Crystallization of FCC and BCC Liquid Metals Studied by Molecular Dynamics Simulation
Abstract
1. Introduction
2. Computational Procedure
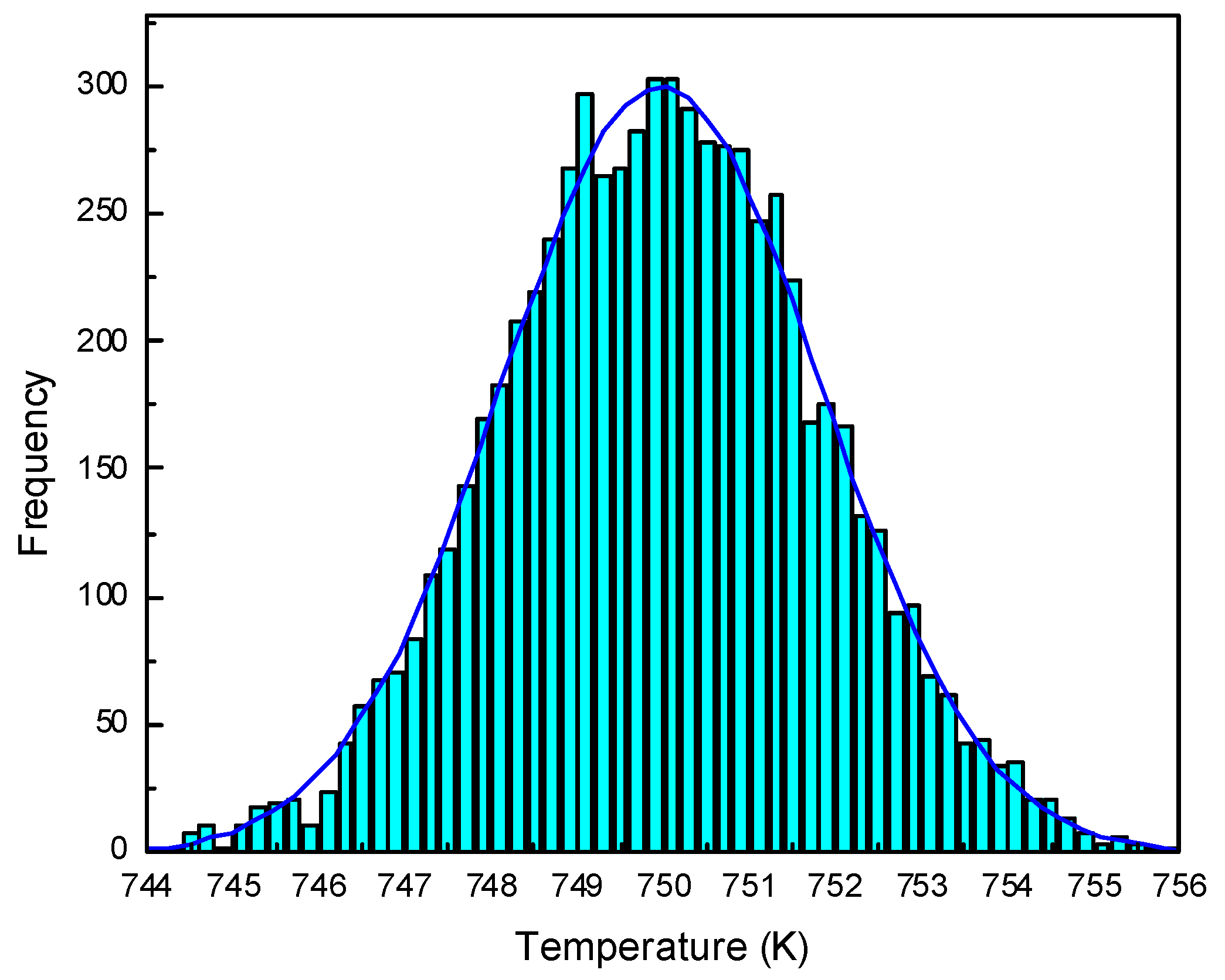
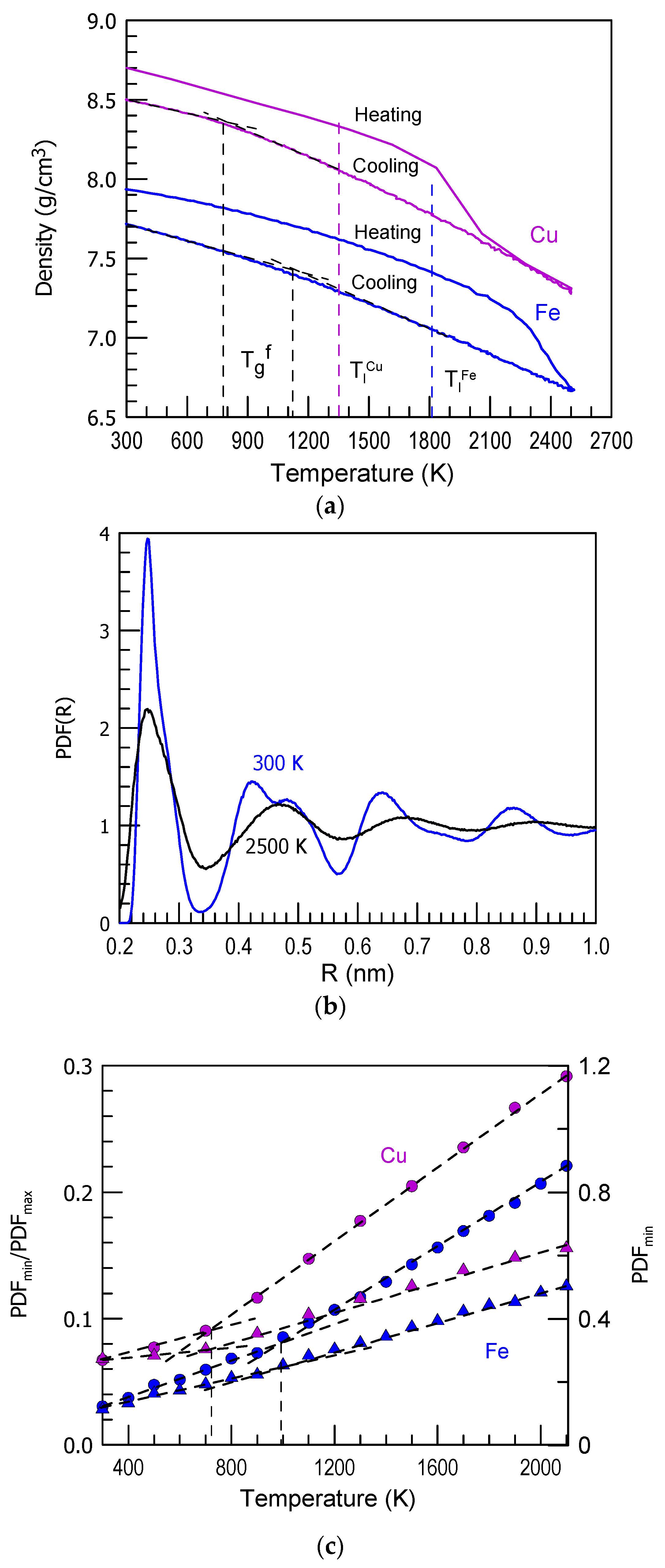
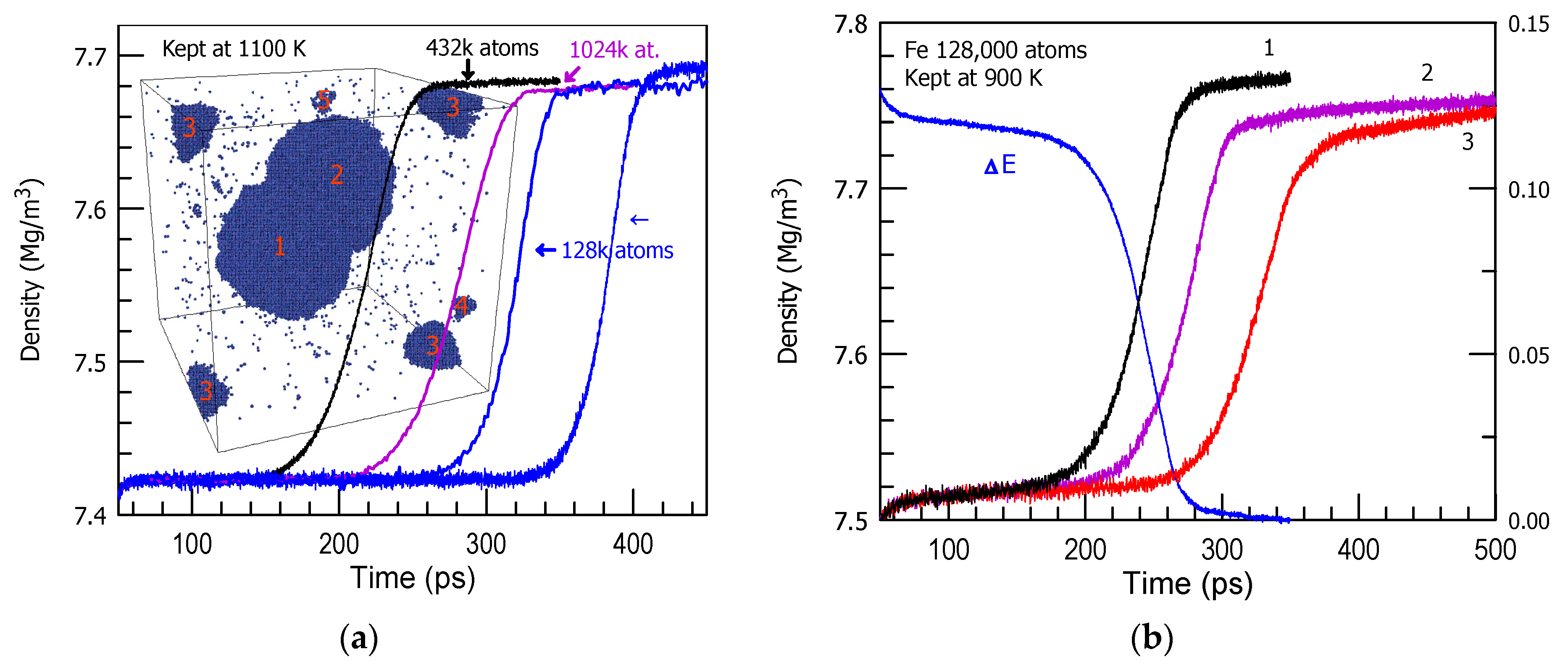
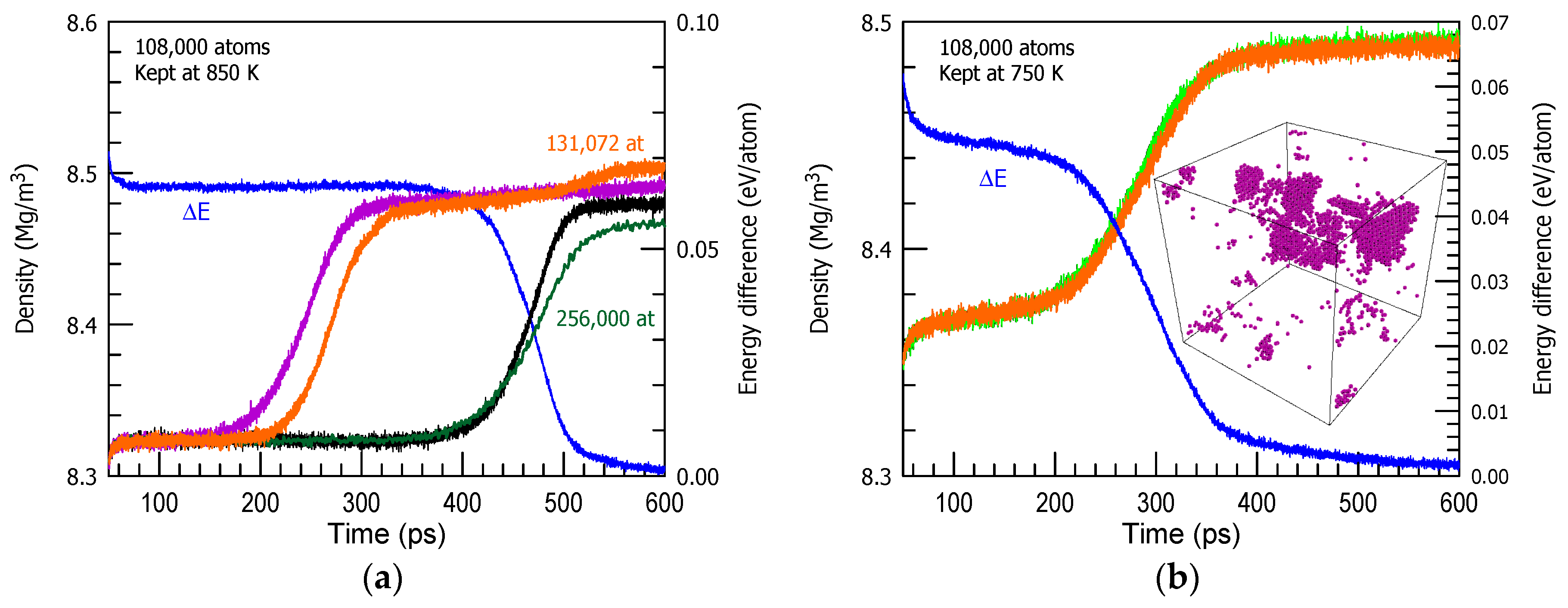
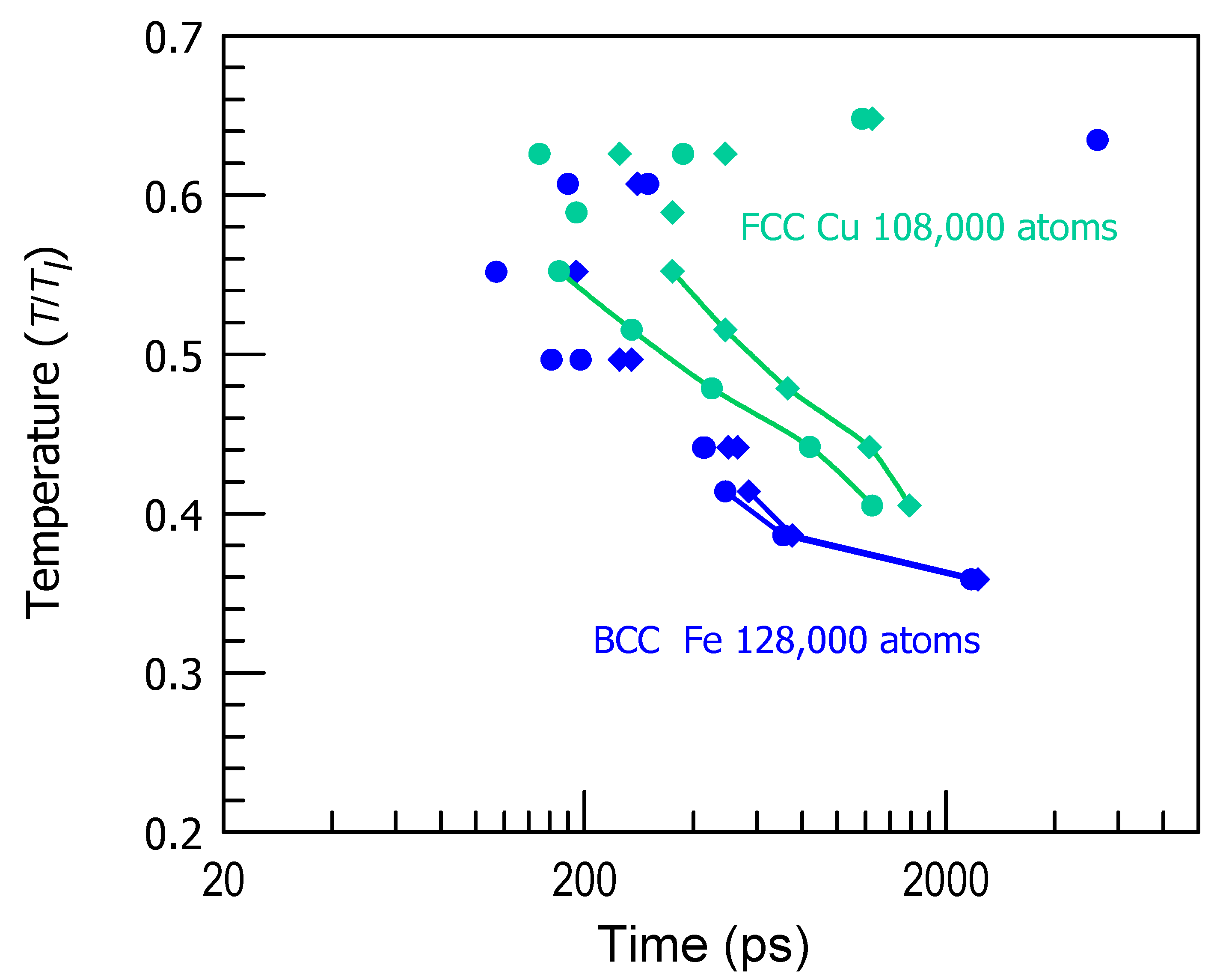
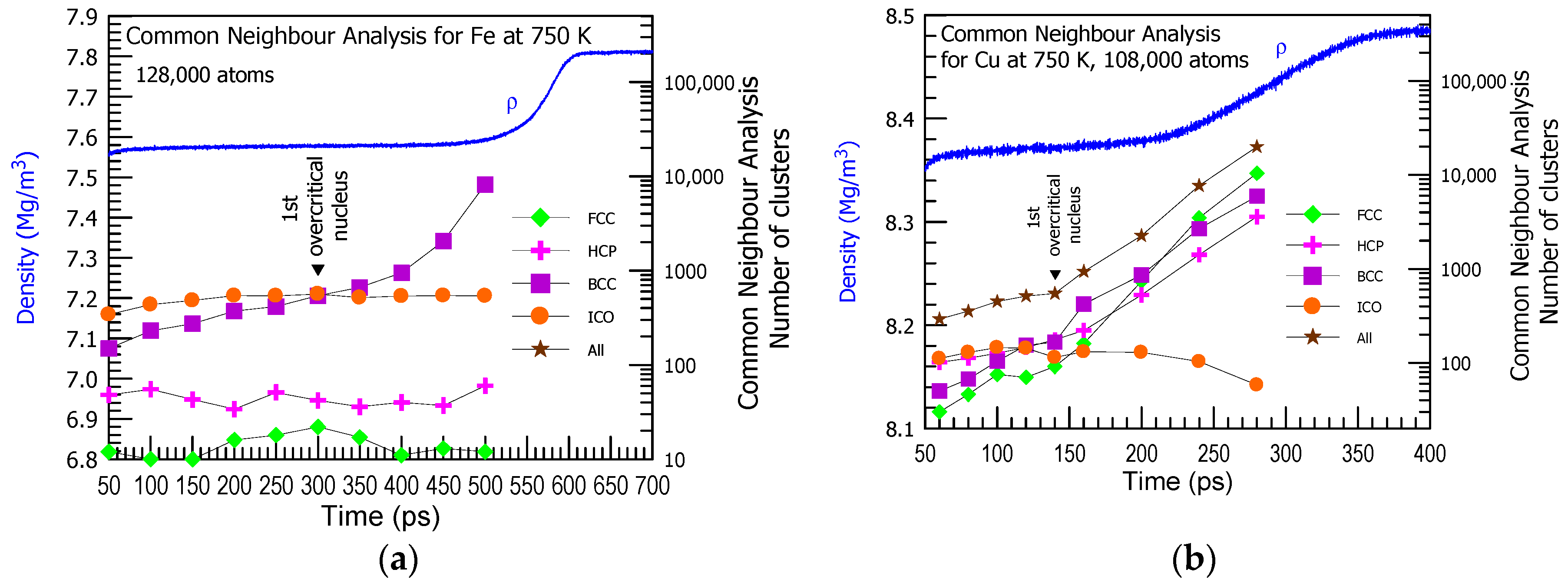
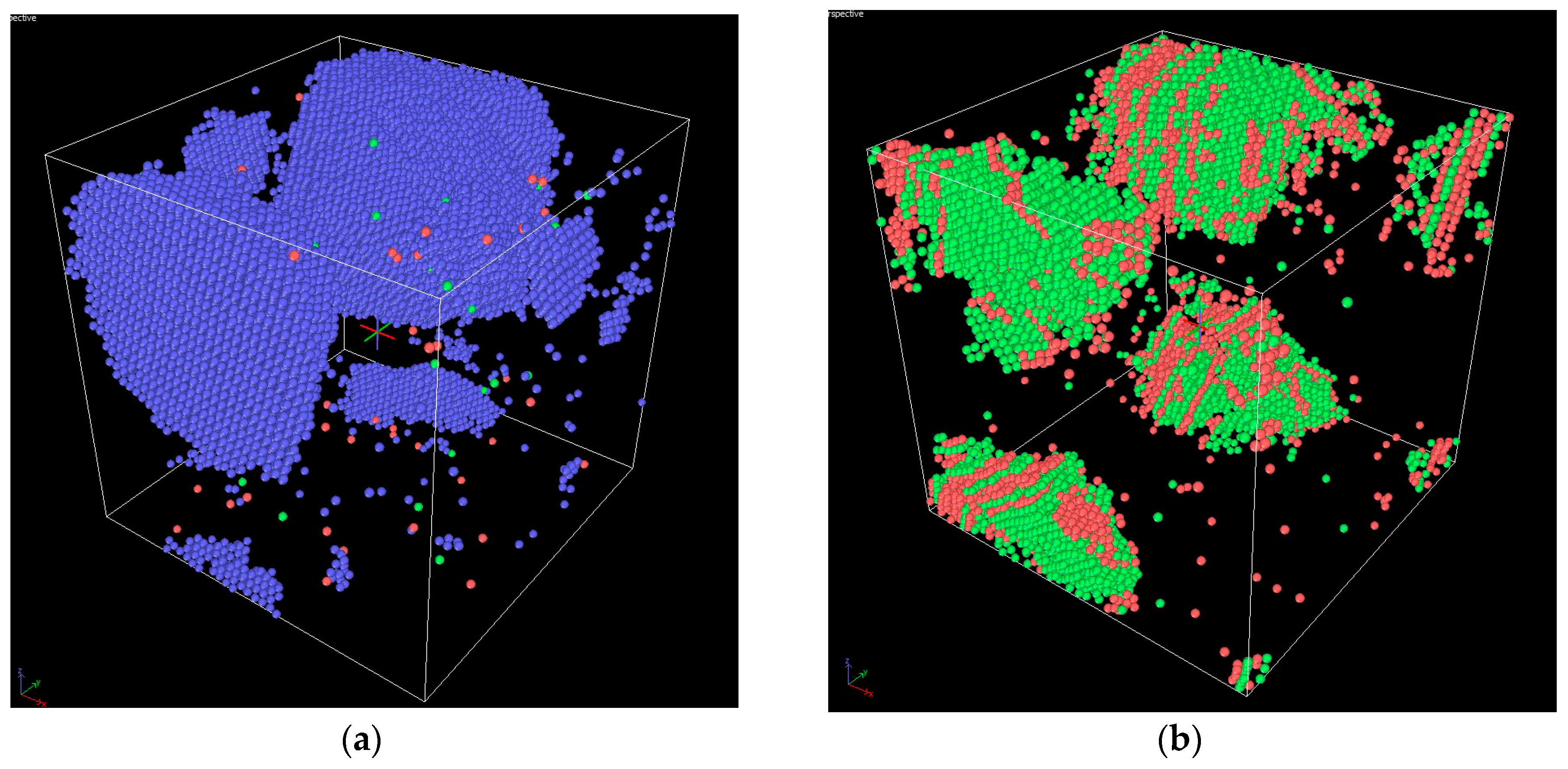
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kelton, K.F.; Greer, A.L. Nucleation in condensed matter applications in materials and biology. Pergamon Mater. Ser. 2010, 15, 1–743. [Google Scholar]
- Orava, J.; Greer, A.L. Fast and slow crystal growth kinetics in glass-forming melts. J. Chem. Phys. 2014, 140, 214504. [Google Scholar] [CrossRef]
- Buckle, E.R. Studies on the freezing of pure liquids. II. The kinetics of homogeneous nucleation in supercooled liquids. Proc. R. Soc. A 1961, 261, 189–196. [Google Scholar]
- Van Duijneveldt, J.S.; Frenkel, D. Computer simulation study of free energy barriers in crystal nucleation. J. Chem. Phys. 1992, 96, 4655. [Google Scholar] [CrossRef]
