Recycling Strategies for Ceramic All-Solid-State Batteries—Part I: Study on Possible Treatments in Contrast to Li-Ion Battery Recycling
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Concept
2.2. Assembling Process
2.3. Material Demand
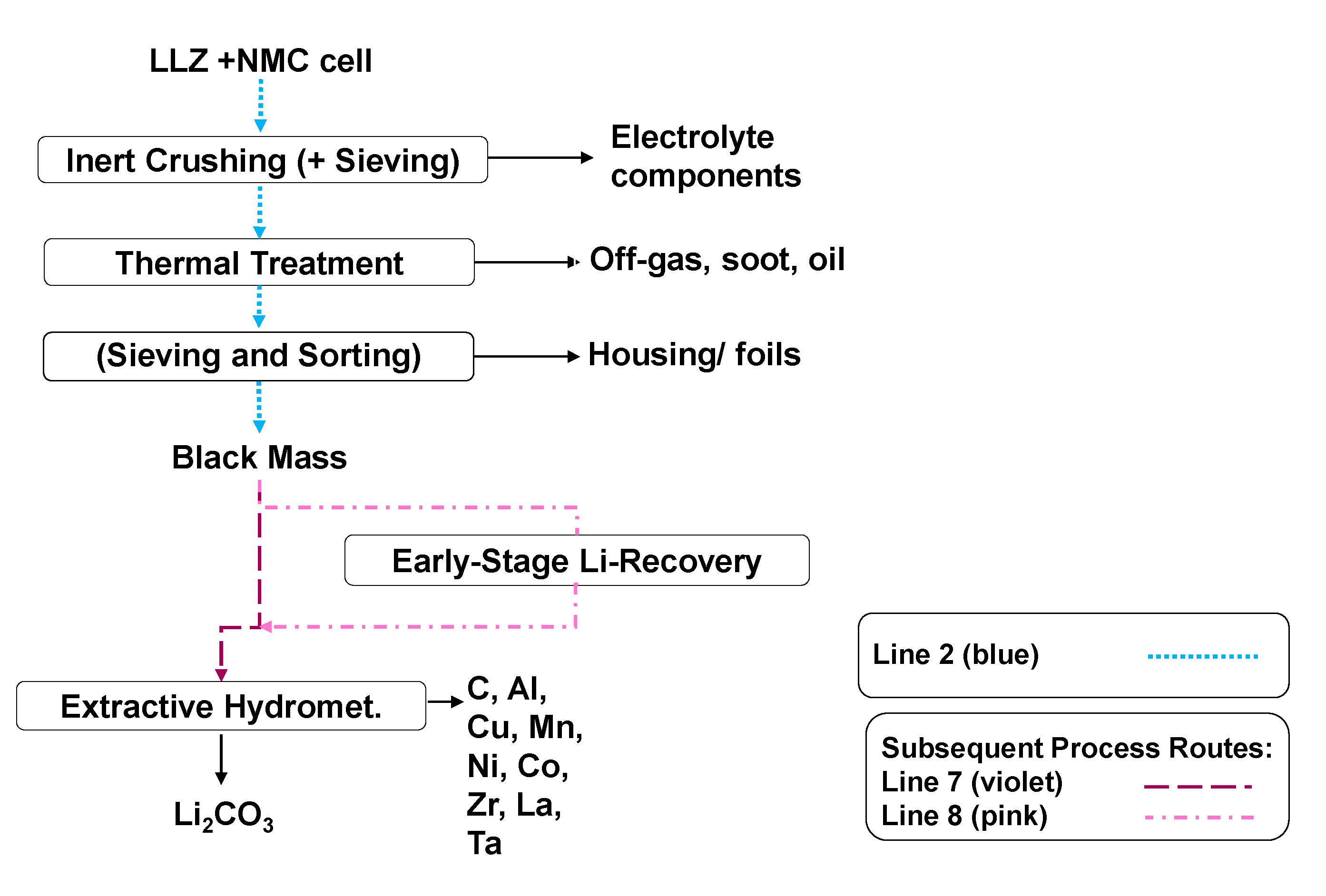
3. Recycling Approach
- Scenario 1
- Scenario 2
4. Conclusions
5. Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Olivetti, E.A.; Ceder, G.; Gaustad, G.G.; Fu, X. Lithium-ion battery supply chain considerations: Analysis of potential bottlenecks in critical metals. Joule 2017, 1, 229–243. [Google Scholar] [CrossRef]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling lithium-ion batteries from electric vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhu, Z.; Lin, X.; Zhang, Y.; He, Y.; Cao, H.; Sun, Z. A Mini-Review on Metal Recycling from Spent Lithium Ion Batteries. Engineering 2018, 4, 361–370. [Google Scholar] [CrossRef]
- Zhao, S.; He, W.; Li, G. Recycling Technology and Principle of Spent Lithium-Ion Battery. In Recycling of Spent Lithium-Ion Batteries; An, L., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–26. [Google Scholar]
- Träger, T.; Friedrich, B.; Weyhe, R. Recovery Concept of Value Metals from Automotive Lithium-Ion Batteries. Chem. Ing. Tech. 2015, 87, 1550–1557. [Google Scholar] [CrossRef]
- Diaz, F.; Wang, Y.; Moorthy, T.; Friedrich, B. Degradation Mechanism of Nickel-Cobalt-Aluminum (NCA) Cathode Material from Spent Lithium-Ion Batteries in Microwave-Assisted Pyrolysis. Metals 2018, 8, 565. [Google Scholar] [CrossRef]
- Ekberg, C.; Petranikova, M. Lithium Batteries Recycling. In Lithium Process Chemistry. Resources, Extraction, Batteries, and Recycling; Chagnes, A., Światowska, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 233–267. [Google Scholar]
- Wang, H.; Vest, M.; Friedrich, B. Hydrometallurgical processing of Li-Ion battery scrap from electric vehicles. In Proceedings of the EMC 2011, Düsseldorf, Germany, 26–29 June 2011; Harre, J., Ed.; GDMB: Clausthal-Zellerfeld, Germany, 2011. [Google Scholar]
- Lombardo, G.; Ebin, B.; Foreman, M.R.S.J.; Steenari, B.-M.; Petranikova, M. Chemical Transformations in Li-Ion Battery Electrode Materials by Carbothermic Reduction. ACS Sustain. Chem. Eng. 2019, 7, 13668–13679. [Google Scholar] [CrossRef]
- Zhang, G.; Du, Z.; He, Y.; Wang, H.; Xie, W.; Zhang, T. A Sustainable Process for the Recovery of Anode and Cathode Materials Derived from Spent Lithium-Ion Batteries. Sustainability 2019, 11, 2363. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Recovery of valuable metals from cathodic active material of spent lithium ion batteries: Leaching and kinetic aspects. Waste Manag. 2015, 45, 306–313. [Google Scholar] [CrossRef]
- Sun, L.; Qiu, K. Vacuum pyrolysis and hydrometallurgical process for the recovery of valuable metals from spent lithium-ion batteries. J. Hazard. Mater. 2011, 194, 378–384. [Google Scholar] [CrossRef]
- Hanisch, C.; Loellhoeffel, T.; Diekmann, J.; Markley, K.J.; Haselrieder, W.; Kwade, A. Recycling of lithium-ion batteries: A novel method to separate coating and foil of electrodes. J. Clean. Prod. 2015, 108, 301–311. [Google Scholar] [CrossRef]
- Or, T.; Gourley, S.W.D.; Kaliyappan, K.; Yu, A.; Chen, Z. Recycling of mixed cathode lithium-ion batteries for electric vehicles: Current status and future outlook. Carbon Energy 2020, 2, 6–43. [Google Scholar] [CrossRef]
