Microstructure and Properties of Copper–Graphite Composites Fabricated by Spark Plasma Sintering Based on Two-Step Mixing
Abstract
:1. Introduction
2. Experiment
2.1. Materials and Preparation of Composites Powder
2.2. Fabrication of Copper and Graphite Composite
2.3. Characterization of CGC
3. Results and Discussion
3.1. Characterization of CGC Powder
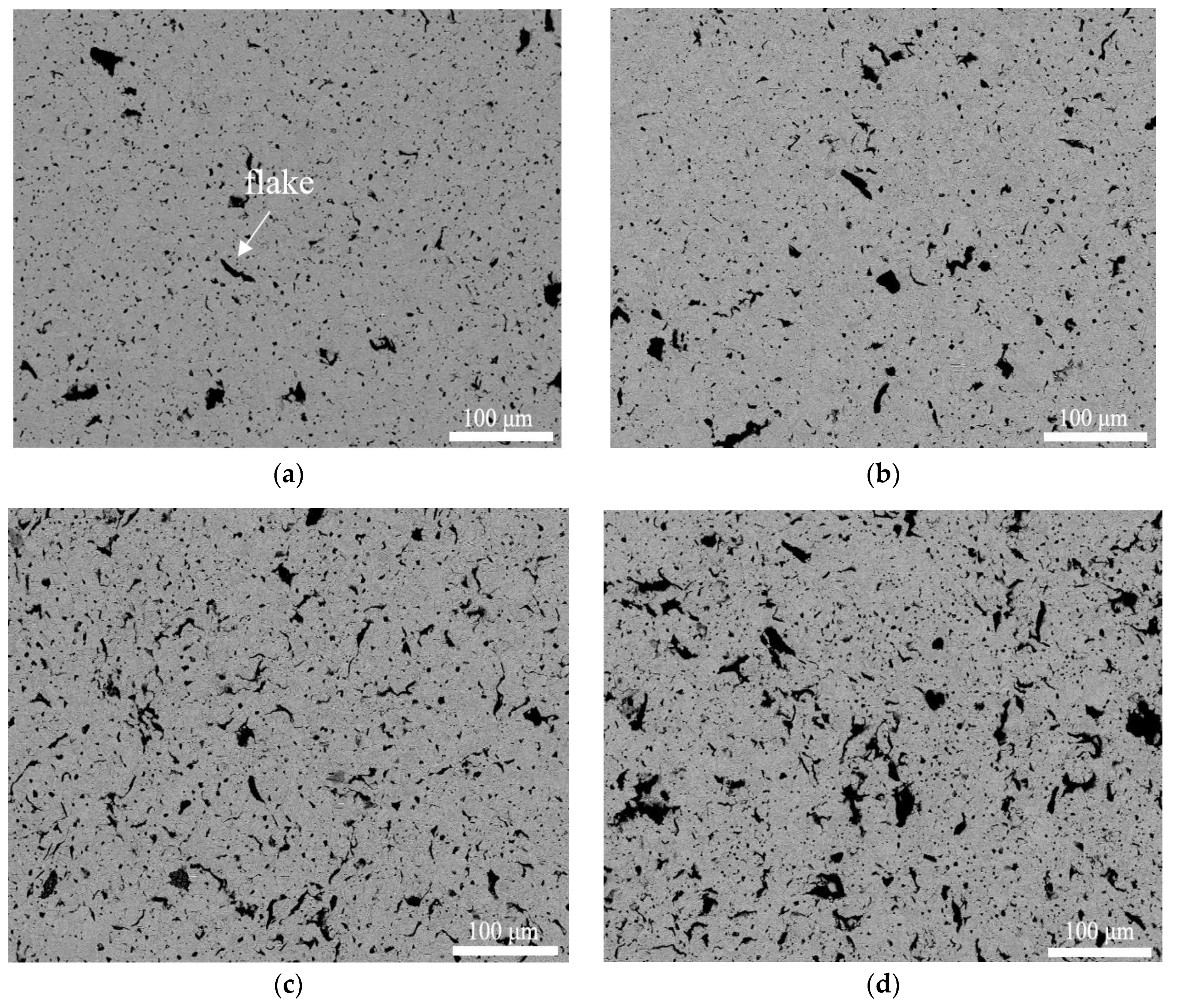
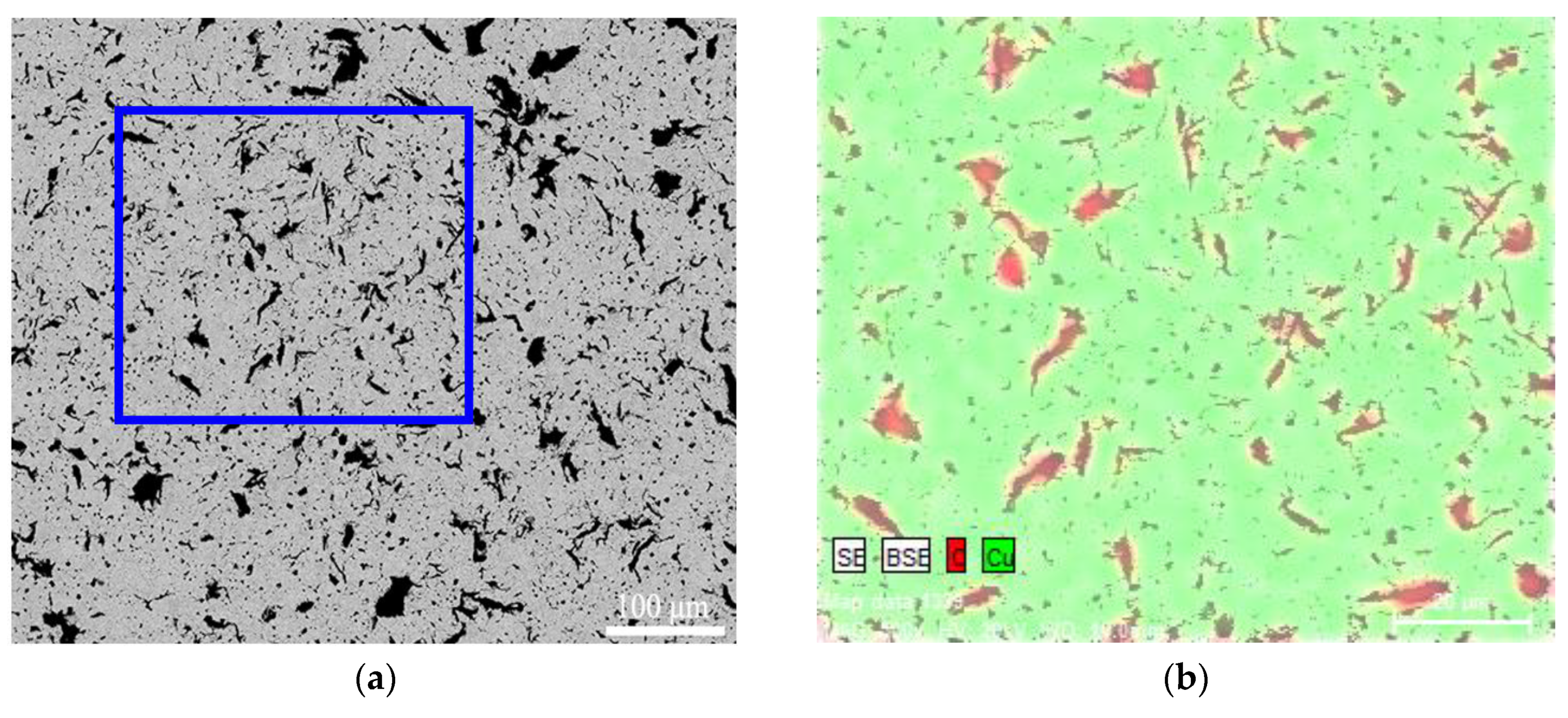