- E, J.C.; Wang, L.; Cai, Y.; Wu, H.A.; Luo, S.N. Crystallization in supercooled liquid Cu: Homogeneous nucleation and growth. J. Chem. Phys. 2015, 142, 064704. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, C.L.; Ketov, S.V.; Akagi, K.; Tsarkov, A.A.; Ikuhara, Y.; Louzguine-Luzgin, D.V. Local chemical ordering within the incubation period as a trigger for nanocrystallization of a highly supercooled Ti-based liquid. Mater. Des. 2018, 156, 504–513. [Google Scholar] [CrossRef]
- Evteev, A.V.; Kosilov, A.T.; Levchenko, E.V. Atomic mechanisms of pure iron vitrification. J. Exp. Theor. Phys. 2004, 99, 522–529. [Google Scholar] [CrossRef]
- Okita, S.; Verestek, W.; Sakane, S.; Takaki, T.; Ohnoe, M.; Shibuta, Y. Molecular dynamics simulations investigating consecutive nucleation, solidification and grain growth in a twelve-million-atom Fe-system. J. Cryst. Growth 2017, 474, 140–145. [Google Scholar] [CrossRef]
- Jakse, N.; Pasturel, A. Ab initio molecular dynamics simulations of local structure of supercooled Ni. J. Chem. Phys. 2004, 120, 6124. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Belosludov, R.; Saito, M.; Kawazoe, Y.; Inoue, A. Glass-transition behavior of Ni. Calculation, prediction and experiment. J. Appl. Phys. 2008, 104, 123529. [Google Scholar] [CrossRef]