- Pagliaro, M.; Meneguzzo, F. Lithium battery reusing and recycling: A circular economy insight. Heliyon 2019, 5, e01866. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Song, W.; Mo, W.-T.; Zhou, L.; Liu, J.-W. Lithium fluoride recovery from cathode material of spent lithium-ion battery. RSC Adv. 2018, 8, 8990–8998. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, N.; Jie, Y.; Hu, F.; Li, Y.; Wilson, B.P.; Xi, Y.; Lai, Y.; Yang, S. Toxicity Identification and Evolution Mechanism of Thermolysis-Driven Gas Emissions from Cathodes of Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2019, 7, 18228–18235. [Google Scholar] [CrossRef]
- Wang, H.; Friedrich, B. Development of a Highly Efficient Hydrometallurgical Recycling Process for Automotive Li–Ion Batteries. J. Sustain. Metall. 2015, 1, 168–178. [Google Scholar] [CrossRef]
- Yin, H.; Xing, P. Pyrometallurgical Routes Pyrometallurgical Routes for the Recycling of Spent Lithium-Ion Batteries. In Recycling of Spent Lithium-Ion Batteries; An, L., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 57–83. [Google Scholar]
- Pinegar, H.; Smith, Y.R. Recycling of End-of-Life Lithium Ion Batteries, Part I: Commercial Processes. J. Sustain. Metall. 2019, 5, 402–416. [Google Scholar] [CrossRef]
- Georgi-Maschler, T.; Friedrich, B.; Weyhe, R.; Heegn, H.; Rutz, M. Development of a recycling process for Li-ion batteries. J. Power Sources 2012, 207, 173–182. [Google Scholar] [CrossRef]
- Heelan, J.; Gratz, E.; Zheng, Z.; Wang, Q.; Chen, M.; Apelian, D.; Wang, Y. Current and Prospective Li-Ion Battery Recycling and Recovery Processes. JOM 2016, 68, 2632–2638. [Google Scholar] [CrossRef]
- Chagnes, A.; Pospiech, B. A brief review on hydrometallurgical technologies for recycling spent lithium-ion batteries. J. Chem. Technol. Biotechnol. 2013, 88, 1191–1199. [Google Scholar] [CrossRef]
- Swain, B. Recovery and recycling of lithium: A review. Sep. Purif. Technol. 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Kwade, A. Project Website InnoRec. 2019. Available online: https://www.prozell-cluster.de/en/projects/innorec/ (accessed on 24 March 2020).
- VDI/VDE Innovation + Technik GmbH. Project Website Mercator. 2019. Available online: https://www.erneuerbar-mobil.de/en/node/1232 (accessed on 16 November 2020).
- Stallmeister, C.; Schwich, L.; Friedrich, B. Early-Stage Li-Removal -Vermeidung von Lithiumverlusten im Zuge der Thermischen und Chemischen Recyclingrouten von Batterien. In Recycling und Sekundärrohstoffe, Band 13; Thomé-Kozmiensky, E., Holm, O., Friedrich, B., Goldmann, D., Eds.; Thomé-Kozmiensky Verlag GmbH: Neuruppin, Germany, 2020; pp. 545–557. [Google Scholar]
- Hu, J.; Zhang, J.; Li, H.; Chen, Y.; Wang, C. A promising approach for the recovery of high value-added metals from spent lithium-ion batteries. J. Power Sources 2017, 351, 192–199. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Xu, Z. Environmentally-friendly oxygen-free roasting/wet magnetic separation technology for in situ recycling cobalt, lithium carbonate and graphite from spent LiCoO2/graphite lithium batteries. J. Hazard. Mater. 2016, 302, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Bertau, M.; Martin, G. Integrated Direct Carbonation Process for Lithium Recovery from Primary and Secondary Resources. MSF 2019, 959, 69–73. [Google Scholar] [CrossRef]
- Vanderbruggen, A.; Rudolph, M. Flotation of Spherodized Graphite from Spent Lithium Ion Batteries. 2020. Available online: https://www.researchgate.net/publication/338630299_Recovery_of_spheroidized_graphite_from_spent_lithium_ion_batteries_Talk_at_AABC_Europe (accessed on 16 November 2020).
