Chem. Res. 2008, 47, 2146–2154. [Google Scholar] [CrossRef]
- United States Geological Survey. Magnesium Statistics and Information. 2020. Available online: https://www.usgs.gov/centers/nmic/magnesium-statistics-and-information (accessed on 3 May 2020).
- Gao, F.; Nie, Z.; Wang, Z.; Gong, X.; Zuo, T. Assessing environmental impact of magnesium production using Pidgeon process in China. Trans. Nonferr. Met. Soc. 2008, 18, 749–754. [Google Scholar] [CrossRef]
- Li, R.; Zhang, S.; Guo, L.; Wei, J. Numerical study of magnesium (Mg) production by the Pidgeon process: Impact of heat transfer on Mg reduction process. J. Heat Mass Transf. 2013, 59, 328–337. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.; Zhang, S.; Guo, L. Experimental and numerical studies on a one-step method for the production of Mg in the silicothermic reduction process. Int. J. Miner. Process. 2015, 54, 8883–8892. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.; Zhang, S.; Guo, L. The effects of hydration activity of calcined dolomite (HCD) on the silicothermic reduction process. Int. J. Miner. Process. 2015, 142, 154–160. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, C.; Zhang, S.; Guo, L. The effect of CaF2 on the magnesium production with silicothermal process. Int. J. Miner. Process. 2015, 142, 147–153. [Google Scholar] [CrossRef]
- Li, R.; Zhang, C.; Zhang, S.; Guo, L. Experimental and numerical modeling studies on production of Mg by vacuum silicothermic reduction of CaO·MgO. Metall. Mater. Trans. B 2014, 45, 236–250. [Google Scholar] [CrossRef]
- Fu, D.; Feng, N.; Wang, Y. Study on the kinetics and mechanism of grain growth during the thermal decomposition of magnesite. Bull. Korean Chem. Soc. 2012, 33, 2483–2488. [Google Scholar] [CrossRef] [Green Version]
- Fu, D.; Wang, Y.; Peng, J.; Di, Y.; Tao, S.; Feng, N. Mechanism of extracting magenesium from mixture of calcined magnesite and calcined dolomite by vacuum aluminothermic reduction. Trans. Nonferr. Met. Soc. 2014, 24, 2677–2686. [Google Scholar] [CrossRef]
- Fu, D.; Zhang, T.; Guan, L.; Dou, Z.; Wen, M. Magnesium production by silicothermic reduction of dolime in pre-prepared dolomite pellets. JOM 2016, 68, 3208–3213. [Google Scholar] [CrossRef]
- Fu, D.; Feng, N.; Wang, Y.; Peng, J.; Di, Y. Kinetics of extracting magnesium from mixture of calcined magnesite and calcined dolomite by vacuum aluminothermic reduction. Trans. Nonferr. Met. Soc. 2014, 24, 839–847. [Google Scholar] [CrossRef]
- Fu, D.; Zhang, T.; Dou, Z.; Guan, L. A new energy-efficient and environmentally friendly process to produce magnesium. Can. Metall. Quar. 2017, 56, 418–425. [Google Scholar] [CrossRef]
- Morsi, I.M.; Ali, H.H. Kinetics and mechanism of silicothermic reduction process of calcined dolomite in magnetherm reactor. Int. J. Miner. Process. 2014, 127, 37–43. [Google Scholar] [CrossRef]
- Morsi, I.M.; Barawy, K.; Morsi, M.B. Abdel-gawad SR. silicothermic reduction of dolomite ore under inert atmosphere. Can. Metall. Quar. 2002, 41, 15–28. [Google Scholar] [CrossRef]
- Wulandari, W.; Brooks, G.A.; Rhamdhani, M.A.; Monaghan, B.J. Kinetic analysis of silicothermic process under flowing argon atmosphere. Can. Metall. Quar. 2014, 53, 17–25. [Google Scholar] [CrossRef]
- Che, Y.; Hao, Z.; Zhu, J.; Fu, Z.; He, J.; Song, J. Kinetic mechanism of magnesium production by silicothermy in argon flowing. Thermochim. Acta 2019, 681, 178397. [Google Scholar] [CrossRef]
- Zhang, T.; Dou, Z.; Zhang, Z.; Liu, Y.; Lv, G.; He, J.C. Method for Smelting Magnesium Quickly and Continuously. U.S. Patent 10047413, 14 August 2018. [Google Scholar]
- Yang, C.; Tian, Y.; Qu, T.; Yang, B.; Xu, B.; Dai, Y. Magnesium vapor nucleation in phase transitions and condensation under vacuum conditions. Trans. Nonferr. Met. Soc. 2014, 24, 561–569. [Google Scholar] [CrossRef]