- Fang, H.Z.; Hui, X.; Chen, G.L.; Liu, Z.K. Structural evolution of Cu during rapid quenching by ab initio molecular dynamics. Phys. Lett. A 2008, 372, 5831–5837. [Google Scholar] [CrossRef]
- Kirova, E.; Norman, G.E.; Pisarev, V. Simulation of the glass transition of a thin aluminum melt layer at ultrafast cooling under isobaric conditions. JETP Lett. 2019, 110, 359–363. [Google Scholar] [CrossRef]
- Han, J.J.; Wang, C.P.; Liu, X.J.; Wang, Y.; Liu, Z.-K.; Zhang, T.-Y.; Jiang, J.Z. Abnormal correlation between phase transformation and cooling rate for pure metals. Sci. Rep. 2016, 6, 22391. [Google Scholar] [CrossRef] [PubMed]
- Shibuta, Y.; Sakane, S.; Miyoshi, E.; Okita, S.; Takaki, T.; Ohno, M. Heterogeneity in homogeneous nucleation from billion-atom molecular dynamics simulation of solidification of pure metal. Nat. Commun. 2017, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazy, Y.; Averback, R.S. Kinetic stages in the crystallization of deeply undercooled body-centered-cubic and face-centered-cubic metals. Acta Mater. 2010, 58, 524–530. [Google Scholar] [CrossRef]