- He, Y.; Zhang, T.; Wang, F.; Zhang, G.; Zhang, W.; Wang, J. Recovery of LiCoO2 and graphite from spent lithium-ion batteries by Fenton reagent-assisted flotation. J. Clean. Prod. 2017, 143, 319–325. [Google Scholar] [CrossRef]
- Paulino, J.F.; Busnardo, N.G.; Afonso, J.C. Recovery of valuable elements from spent Li-batteries. J. Hazard. Mater. 2008, 150, 843–849. [Google Scholar] [CrossRef]
- Hosseini-Bab-Anari, E.; Boschin, A.; Mandai, T.; Masu, H.; Moth-Poulsen, K.; Johansson, P. Fluorine-free salts for aqueous lithium-ion and sodium-ion battery electrolytes. RSC Adv. 2016, 6, 85194–85201. [Google Scholar] [CrossRef]
- Davenport, W.G. Extractive Metallurgy of Nickel, Cobalt, and Platinum Group Materials; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Pinegar, H.; Smith, Y.R. Recycling of End-of-Life Lithium-Ion Batteries, Part II: Laboratory-Scale Research Developments in Mechanical, Thermal, and Leaching Treatments. J. Sustain. Metall. 2020, 6, 142–160. [Google Scholar] [CrossRef]
- Janek, J.; Zeier, W.G. A solid future for battery development. Nat. Energy 2016, 1, 1167. [Google Scholar] [CrossRef]
- Kurzweil, P.; Dietlmeier, O.K. Elektrochemische Speicher: Superkondensatoren, Batterien, Elektrolyse-Wasserstoff, Rechtliche Grundlagen, 1. Aufl.; Springer Fachmedien Wiesbaden: Wiesbaden, Germany, 2015. [Google Scholar]
- Tsai, C.-L.; Ma, Q.; Dellen, C.; Lobe, S.; Vondahlen, F.; Windmüller, A.; Grüner, D.; Zheng, H.; Uhlenbruck, S.; Finsterbusch, M.; et al. A garnet structure-based all-solid-state Li battery without interface modification: Resolving incompatibility issues on positive electrodes. Sustain. Energy Fuels 2019, 3, 280–291. [Google Scholar] [CrossRef]
- Troy, S.; Schreiber, A.; Reppert, T.; Gehrke, H.-G.; Finsterbusch, M.; Uhlenbruck, S.; Stenzel, P. Life Cycle Assessment and resource analysis of all-solid-state batteries. Appl. Energy 2016, 169, 757–767. [Google Scholar] [CrossRef]
- Erdmann, L.; Graedel, T.E. Criticality of non-fuel minerals: A review of major approaches and analyses. Environ. Sci. Technol. 2011, 45, 7620–7630. [Google Scholar] [CrossRef] [PubMed]
- Graedel, T.E.; Barr, R.; Chandler, C.; Chase, T.; Choi, J.; Christoffersen, L.; Friedlander, E.; Henly, C.; Jun, C.; Nassar, N.T.; et al. Methodology of metal criticality determination. Environ. Sci. Technol. 2012, 46, 1063–1070. [Google Scholar] [CrossRef]
- Ambrose, H.; Kendall, A. Understanding the future of lithium: Part 1, resource model. J. Ind. Ecol. 2020, 24, 80–89. [Google Scholar] [CrossRef]
- Simon, B.; Ziemann, S.; Weil, M. Criticality of metals for electrochemical energy storage systems—Development towards a technology specific indicator. Metall. Res. Technol. 2015, 111, 191–200. [Google Scholar] [CrossRef]
- Helbig, C.; Bradshaw, A.M.; Wietschel, L.; Thorenz, A.; Tuma, A. Supply risks associated with lithium-ion battery materials. J. Clean. Prod. 2018, 172, 274–286. [Google Scholar] [CrossRef]
- Buchholz, P.; Huy, D.; Sievers, H. DERA-Rohstoffliste 2012, Angebotskonzentration bei Metallen und Industriemineralien—Potenzielle Preis-und Lieferrisiken; Deutsche Rohstoffagentur (DERA) in der Bundesanstalt für Geowissenschaften und Rohstoffe: Berlin, Germany, 2012; Volume 10. [Google Scholar]
- LITHOREC. Recycling von Lithium-Ionen-Batterien—LithoRec II. Abschlussberichte der Beteiligten Verbundpartner. 2012. Available online: https://www.erneuerbar-mobil.de/sites/default/files/2017-01/Abschlussbericht_LithoRec_II_20170116.pdf (accessed on 1 October 2020).
- Deloitte Sustainability; British Geological Survey; Bureau de Recherches Géologiques et Minières; Netherlands Organisation for Applied Scientific Research. Study on the Review of the List of Critical Raw Materials. Criticality Assessments. 2017. Available online: https://op.europa.eu/en/publication-detail/-/publication/08fdab5f-9766-11e7-b92d-01aa75ed71a1/language-en (accessed on 16 November 2020).
