Effect of Mischmetal Introduction on Hydrogen Storage Properties in Impure Hydrogen Gas of Ti-Fe-Mn-Co Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Composition and Structure Analysis
3.2. Activation Performance
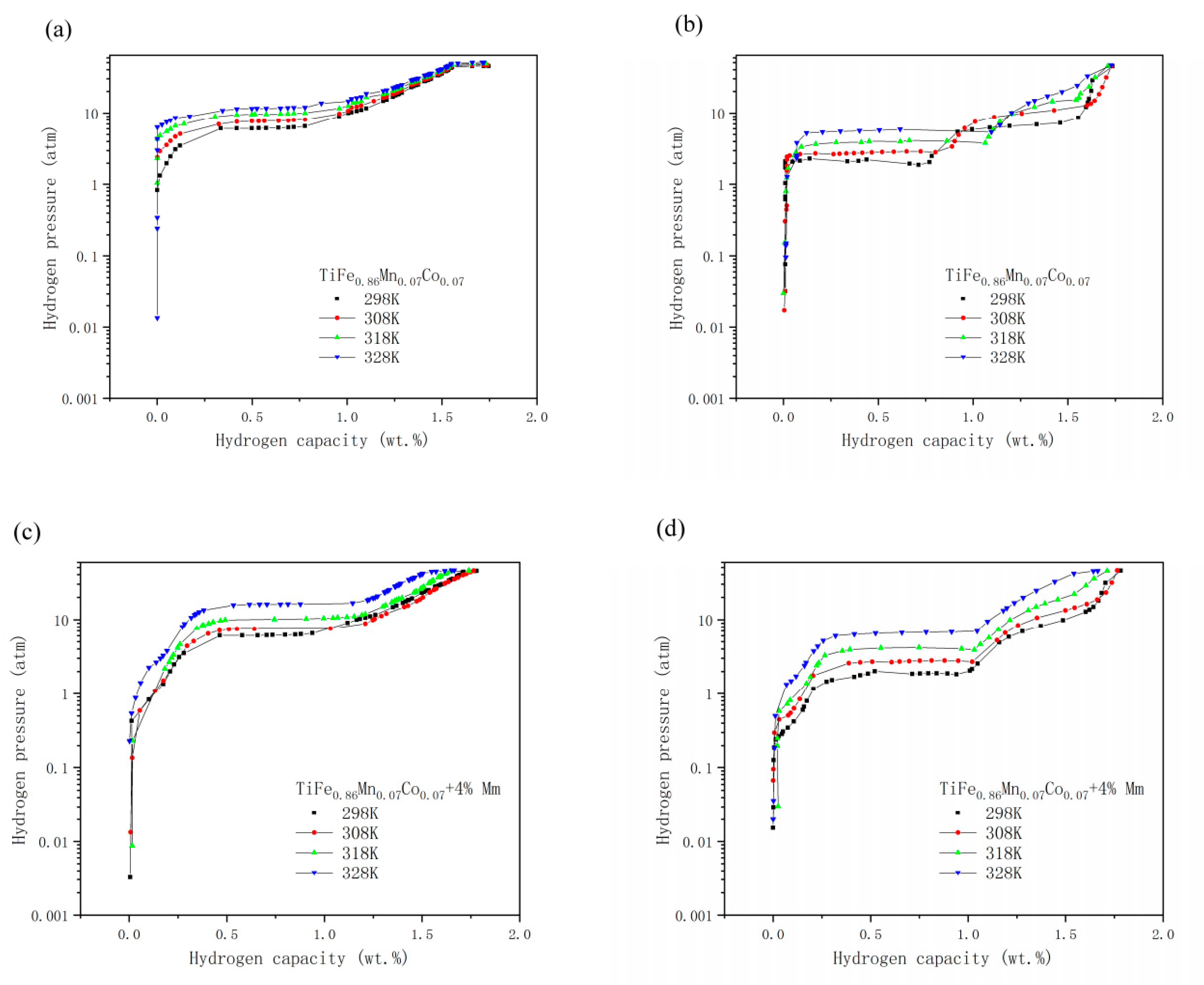
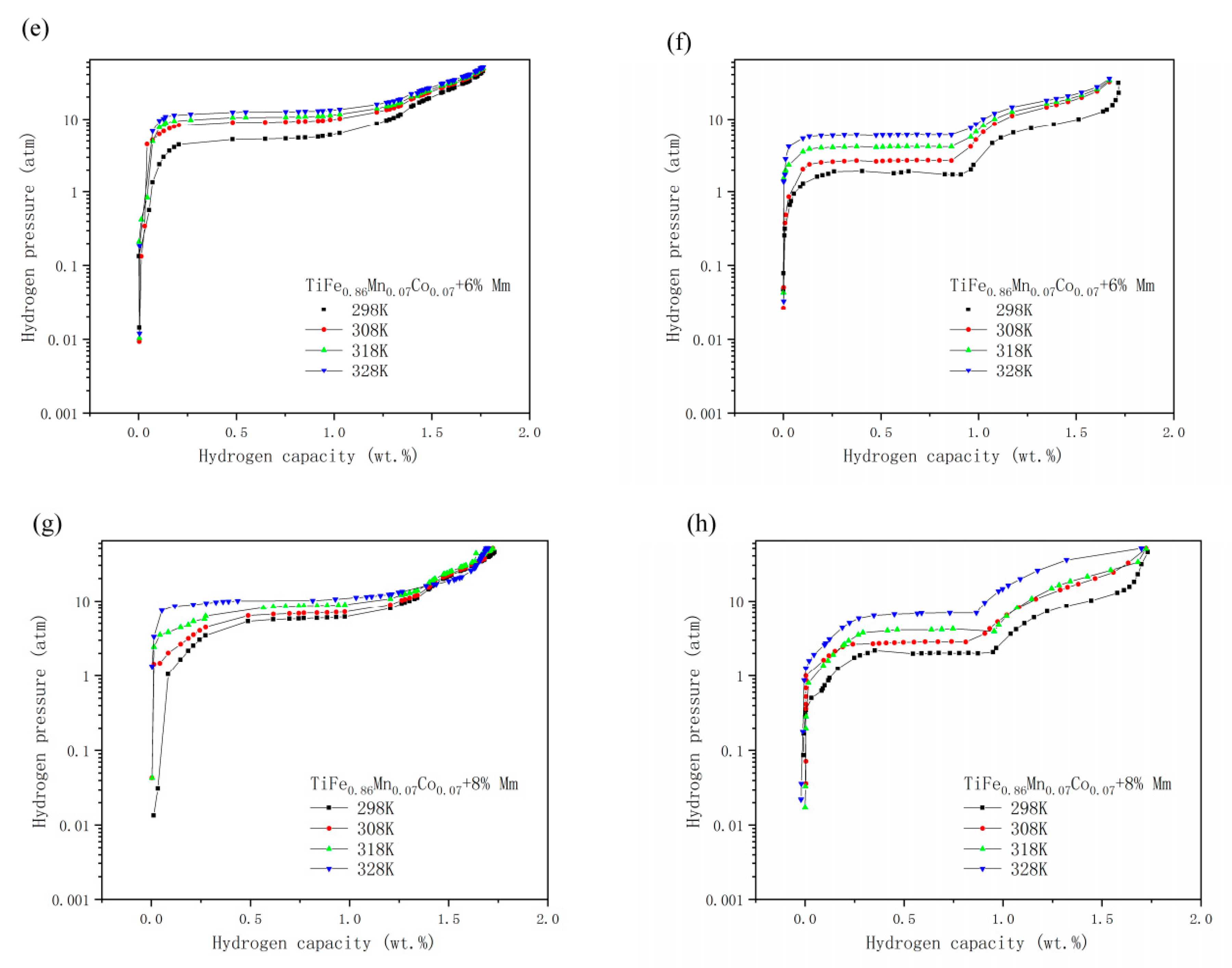
3.3. PCT Tests
3.4. Hydrogen Absorption Cycle Stability Tests
3.5. Cyclic Stability Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Reilly, J.J.; Wiswall, R.H. Formation and Properties of Iron Titanium Hydride. Inorg. Chem. 1974, 13, 77–112. [Google Scholar] [CrossRef]
- Leng, H.; Yu, Z.; Yin, J.; Li, Q.; Wu, Z.; Chou, K.-C. Effects of Ce on the hydrogen storage properties of TiFe0.9Mn0.1 alloy. Int. J. Hydrogen Energy 2017, 42, 23731–23736. [Google Scholar] [CrossRef]
- Qu, H.; Du, J.; Pu, C.; Nyu, Y.; Huang, T.; Li, Z.; Lou, Y.; Wu, Z. Effects of Co introduction on hydrogen storage properties of Ti–Fe–Mn alloys. Int. J. Hydrogen Energy 2015, 40, 2729–2735. [Google Scholar] [CrossRef]
- Gosselin, C.; Huot, J. First Hydrogenation Enhancement in TiFe Alloys for Hydrogen Storage Doped with Yttrium. Metals 2019, 9, 242. [Google Scholar] [CrossRef] [Green Version]
- Ali, W.; Li, M.; Gao, P.; Wu, C.; Li, Q.; Lu, X.; Li, C.-H. Hydrogenation properties of Ti-Fe-Mn alloy with Cu and Y as additives. Int. J. Hydrogen Energy 2017, 42, 2229–2238. [Google Scholar] [CrossRef]
- Ali, W.; Hao, Z.; Li, Z.; Chen, G.; Wu, Z.; Lu, X.; Li, C.-H. Effects of Cu and Y substitution on hydrogen storage performance of TiFe 0.86Mn0.1Y0.1−xCux. Int. J. Hydrogen Energy 2017, 42, 16620–16631. [Google Scholar] [CrossRef]