- Wilde, G.; Sebright, J.L.; Perepezko, J.H. Bulk liquid undercooling and nucleation in gold. Acta Mater. 2006, 54, 4759–4769. [Google Scholar] [CrossRef]
- Bokeloh, J.; Wilde, G.; Rozas, R.E.; Benjamin, R.; Horbach, J. Nucleation barriers for the liquid-to-crystal transition in simple metals: Experiment vs. Simulation. Eur. Phys. J. Spec. Top. 2014, 223, 511–526. [Google Scholar] [CrossRef]
- Plimpton, S.J. Fast parallel algorithms for short-range molecular dynamics. J. Comp. Phys. 1995, 117, 1. [Google Scholar] [CrossRef]
- Mendelev, M.I.; Kramer, M.J.; Becker, C.A.; Asta, M. Analysis of semi-empirical interatomic potentials appropriate for simulation of crystalline and liquid Al and Cu. Philos. Mag. 2008, 88, 1723–1750. [Google Scholar] [CrossRef]
- Mendelev, M.I.; Han, S.; Srolovitz, D.J.; Ackland, G.J.; Sun, D.Y.; Asta, M. Development of new interatomic potentials appropriate for crystalline and liquid iron. Philos. Mag. A 2003, 83, 3977–3994. [Google Scholar] [CrossRef]
- Nose, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511. [Google Scholar] [CrossRef]