- Joint Research Centre. The European Commission’s In-House Science Service: Annual Report 2013; Report EUR 26372 EN; Publications Office of the European Union: Luxembourg, 2014. [Google Scholar]
- Schultz, D.; Kuckshinrichs, W. The need for lithium—An upcoming problem of electrochemical energy storages? J. Energy Chall. Mech. 2016, 3, 180–185. [Google Scholar]
- Piana, G.; Ricciardi, M.; Bella, F.; Cucciniello, R.; Proto, A.; Gerbaldi, C. Poly(glycidyl ether)s recycling from industrial waste and feasibility study of reuse as electrolytes in sodium-based batteries. Chem. Eng. J. 2020, 382, 122934. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Diao, W.-Y.; Fan, C.-Y.; Wu, X.-L.; Zhang, J.-P. Benign Recycling of Spent Batteries towards All-Solid-State Lithium Batteries. Chemistry 2019, 25, 8975–8981. [Google Scholar] [CrossRef]
- Tsai, C.-L.; Roddatis, V.; Chandran, C.V.; Ma, Q.; Uhlenbruck, S.; Bram, M.; Heitjans, P.; Guillon, O. Li7La3Zr2O12 Interface Modification for Li Dendrite Prevention. ACS Appl. Mater. Interfaces 2016, 8, 10617–10626. [Google Scholar] [CrossRef]
- Uhlenbruck, S.; Dornseiffer, J.; Lobe, S.; Dellen, C.; Tsai, C.-L.; Gotzen, B.; Sebold, D.; Finsterbusch, M.; Guillon, O. Cathode-electrolyte material interactions during manufacturing of inorganic solid-state lithium batteries. J. Electroceram. 2017, 38, 197–206. [Google Scholar] [CrossRef]
- Park, K.; Yu, B.-C.; Jung, J.-W.; Li, Y.; Zhou, W.; Gao, H.; Son, S.; Goodenough, J.B. Electrochemical Nature of the Cathode Interface for a Solid-State Lithium-Ion Battery: Interface between LiCoO2 and Garnet-Li7La3Zr2O12. Chem. Mater. 2016, 28, 8051–8059. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, T.; Shen, Y.; Lin, Y.; Nan, C.-W. Chemical compatibility between garnet-like solid state electrolyte Li6.75La3Zr1.75Ta0.25O12 and major commercial lithium battery cathode materials. J. Mater. 2016, 2, 256–264. [Google Scholar] [CrossRef]