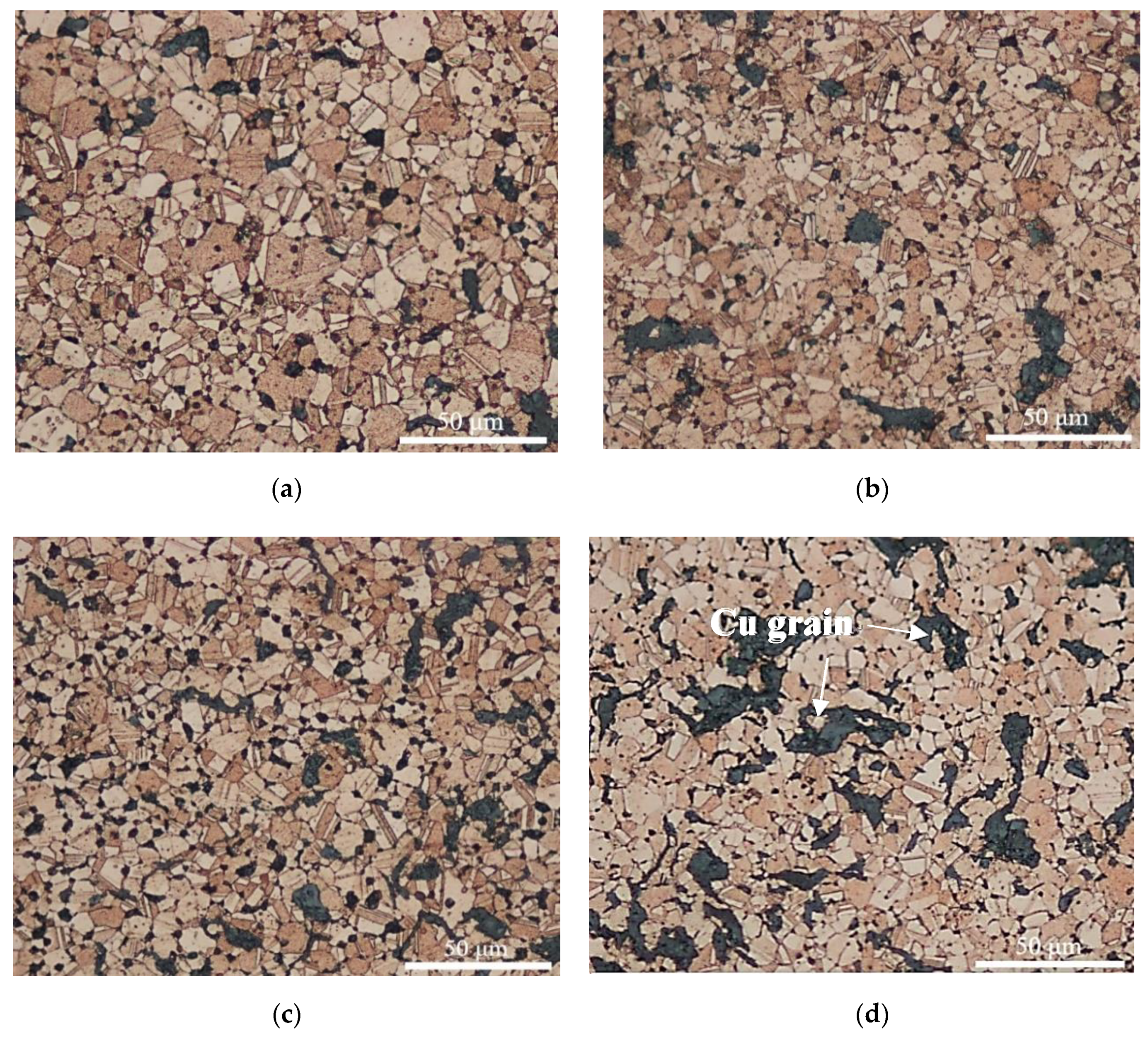
3.2. Microstructure of CGC
3.3. Effect of the Graphite Content on Properties of CGC
3.3.1. Relative Density
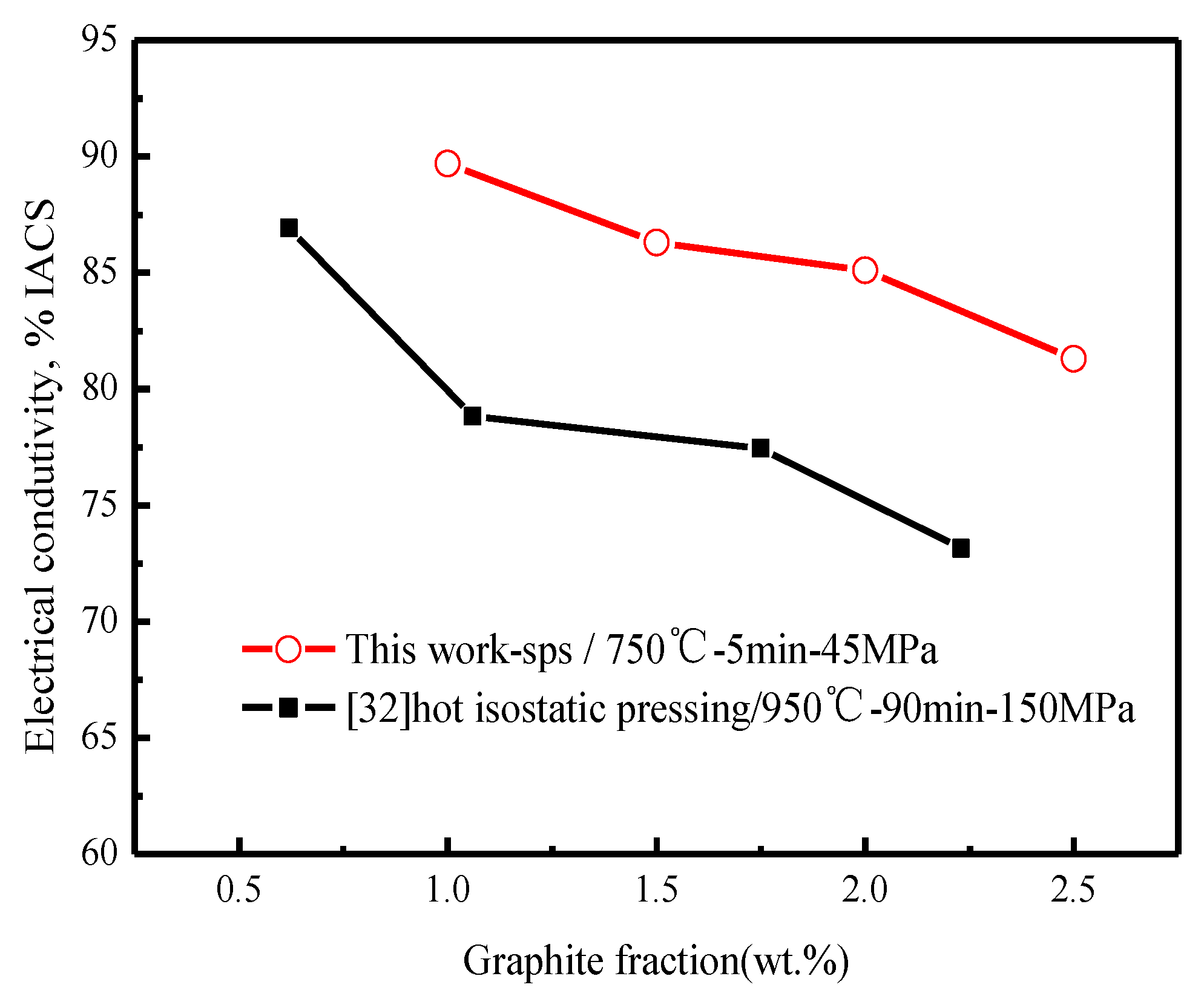
3.3.2. Electrical Conductivity
3.3.3. Micro-Hardness
3.3.4. Friction and Wear Characteristics
4. Conclusions
- (1)
- During ball milling, Cu powders were rolled into Cu flakes and its size decreased with the increase in graphite content.