- Wu, C.L.; Yan, Y.G.; Chen, Y.G.; Tao, M.D.; Zheng, Z. Effect of rare earth (RE) elements on V-based hydrogen storage alloys. Int. J. Hydrogen Energy 2008, 33, 93–97. [Google Scholar] [CrossRef]
- Jain, P.; Gosselin, C.; Skryabina, N.; Fruchart, D.; Huot, J. Hydrogenation properties of TiFe with Zr7Ni10 alloy as additive. J. Alloy. Compd. 2015, 636, 375–380. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, X.; Lionel, G.; Hu, R.; Xue, X.; Fu, H. On the poisoning effect of O2 and N2 for the Zr0.9Ti0.1V2 hydrogen storage alloy. J. Power Sources 2012, 202, 217–224. [Google Scholar] [CrossRef]
- Shwartz, A.; Shamir, N.; Zlakind, S.; Edry, I.; Haim, I.; Mintz, M.H. Initial oxidation of TiFe1−xMnx (x = 0–0.3) by low dose exposures to H2O and O2. J. Alloy. Compd. 2014, 610, 6–10. [Google Scholar] [CrossRef]
- Sandrock, G.; Goodell, P. Surface poisoning of LaNi5, FeTi and (Fe,Mn) Ti by O2, CO and H2O. J. Less Common Met. 1980, 73, 161–168. [Google Scholar] [CrossRef]
- Shihai, G.; Guoqing, W.; Dongliang, Z.; Yanghuan, Z.; Xinlin, W. Study on Hydrogen in Mixed Gas Separated by Rare Earth Hydrogen Storage Alloys. Rare Metal. Mat. Eng. 2011, 40, 189–194. [Google Scholar] [CrossRef]
- Ulmer, U.; Oertel, D.; Diemant, T.; Minella, C.M.; Bergfeldt, T.; Dittmeyer, R.; Behm, R.J.; Fichtner, M. Performance Improvement of V-Fe-Cr-Ti Solid State Hydrogen Storage Materials in Impure Hydrogen Gas. ACS Appl. Mater. Interfaces 2017, 10, 1662–1671. [Google Scholar] [CrossRef] [PubMed]