- DeWees, R.; Wang, H. Synthesis and Properties of NaSICON-type LATP and LAGP Solid Electrolytes. ChemSusChem 2019, 12, 3713–3725. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Iwasaki, S.; Ishii, Y.; Motoyama, M.; West, W.C.; Yamamoto, Y.; Iriyama, Y. Preparation of thick-film electrode-solid electrolyte composites on Li7La3Zr2O12 and their electrochemical properties. J. Power Sources 2016, 303, 65–72. [Google Scholar] [CrossRef]
- Thielmann, A.; Neef, C.; Hettesheimer, T.; Döscher, H.; Wietschel, M.; Tübke, J. Energiespeicher-Roadmap. 2017. Available online: https://www.isi.fraunhofer.de/content/dam/isi/dokumente/cct/lib/Energiespeicher-Roadmap-Dezember-2017.pdf (accessed on 23 March 2020).
- United States Geological Survey. U.S. Geological Survey: Mineral Commodity Summaries; United States Geological Survey: Reston, VA, USA, 2020.
- Reichl, C.; Schatz, M.; Zsak, G. World-Mining-Data Welt-Bergbau-Daten. 2014. Available online: https://www.univie.ac.at/Mineralogie/docs/Weltbergbaudaten_2014.pdf (accessed on 1 October 2020).
- Brown, T.J.; Idoine, N.E.; Wrighton, C.E.; Raycraft, E.R.; Hobbs, S.F.; Shaw, R.A.; Everett, P.; Kresse, C.; Deady, E.A.; Bide, T. World Mineral Production 2014–2018; British Geological Survey: Nottingham, UK, 2019. [Google Scholar]
- Available online: https://www.statista.com/statistics/280060/share-of-global-rare-earth-supply-by-element-2015/ (accessed on 1 October 2020).
- Peters, L.; Schier, C.; Friedrich, B. Lithium- und Kobalt-Rückgewinnung aus Elektrolichtbogenofenschlacken des Batterie-Recyclings. In Mineralische Nebenprodukte und Abfälle. Aschen, Schlacken, Stäube und Baurestmassen; Thiel, S., Thomé-Kozmiensky, E., Friedrich, B., Pretz, T., Quicker, P., Senk, D., Wotruba, H., Eds.; Thomé-Kozmiensky Verlag GmbH: Neuruppien, Germany, 2018; pp. 338–359. [Google Scholar]
- Wang, H.; Friedmann, D.; Vest, M.; Friedrich, B. Innovative Recycling of Li-Based Electric Vehicle Batteries, General Hydrometallurgy, General Pyrometallurgy/Vessel Integrity/Process Gas Treatment, Recycling. Posters, Authors Index, Keywords Index; GDMB Verlag: Clausthal-Zellerfeld, Germany, 2013. [Google Scholar]