- Hoover, W.G. Canonical dynamics, Equilibrium phase-space distributions. Phys. Rev. A 1985, 31, 1965. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO—The Open Visualization Tool, Modelling Simul. Mater. Sci. Eng. 2010, 18, 015012. [Google Scholar]
- Honeycutt, J.D.; Andersen, H.C. Molecular dynamics study of melting and freezing of small Lennard-Jones clusters. J. Phys. Chem. 1987, 91, 4950–4963. [Google Scholar] [CrossRef]
- Zhong, L.; Wang, J.; Sheng, H.; Zhang, Z.; Mao, S.X. Formation of monatomic metallic glasses through ultrafast liquid quenching. Nature 2014, 512, 177–180. [Google Scholar] [CrossRef]
- Russo, J.; Tanaka, H. The microscopic pathway to crystallization in supercooled liquids. Sci. Rep. 2012, 2, 505. [Google Scholar] [CrossRef]
- Belousov, O.K. Estimation of the critical glass transition rate and the inorganic glass thickness. Russ. Metall. Met. 2009, 2009, 488–497. [Google Scholar] [CrossRef]
- Kim, Y.W.; Lin, H.M.; Kelly, T.F. Amorphous solidification of pure metals in submicron spheres. Acta Metall. 1989, 37, 247–255. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Inoue, A. An extended criterion for estimation of glass-forming ability of metals. J. Mater. Res. 2007, 22, 1378–1383. [Google Scholar] [CrossRef]