- (2)
- The equiaxed grain size of CGC prepared by SPS reduced slightly when adding more graphite powders and the graphite powders were mainly distributed at grain boundaries with the shape of granular and flake.
- (3)
- Compared with other conventional methods, the CGC prepared by two-step mixing and SPS achieved higher relative density, electrical conductivity, and micro-hardness.
- (4)
- As the graphite content increases, the friction coefficient and wear rate of the composite decreases. When the graphite content of CGC was low, the main wear mechanism was plastic deformation, delamination, adhesive and fatigue wear. The adhesive and fatigue wear disappeared gradually with the increasing of graphite content.
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, P.; Zhang, L.; Wei, D.B.; Wu, P.F.; Cao, J.W.; Shijia, C.R.; Qu, X.H.; Fu, K.X. Effect of graphite type on the contact plateaus and friction properties of copper-based friction material for high-speed railway train. Wear 2019, 432, 202927. [Google Scholar]
- Singh, M.K.; Gautam, P.K. Mechanical and electrical behaviour of developed copper based hybrid composites. Mater. Today Proc. 2018, 5, 5692–5700. [Google Scholar]
- Kumar, R.A.; Kumar, K.K.; Radhika, N. Mechanical and wear properties of functionally graded Cu-11Ni-4Si/graphite composite. Silicon 2019, 11, 2613–2624. [Google Scholar]
- Nageswaran, G.; Natarajan, S.; Ramkumar, K.R. Synthesis, structural characterization, mechanical and wear behaviour of Cu-TiO2-Gr hybrid composite through stir casting technique. J. Alloys Compd. 2018, 768, 733–741. [Google Scholar]
- Mahdi, F.M.; Razooqi, R.N.; Irhayyim, S.S. The influence of graphite content and milling time on hardness, compressive strength and wear volume of copper-graphite composites prepared via powder metallurgy. Tikrit J. Eng. Sci. 2017, 24, 47–54. [Google Scholar]
- Zhu, Y.B.; Bai, H.; Xue, C.; Zhou, R.; Xu, Q.F.; Tao, P.F.; Wang, C.; Wang, J.W.; Jiang, N. Thermal conductivity and mechanical properties of a flake graphite/Cu composite with a silicon nanolayer on a graphite surface. RSC Adv. 2016, 6, 98190–98196. [Google Scholar]
- Meher, A.; Chaira, G. Effect of graphite and SiC addition into Cu and SiC particle size effect on fabrication of Cu–Graphite–SiC MMC by powder metallurgy. Trans. Indian Inst. Met. 2017, 70, 2047–2057. [Google Scholar]
- Liu, B.; Zhang, D.Q.; Li, X.F.; He, Z.; Guo, X.H.; Liu, Z.J.; Guo, Q.G. Effect of graphite flakes particle sizes on the microstructure and properties of graphite flakes/copper composites. J. Alloys Compd. 2018, 766, 382–390. [Google Scholar]
- Dixit, M.; Srivastava, R. The effect of copper granules on interfacial bonding and properties of the copper-graphite composite prepared by flake powder metallurgy. Adv. Powder Technol. 2019, 30, 3067–3078. [Google Scholar]
- Kumar, J.; Mondal, S. Microstructure and properties of graphite-reinforced copper matrix composites. J. Brazil. Soc. Mech. Sci. Eng. 2018, 40, 196–205. [Google Scholar]
- Pragatheeswaran, A.; Ravi, R.; Bakshi, S.R. Microstructural and morphological changes during ball milling of Copper-Silver-Graphite flake mixtures. Adv. Powder Technol. 2019, 30, 2759–2767. [Google Scholar]