- Lide, D.R. (Ed.) A ready-reference book of chemical and physical data. In CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Larraz, G.; Orera, A.; Sanjuán, M.L. Cubic phases of garnet-type Li7La3Zr2O12: The role of hydration. J. Mater. Chem. A 2013, 1, 11419. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Y.; Hood, Z.D.; Ma, C.; Yu, S.; Sharafi, A.; Wang, H.; An, K.; Sakamoto, J.; Siegel, D.J.; et al. Elucidating the mobility of H+ and Li+ ions in (Li 6.25−xHxAl0.25 )La3Zr2O12 via correlative neutron and electron spectroscopy. Energy Environ. Sci. 2019, 12, 945–951. [Google Scholar] [CrossRef]
- Ma, C.; Rangasamy, E.; Liang, C.; Sakamoto, J.; More, K.L.; Chi, M. Excellent stability of a lithium-ion-conducting solid electrolyte upon reversible Li(+)/H(+) exchange in aqueous solutions. Angew. Chem. Int. Ed. Engl. 2015, 54, 129–133. [Google Scholar] [CrossRef]
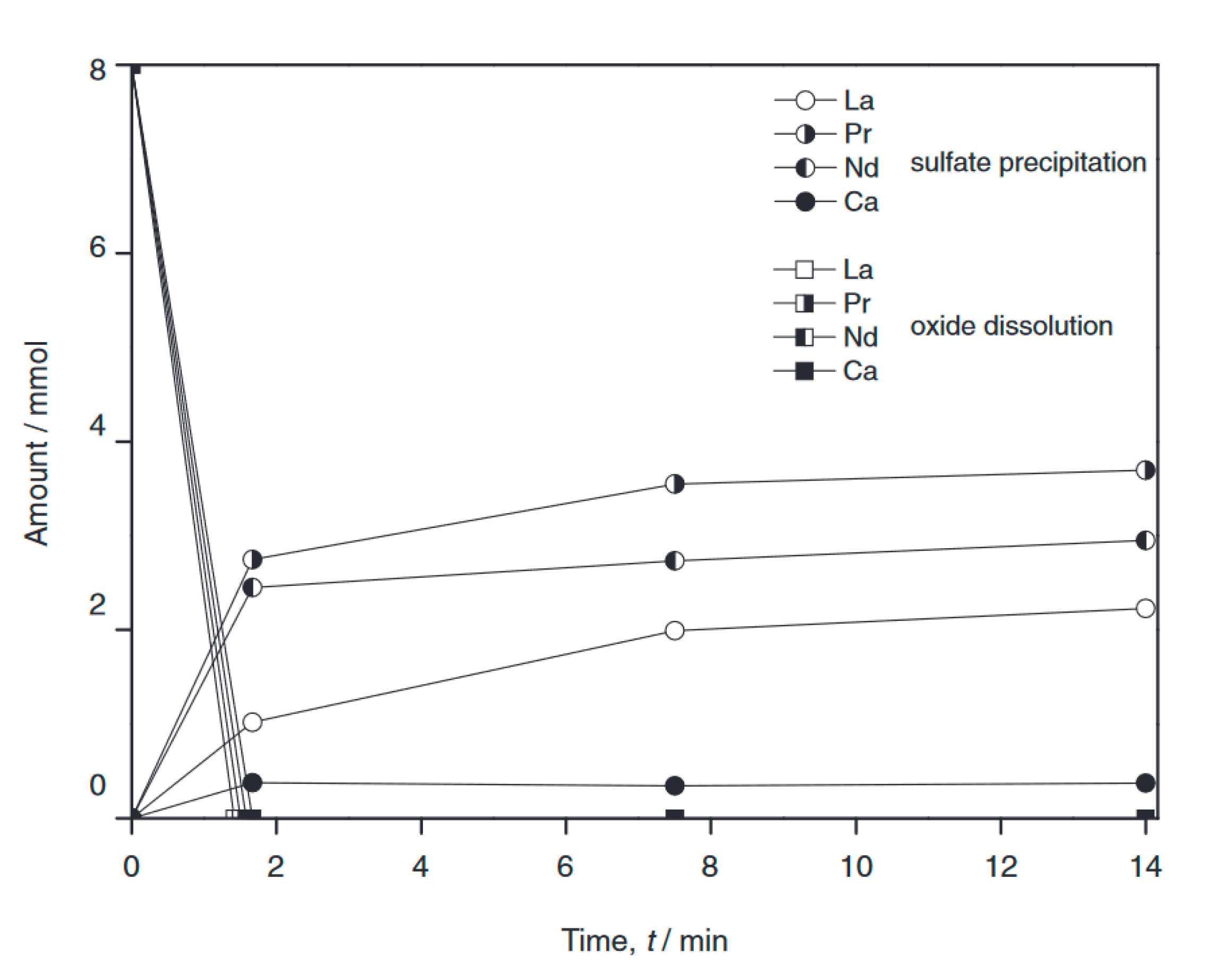
- Ma, Y.; Stopic, S.; Wang, X.; Forsberg, K.; Friedrich, B. Basic Sulfate Precipitation of Zirconium from Sulfuric Acid Leach Solution. Metals 2020, 10, 1099. [Google Scholar] [CrossRef]
- Ferreira, C.A.; Formiga, T.S.; Morais, C.A. Study of the Zircon Processing Aiming the Recovery of Zirconium and Silica. In Proceedings of the 24th International Mining Congress and Exhibition of Turkey, IMCET 2015, Antalya, Turkey, 14–17 April 2015; pp. 1351–1356. [Google Scholar]
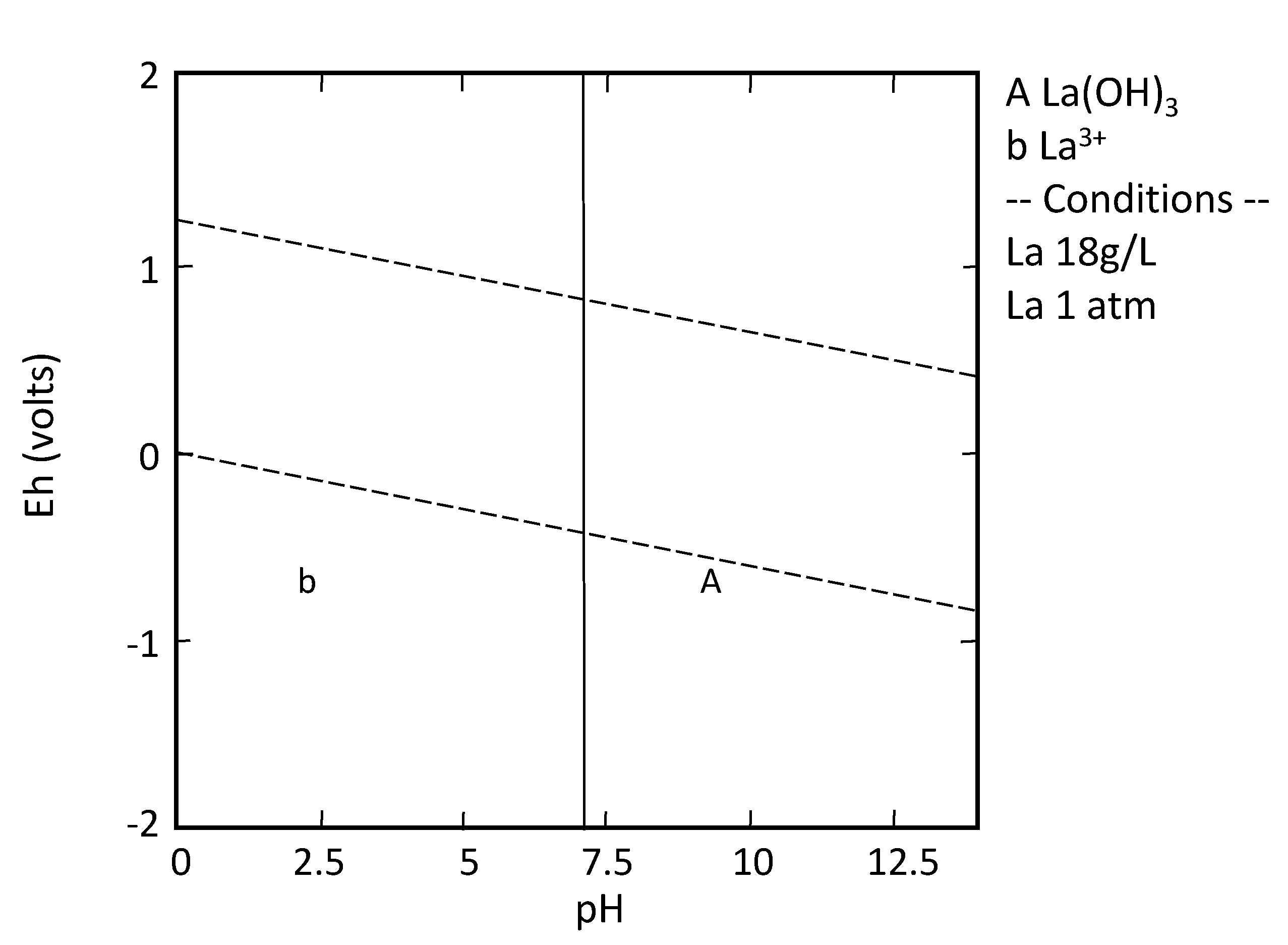
- Orhanovic, Z.; Pokric, B.; Füredi, H.; Branica, M. Precipitation and Hydrolysis of Metallic Ions. III. Studies on the Solubility of Yttrium and Some Rare Earth Hydroxides. Croat. Chem. Acta 1966, 38, 269–276. [Google Scholar]
- Um, N.; Hirato, T. Dissolution Behavior of La2O3, Pr2O3, Nd2O3, CaO and Al2O3 in Sulfuric Acid Solutions and Study of Cerium Recovery from Rare Earth Polishing Powder Waste via Two-Stage Sulfuric Acid Leaching. Mater. Trans. 2013, 54, 713–719. [Google Scholar] [CrossRef]