- Ojovan, M.I.; Louzguine-Luzgin, D.V. Revealing structural changes at glass transition via radial distribution functions. J. Phys. Chem. B 2020, 124, 3186–3194. [Google Scholar] [CrossRef] [PubMed]
- Wendt, H.H.; Abraham, F.F. Empirical criterion for the glass transition region based on Monte Carlo simulations. Phys. Rev. Lett. 1978, 41, 1244–1246. [Google Scholar] [CrossRef]
- Sosso, G.S.; Chen, J.; Cox, S.J.; Fitzner, M.; Pedevilla, P.; Zen, A.; Michaelides, A. Nucleation in liquids: Open questions and future challenges in molecular dynamics simulations. Chem. Rev. 2016, 116, 7078–7116. [Google Scholar] [CrossRef] [PubMed]
- Louzguine-Luzgin, D.V.; Miyama, M.; Nishio, K.; Tsarkov, A.A.; Greer, A.L. Vitrification and nanocrystallization of pure liquid Ni studied using molecular-dynamics simulation. J. Chem. Phys. 2019, 151, 124502. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Sun, Y.; Zhang, F.; Wang, C.Z.; Ho, K.M.; Mendelev, M.I. Nucleation of stoichiometric compounds from liquid: Role of the kinetic factor. Phys. Rev. Mater. 2018, 2, 023401. [Google Scholar] [CrossRef]
- Kurtuldu, G.; Sicco, A.; Rappaz, M. Icosahedral quasicrystal-enhanced nucleation of the fcc phase in liquid gold alloys. Acta. Mater. 2014, 70, 240–248. [Google Scholar] [CrossRef]