- Zhao, Q.; Gan, X.; Zhou, K. Enhanced properties of carbon nanotube-graphite hybrid-reinforced Cu matrix composites via optimization of the preparation technology and interface structure. Powder Technol. 2019, 355, 408–416. [Google Scholar]
- Chen, J.; Ren, S.; Qu, X. Properties and microstructure of nickel-coated graphite flakes/copper composites fabricated by spark plasma sintering. Carbon 2017, 121, 25–34. [Google Scholar]
- Zhang, R.; He, X.; Chen, H.; Qu, X. Effect of alloying element Zr on the microstructure and properties of graphite flake/Cu composites fabricated by vacuum hot pressing. J. Alloys Compd. 2019, 770, 267–275. [Google Scholar]
- Kovacik, J.; Emmer, S.; Bielek, J. Thermal conductivity of Cu-graphite composites. Int. J. Ther. Sci. 2015, 90, 298–302. [Google Scholar]
- Araia, S.; Osaki, T.; Hirota, M.; Uejima, M. Fabrication of copper/single-walled carbon nanotube composite film with homogeneously dispersed nanotubes by electroless deposition. Mater. Today Commun. 2016, 7, 101–107. [Google Scholar]
- Li, J.; Zhang, L.; Zhou, K. Sliding wear behavior of copper-based composites reinforced with graphene nanosheets and graphite. Trans. Nonferrous Met. Soc. China 2015, 25, 3354–3362. [Google Scholar]
- Singh, G.; Pandey, P.M. Topological ordered copper graphene composite foam: Fabrication and compression properties study. Mater. Lett. 2019, 257, 126712. [Google Scholar]
- Mallakpour, S.; Jarahiyan, A. Utilization of ultrasonic irradiation as a green and effective strategy to prepare poly(N-vinyl-2-pyrrolidone)/modified nano-copper (II) oxide nanocomposites. Ultrason. Sonochem. 2017, 37, 128–135. [Google Scholar]
- Rajkumar, K.; Aravindan, S. Tribological behavior of microwave processed copper-nanographite composites. Tribol. Int. 2013, 57, 282–296. [Google Scholar]
- Sarmadi, H.; Kokabi, A.H.; Reihani, S.M. Seyed Reihani. Friction and wear performance of copper–graphite surface composites fabricated by friction stir processing (FSP). Wear 2013, 304, 1–12. [Google Scholar]
- Dash, K.; Ray, B.C.; Chaira, D. Synthesis and characterization of copper-alumina metal matrix composite by conventional and spark plasma sintering. J. Alloys Compd. 2012, 516, 78–84. [Google Scholar]
- Ogunbiyia, O.F.; Jamiru, T.; Sadiku, E.R.; Adesina, O.T.; Beneke, L.; Adegbola, T.A. Spark plasma sintering of nickel and nickel based alloys: A Review. Proc. Manuf. 2019, 35, 1324–1329. [Google Scholar]
- Chen, B.; Li, Z.; Shen, J.; Li, S.; Jia, L.; Umeda, J.; Kondoh, K.; Li, J.S.; Umeda, K.; Kondoh, J.S. Mechanical properties and strain hardening behavior of aluminum matrix composites reinforced with few-walled carbon nanotubes. J. Alloys Compd. 2020, 826, 154075. [Google Scholar]
- Shkodich, N.F.; Spasova, M.; Farle, M.; Kovalev, D.Y.; Nepapushev, A.A.; Kuskov, K.V.; Vergunova, Y.S.; Scheck, Y.B.; Rogachev, A.S. Structural evolution and magnetic properties of high-entropy CuCrFeTiNi alloys prepared by high-energy ball milling and spark plasma sintering. J. Alloys Compd. 2020, 816, 152611. [Google Scholar]
- Ghayebloo, M.; Alizadeh, P.; Melo, R.M. Fabrication of ZrO2-Bearing lithium-silicate glass-ceramics by pressureless sintering and spark plasma sintering. J. Mech. Behav. Biomed. Mater. 2020, 105, 103709. [Google Scholar]