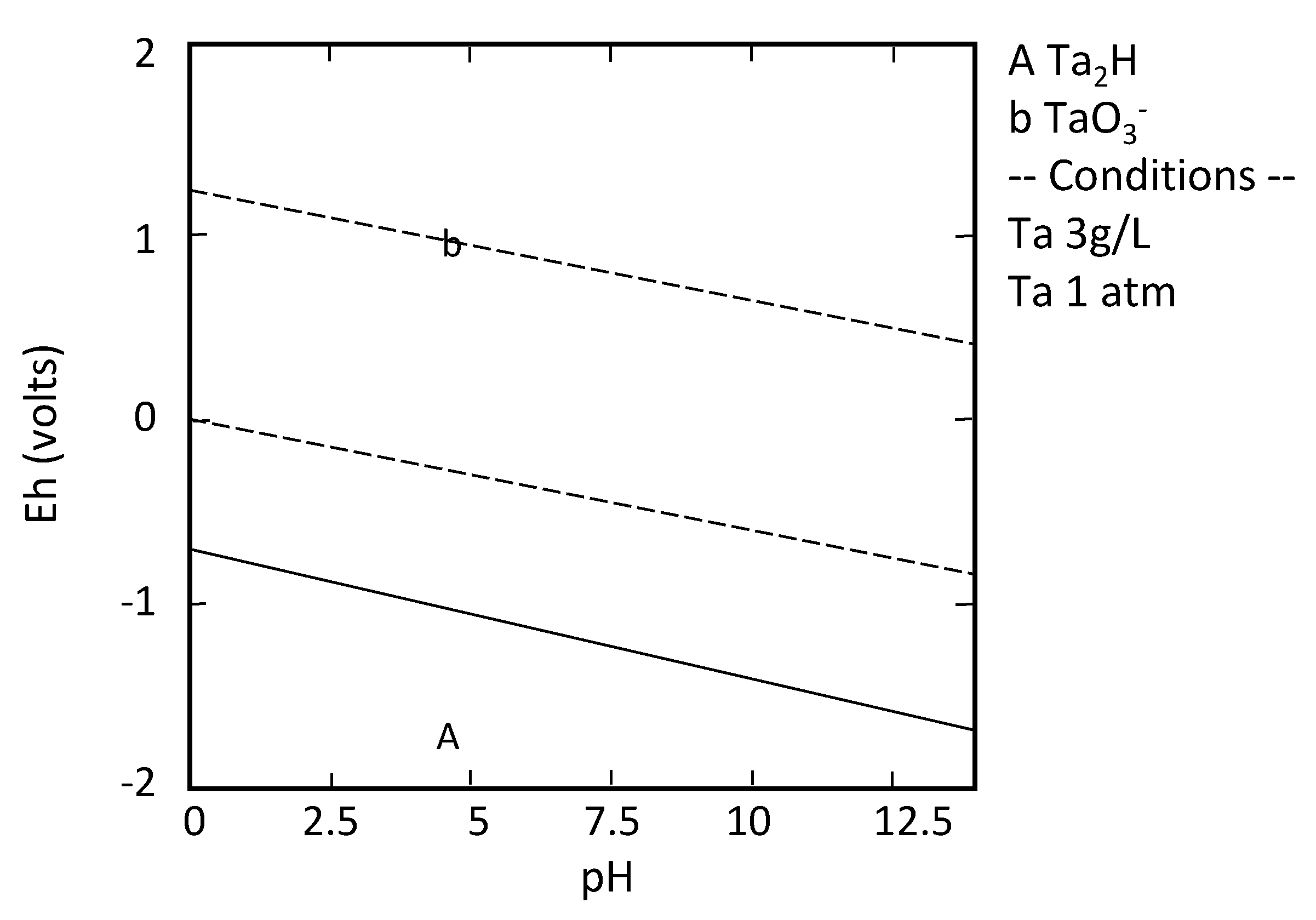
- Chen, W.-S.; Ho, H.-J.; Lin, K.-Y. Hydrometallurgical Process for Tantalum Recovery from Epoxy-Coated Solid Electrolyte Tantalum Capacitors. Materials 2019, 12, 1220. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.J.H.; Brown, D.; Bailar, J.C.; Emeléus, H.J.; Nyholm, R. The Chemistry of Vanadium, Niobium and Tantalum: Pergamon Texts in Inorganic Chemistry; Elsevier: Amsterdam, The Netherlands, 2013; Volume 20. [Google Scholar]
- Deblonde, G.J.-P.; Chagnes, A.; Bélair, S.; Cote, G. Solubility of niobium(V) and tantalum(V) under mild alkaline conditions. Hydrometallurgy 2015, 156, 99–106. [Google Scholar] [CrossRef]
- Deblonde, G.J.-P.; Chagnes, A.; Weigel, V.; Cote, G. Direct precipitation of niobium and tantalum from alkaline solutions using calcium-bearing reagents. Hydrometallurgy 2016, 165, 345–350. [Google Scholar] [CrossRef]





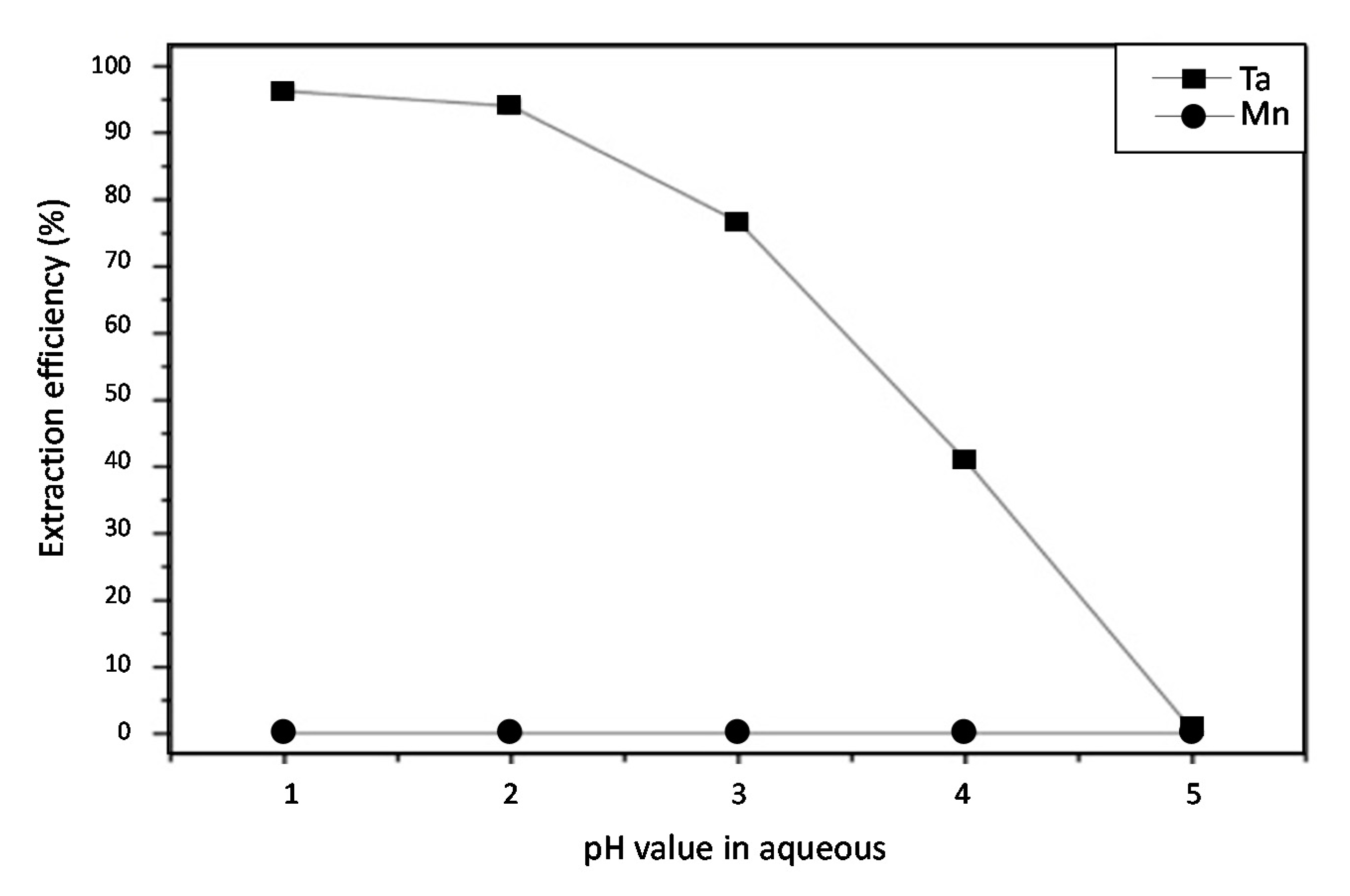
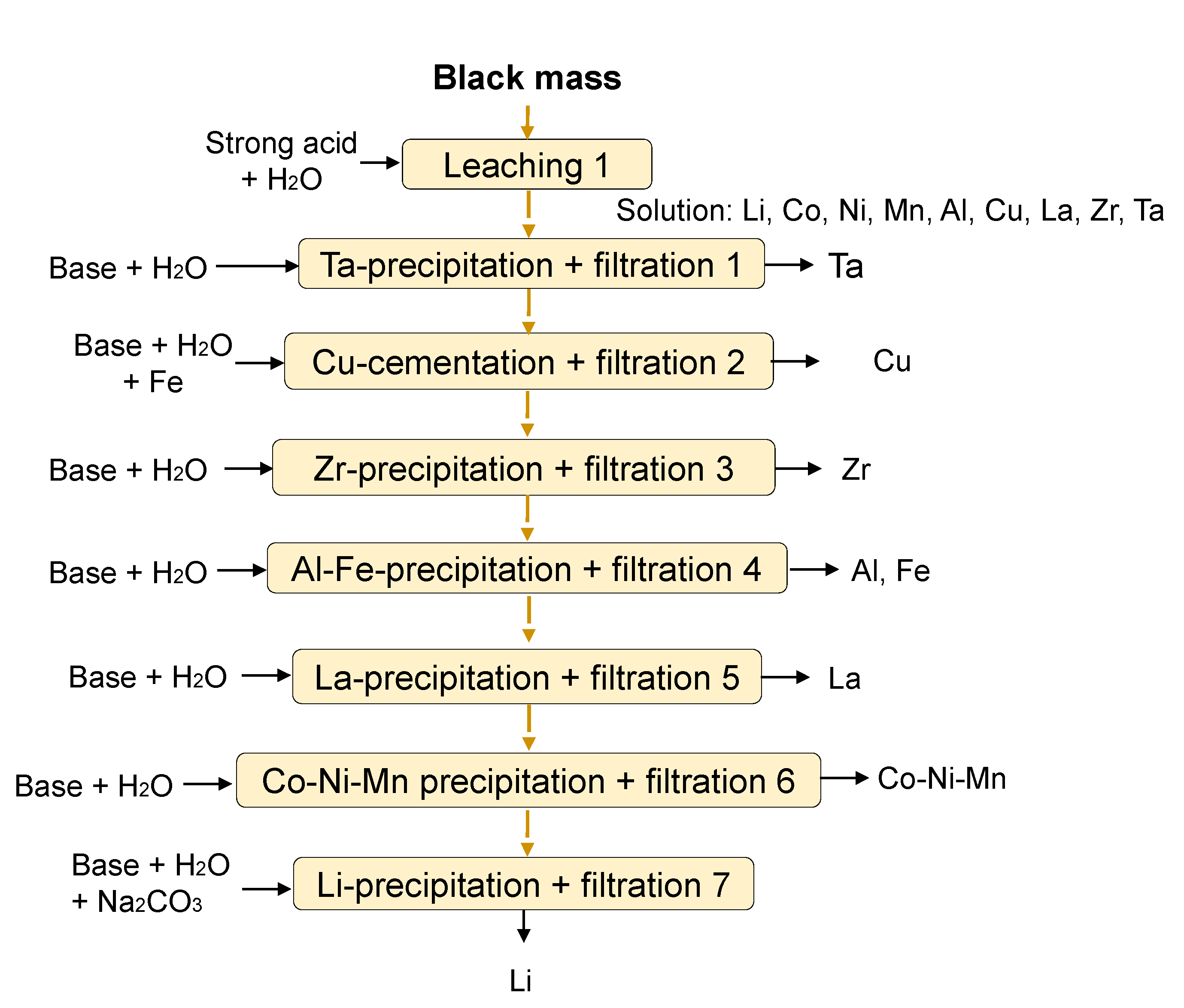

| Cell Number | Cathode Materials | Energy Density (Wh/kg) |
|---|---|---|
| 1.1 | LLZ + LCO | 309 |
| 1.2 | LLZ + NMC | 406 |
| 2.1 | LATP + LCO | 352 |
| 2.2 | LATP + NMC | 463 |
| World Production (T) | Material Demand for 1 TWh ASB in 105 T | Share (%) on Current World Production in 2030 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cell 1.1 | Cell 1.2 | Cell 2.1 | Cell 2.2 | Cell 1.1 | Cell 1.2 | Cell 2.1 | Cell 2.2 | ||
| LI | 77,000 [60] | 1.93 | 1.47 | 1.57 | 1.19 | 251 | 191 | 204 | 155 |
| NI | 2,700,000 [60] | − | 6.35 | − | 6.35 | − | 24 | − | 24 |
| CO | 140,000 [60] | 10.56 | 0.80 | 10.56 | 0.80 | 755 | 57 | 754 | 57 |
| MN | 15,414,509 [61] 53,000,000 a [62] | − | 0.74 | − | 0.74 | − | 0.5 | − | 0.5 |
| AL | 64,000,000 [60] | 0.93 | 0.72 | 1.11 | 0.86 | 0.1 | 0.1 | 0.2 | 0.1 |
| TI | 4,394,500 [62] | − | − | 0.95 | 0.73 | − | − | 2.2 | 1.7 |