- Rappaz, M.; Kurtuldu, G. Quasicrystal-enhanced nucleation during the solidification of fcc metallic alloys: A tentative thermodynamic approach. J. Phase Equilib. Diffus. 2016, 37, 2–3. [Google Scholar] [CrossRef][Green Version]
- Kirova, E.; Pisarev, V. System size effect on crystal nuclei morphology in supercooled metallic melt. J. Cryst. Growth 2019, 528, 125266. [Google Scholar] [CrossRef]
- Herlach, D.M. Containerless undercooling and solidification of pure metals. Annu. Rev. Mater. Sci. 1991, 21, 23–44. [Google Scholar] [CrossRef]
- Bai, X.-M.; Li, M. Comparing crystal–melt interfacial free energies through homogeneous nucleation rates. J. Phys. Condens. Matter 2008, 20, 375103. [Google Scholar] [CrossRef]
- Herlach, D.M. Non-equilibrium solidification of undercooled metallic melts. Mater. Sci. Eng. R 1994, 12, 177–272. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Inoue, A. Observation of linear defects in Al particles below 7 nm in size. J. Mater. Res. 2006, 21, 1347. [Google Scholar] [CrossRef]
- Wang, Z.; Ketov, S.V.; Chen, C.L.; Shen, Y.; Ikuhara, Y.; Tsarkov, A.A.; Louzguine-Luzgin, D.V.; Perepezko, J.H. Nucleation and thermal stability of an icosahedral nanophase during the early crystallization stage in Zr-Co-Cu-Al metallic glasses. Acta Mater. 2017, 132, 298–306. [Google Scholar] [CrossRef]
| Metal | T (K) | UN (m/s) |
|---|---|---|
| Fe | 1100 | 48 |
| 900 | 20 | |
| Cu | 850 | 18 |
| 750 | 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Louzguine-Luzgin, D.V.; Bazlov, A.I. Crystallization of FCC and BCC Liquid Metals Studied by Molecular Dynamics Simulation. Metals 2020, 10, 1532. https://doi.org/10.3390/met10111532
Louzguine-Luzgin DV, Bazlov AI. Crystallization of FCC and BCC Liquid Metals Studied by Molecular Dynamics Simulation. Metals. 2020; 10(11):1532. https://doi.org/10.3390/met10111532
Chicago/Turabian StyleLouzguine-Luzgin, Dmitri V., and Andrey I. Bazlov. 2020. "Crystallization of FCC and BCC Liquid Metals Studied by Molecular Dynamics Simulation" Metals 10, no. 11: 1532. https://doi.org/10.3390/met10111532
APA StyleLouzguine-Luzgin, D. V., & Bazlov, A. I. (2020). Crystallization of FCC and BCC Liquid Metals Studied by Molecular Dynamics Simulation. Metals, 10(11), 1532. https://doi.org/10.3390/met10111532