- Xiong, N.; Tian, Y.; Yang, B.; Xu, B.; Liu, D.; Dai, Y. Volatilization and condensation behaviours of Mg under vacuum. Vacuum 2018, 156, 463–468. [Google Scholar] [CrossRef]
- Xiong, N.; Tian, Y.; Yang, B.; Xu, B.; Dai, T.; Dai, Y. Results of recent investigations of magnesia carbothermal reduction in vacuum. Vacuum 2019, 160, 213–225. [Google Scholar] [CrossRef]
- Xiong, N.; Tian, Y.; Chen, X.; Li, K.; Xu, B.; Yang, B.; Dai, Y. Dynamic simulation and experimental study of magnesia formed between magnesium vapor and CO under vacuum. JOM 2019, 71, 2791–2797. [Google Scholar] [CrossRef]
- Yang, C.; Tian, Y.; Qu, T.; Yang, B.; Xu, B.; Dai, Y. Production of magnesium during carbothermal reduction of magnesium oxide by differential condensation of magnesium and alkali vapours. J. Magnes. Alloys 2013, 1, 323–329. [Google Scholar] [CrossRef]
- Izmailov, A.F.; Myerson, A.S.; Arnold, S. A statistical understanding of nucleation. J. Cryst. Growth 1999, 196, 234–242. [Google Scholar] [CrossRef]
- Leubner, I.H. Particle nucleation and growth models. Curr. Opin. Colloid Interface Sci. 2000, 5, 151–159. [Google Scholar] [CrossRef]
- Terry, A.R. Nano-sized cluster nucleation. Adv. Colloid Interface Sci. 2001, 91, 473–499. [Google Scholar]
- Lummen, N.; Kraska, T. Homogeneous nucleation of iron from supersaturated vapor investigated by molecular dynamics simulation. J. Aerosol Sci. 2005, 36, 1409–1426. [Google Scholar] [CrossRef]
- Hischier, I.; Chubukov, B.A.; Wallace, M.A.; Fisher, R. A novel experimental method to study metal vapor condensation/oxidation: Mg in CO and CO2 at reduced pressures. Sol. Energy 2016, 139, 389–397. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y. Vacuum Metallurgy of Nonferrous Metals; Metallurgical Industry: Beijing, China, 2000. [Google Scholar]
- Haynes, W.M. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Zhang, R.; Khalizov, A.; Wang, L.; Hu, M.; Xu, W. Nucleation and growth of nanoparticles in the atmosphere. Chem. Rev. 2012, 112, 1957–2011. [Google Scholar] [CrossRef]
- Xu, Z. Material Thermodynamics; Higher Education Press: Beijing, China, 2009. [Google Scholar]
- Tadros, T. Encyclopedia of Colloid and Interface Science; Springer: Berlin, Germany, 2013. [Google Scholar]
- Bohdansky, J.; Schins, H. Surface tension and density of the liquid earth alkaline metals Mg, Ca, Sr, Ba. J. Inorg. Nucl. Chem. 1968, 30, 2331–2337. [Google Scholar] [CrossRef]
- Mcgonigal, P.J.; Kirshenbaum, A.D.; Grosse, A.V. The liquid temperature range, density, and critical constants of magnesium. J. Phys. Chem. 1962, 66, 737–740. [Google Scholar] [CrossRef]



| A | B | C | D | Temperature Range (K) |
|---|---|---|---|---|
| −7780 | −0.86 | - | 13.54 | 298–923 |
| −7550 | −1.411 | - | 14.92 | 923–1393 |
| T/K | rk/m | N |
|---|---|---|
| 1000 | 1.08543 × 10−8 | 2.08105 × 105 |
| 980 | 4.33604 × 10−9 | 1.3311 × 104 |
| 960 | 2.70944 × 10−9 | 3.259 × 103 |
| 940 | 1.97041 × 10−9 | 1.258 × 103 |
| 920 | 1.57239 × 10−9 | 6.41 × 102 |
| 900 | 1.27381 × 10−9 | 3.42 × 102 |
| T/K | rk/m |
|---|---|
| 770 | 1.02 × 10−8 |
| 790 | 2.95 × 10−9 |
| Composition/(Mass Percent) | ||||
|---|---|---|---|---|
| No. | Mg | O | C | Ca |
| A | 88.3 | 1.6 | 10.1 | - |
| B | 52.7 | 3.6 | 43.7 | - |
| C | 90.9 | 0.7 | 8.4 | - |
| D | 88.9 | 0.8 | 10.3 | - |
| E | 45.3 | 14.4 | 39.5 | 0.8 |
| F | 93.7 | 0.5 | 5.8 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.; Fu, D.; Guo, J.; Ji, Z.; Dou, Z.; Zhang, T. Nucleation and Condensation of Magnesium Vapor in Argon Carrier. Metals 2020, 10, 1441. https://doi.org/10.3390/met10111441
Han J, Fu D, Guo J, Ji Z, Dou Z, Zhang T. Nucleation and Condensation of Magnesium Vapor in Argon Carrier. Metals. 2020; 10(11):1441. https://doi.org/10.3390/met10111441
Chicago/Turabian StyleHan, Jibiao, Daxue Fu, Junhua Guo, Zonghui Ji, Zhihe Dou, and Ting’an Zhang. 2020. "Nucleation and Condensation of Magnesium Vapor in Argon Carrier" Metals 10, no. 11: 1441. https://doi.org/10.3390/met10111441