| P | 36,650,402 [61] | − | − | 1.23 | 0.95 | − | − | 0.34 | 0.26 |
| LA | 56,700 b [60] | 5.11 | 3.95 | 0.85 | 0.66 | 901 | 697 | 150 | 116 |
| ZR | 112,471 c [62] | 1.79 | 1.39 | 0.30 | 0.23 | 159 | 123 | 27 | 20 |
| TA | 1800 [60] | 0.89 | 0.69 | 0.15 | 0.11 | 4928 | 3816 | 833 | 611 |
| CU | 20,000,000 [60] | 3.07 | 2.38 | 3.07 | 2.38 | 1.5 | 1.2 | 1.5 | 1.2 |
| Criteria of Scenario | Scenario 1 | Scenario 2 |
|---|---|---|
| Characteristics | LLZ is fully dissolved | Only Li is dissolved, the other LLZ components remain solid |
| Leaching conditions | High temperature/aggressive leaching | Moderate leaching |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwich, L.; Küpers, M.; Finsterbusch, M.; Schreiber, A.; Fattakhova-Rohlfing, D.; Guillon, O.; Friedrich, B. Recycling Strategies for Ceramic All-Solid-State Batteries—Part I: Study on Possible Treatments in Contrast to Li-Ion Battery Recycling. Metals 2020, 10, 1523. https://doi.org/10.3390/met10111523
Schwich L, Küpers M, Finsterbusch M, Schreiber A, Fattakhova-Rohlfing D, Guillon O, Friedrich B. Recycling Strategies for Ceramic All-Solid-State Batteries—Part I: Study on Possible Treatments in Contrast to Li-Ion Battery Recycling. Metals. 2020; 10(11):1523. https://doi.org/10.3390/met10111523
Chicago/Turabian StyleSchwich, Lilian, Michael Küpers, Martin Finsterbusch, Andrea Schreiber, Dina Fattakhova-Rohlfing, Olivier Guillon, and Bernd Friedrich. 2020. "Recycling Strategies for Ceramic All-Solid-State Batteries—Part I: Study on Possible Treatments in Contrast to Li-Ion Battery Recycling" Metals 10, no. 11: 1523. https://doi.org/10.3390/met10111523
APA StyleSchwich, L., Küpers, M., Finsterbusch, M., Schreiber, A., Fattakhova-Rohlfing, D., Guillon, O., & Friedrich, B. (2020). Recycling Strategies for Ceramic All-Solid-State Batteries—Part I: Study on Possible Treatments in Contrast to Li-Ion Battery Recycling. Metals, 10(11), 1523. https://doi.org/10.3390/met10111523