- Fromm, E. Effect of Oxide Layers on the Absorption Kinetics of Hydrogen by Metals at Room Temperature. Z. Phys. Chem. 1986, 147, 61–75. [Google Scholar] [CrossRef]
- Modi, P.; Aguey-Zinsou, K.-F. Titanium-iron-m manganese (TiFe0.85Mn0.15) alloy for hydrogen storage: Reactivation upon oxidation. Int. J. Hydrogen Energy 2019, 44, 16757–16764. [Google Scholar] [CrossRef]
- Yao, Z.; Liu, L.; Xiao, X.; Wang, C.; Jiang, L.; Chen, L. Effect of rare earth doping on the hydrogen storage performance of Ti1.02Cr1.1Mn0.3Fe0.6alloy for hybrid hydrogen storage application. J. Alloy. Compd. 2018, 731, 524–530. [Google Scholar] [CrossRef]
- Groß, A. Hydrogen Dissociation on Metal Surfaces—A Model System for Reactions on Surfaces. Appl. Phys. A Mater. Sci. Process. 1998, 67, 627–635. [Google Scholar] [CrossRef] [Green Version]
- Karen, P.; Kjekshus, A. Phase Diagrams and Thermodynamic Properties. In Handbook on the Physics and Chemistry of Rare Earths; Gschneider, K.A., Eyring, L., Maple, M.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 30, pp. 229–373. [Google Scholar]











| x | Chemical Composition | Space Group | Lattice Parameter(Å) |
|---|---|---|---|
| 0 | TiFe0.8587Mn0.0702Co0.0695 | Pm-3m | 2.97657 |
| 4 | TiFe0.8487Mn0.0710Co0.0691 + 4.123% Mm | Pm-3m | 2.97226 |
| 6 | TiFe0.8543Mn0.0721Co0.0689 + 5.935% Mm | Pm-3m | 2.97302 |
| 8 | TiFe0.8562Mn0.0699Co0.0703 + 8.024% Mm | Pm-3m | 2.97239 |
| x | Activate Times | Initial Pressure (atm) | Pressure after 3 h (atm) | Δp (atm) |
|---|---|---|---|---|
| 4 | 1 | 47.00 | 39.41 | 7.59 |
| 2 | 47.21 | 34.50 | 12.71 | |
| 3 | 48.06 | 35.24 | 12.82 | |