- Zhang, L.; Zhang, Y.Q.; Jiang, Y.H.; Zhou, R. Mechanical behaviors of porous Ti with high porosity and large pore size prepared by one-step spark plasma sintering technique. Vacuum 2015, 122, 187–194. [Google Scholar]
- Moustafa, S.F.; EI-Badry, S.A.; Sanad, A.M.; Kieback, B. Friction and wear of copper-graphite composites made with Cu-coated and uncoated graphite powders. Wear 2002, 253, 699–710. [Google Scholar]
- Zou, H.H.; Ran, X.; Zhu, W.W.; Wang, Y.; Zhan, S.Q.; Hao, Z.K. Tribological behavior of copper–graphite composites reinforced with Cu-coated or uncoated SiO2 particles. Materials 2018, 11, 2414. [Google Scholar]
- Kovacik, J.; Bielekt, J. Electrical conductivity of Cu/Graphite composite material as a funtion of structural characteristics. Scr. Matm. 1996, 35, 151–156. [Google Scholar]
- Kirkpatrick, S. Percolation ancl Conduction. Rev. Mod. Phys. 1973, 45, 574–598. [Google Scholar]
- Mazloum, A.; Kovacik, J.; Zagrai, A.; Sevostianov, I. Copper-graphite composite: Shear modulus, electrical resistivity, and cross-property connections. Int. J. Eng. Sci. 2020, 149, 103232. [Google Scholar]
- Ayyappadas, C.; Muthuchamy, A.; Raja Annamalai, A.; Agrawal, D.K. An investigation on the effect of sintering mode on various properties of copper-graphene metal matrix composite. Adv. Powder Technol. 2017, 28, 1760–1768. [Google Scholar]
- Nayak, D.; Debata, M. Effect of composition and milling time on mechanical and wear performance of copper-graphite composites processed by powder metallurgy route. Powder Metal. 2014, 57, 265–273. [Google Scholar]
- Mahdi, F.M.; Razooqi, R.N.; Irhayyim, S.S. Effcet of graphite content and milling time on physical properties of copper-graphite composites prepared by powder metallurgy route. Aust. J. Basic Appl. Sci. 2013, 7, 245–255. [Google Scholar]
- Blau, P.J.; Yust, C.S. Microfriction studies of model self-lubricating surfaces. Surf. Coat. Technol. 1993, 62, 380–387. [Google Scholar]





| Graphite Content | Relative Density | Graphite Content | Relative Density | Graphite Content | Relative Density | Graphite Content | Relative Density |
|---|---|---|---|---|---|---|---|
| 1.0% | 98.78% | 5% | 88.59% | 8% | 89% | 3.0% | 93.50% |
| 1.5% | 98.36% | 10% | 86.38% | 15% | 88% | ||
| 2.0% | 97.82% | 15% | 81.10% | 20% | 86% | ||
| 2.5% | 96.56% | ||||||
| SPS/750 °C | CS */1000 °C | CS/900 °C | VS */900 °C | ||||
| This work | [9] | [28] | [29] | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Sun, K.; Zeng, L.; Wang, J.; Xiao, X.; Liu, J.; Guo, C.; Ding, Y. Microstructure and Properties of Copper–Graphite Composites Fabricated by Spark Plasma Sintering Based on Two-Step Mixing. Metals 2020, 10, 1506. https://doi.org/10.3390/met10111506
Liu J, Sun K, Zeng L, Wang J, Xiao X, Liu J, Guo C, Ding Y. Microstructure and Properties of Copper–Graphite Composites Fabricated by Spark Plasma Sintering Based on Two-Step Mixing. Metals. 2020; 10(11):1506. https://doi.org/10.3390/met10111506
Chicago/Turabian StyleLiu, Jinping, Ke Sun, Longfei Zeng, Jing Wang, Xiangpeng Xiao, Jinming Liu, Chengjun Guo, and Yi Ding. 2020. "Microstructure and Properties of Copper–Graphite Composites Fabricated by Spark Plasma Sintering Based on Two-Step Mixing" Metals 10, no. 11: 1506. https://doi.org/10.3390/met10111506