| 6 | 1 | 47.82 | 41.17 | 6.65 |
| 2 | 47.49 | 35.86 | 11.63 | |
| 3 | 48.00 | 35.66 | 12.34 | |
| 8 | 1 | 47.03 | 37.58 | 9.45 |
| 2 | 48.04 | 35.52 | 12.52 | |
| 3 | 48.05 | 35.58 | 12.47 |
| x | Cmax (wt%) | ΔHd (kJ/mol) | ΔSd (J/K·mol) | Standard Error | R-Square | |
|---|---|---|---|---|---|---|
| Intercept | Slope | |||||
| 4 | 1.76 | −34.59 | −121.15 | 0.4268 | 0.13334 | 0.99693 |
| 6 | 1.73 | −35.86 | −124.82 | 0.3024 | 0.09447 | 0.99856 |
| 8 | 1.72 | −33.07 | −116.47 | 0.9240 | 0.28868 | 0.98436 |
| Alloys | Zone | Ti wt% | Fe wt% | Mn wt% | Co wt% | La wt% | Ce wt% | O wt% |
|---|---|---|---|---|---|---|---|---|
| TiFe0.86Mn0.07Co0.07 + 4% | A | 0.53 | 0.51 | 0.04 | 0.04 | 42.13 | 42.06 | 14.69 |
| B | 43.20 | 43.93 | 4.01 | 3.71 | 0.54 | 0.41 | 4.20 | |
| C | 0.49 | 0.50 | 0.04 | 0.03 | 42.05 | 42.21 | 14.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, R.; Pu, C.; Xu, X.; Xu, Y.; Li, Z.; Wu, Z. Effect of Mischmetal Introduction on Hydrogen Storage Properties in Impure Hydrogen Gas of Ti-Fe-Mn-Co Alloys. Metals 2020, 10, 1574. https://doi.org/10.3390/met10121574
Shen R, Pu C, Xu X, Xu Y, Li Z, Wu Z. Effect of Mischmetal Introduction on Hydrogen Storage Properties in Impure Hydrogen Gas of Ti-Fe-Mn-Co Alloys. Metals. 2020; 10(12):1574. https://doi.org/10.3390/met10121574
Chicago/Turabian StyleShen, Ruochen, Chaohui Pu, Xiaoou Xu, Youpeng Xu, Zhilin Li, and Zhu Wu. 2020. "Effect of Mischmetal Introduction on Hydrogen Storage Properties in Impure Hydrogen Gas of Ti-Fe-Mn-Co Alloys" Metals 10, no. 12: 1574. https://doi.org/10.3390/met10121574




