Recovery of K2SO4 and Separation of SiO2/Al2O3 from Brown Corundum Fly Ash
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Progress
2.3. Analysis Method
3. Results
3.1. Basic Properties of BCFA
3.1.1. Dissolution Characteristics of BCFA
3.1.2. Particle Size Separation of BCFA
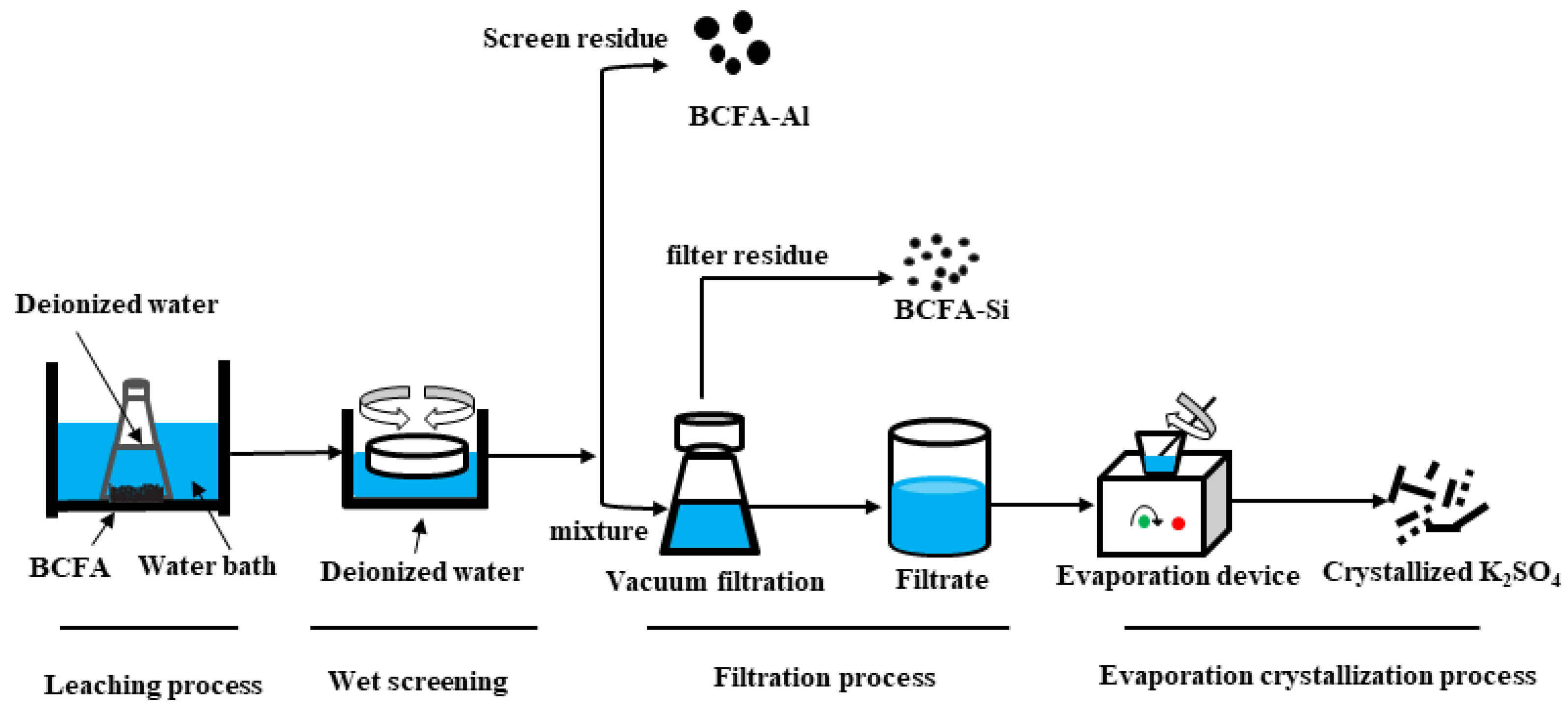
3.2. A Pollution-Free Progress to Recover K2SO4 and Separate Alumina/Silica in BCFA
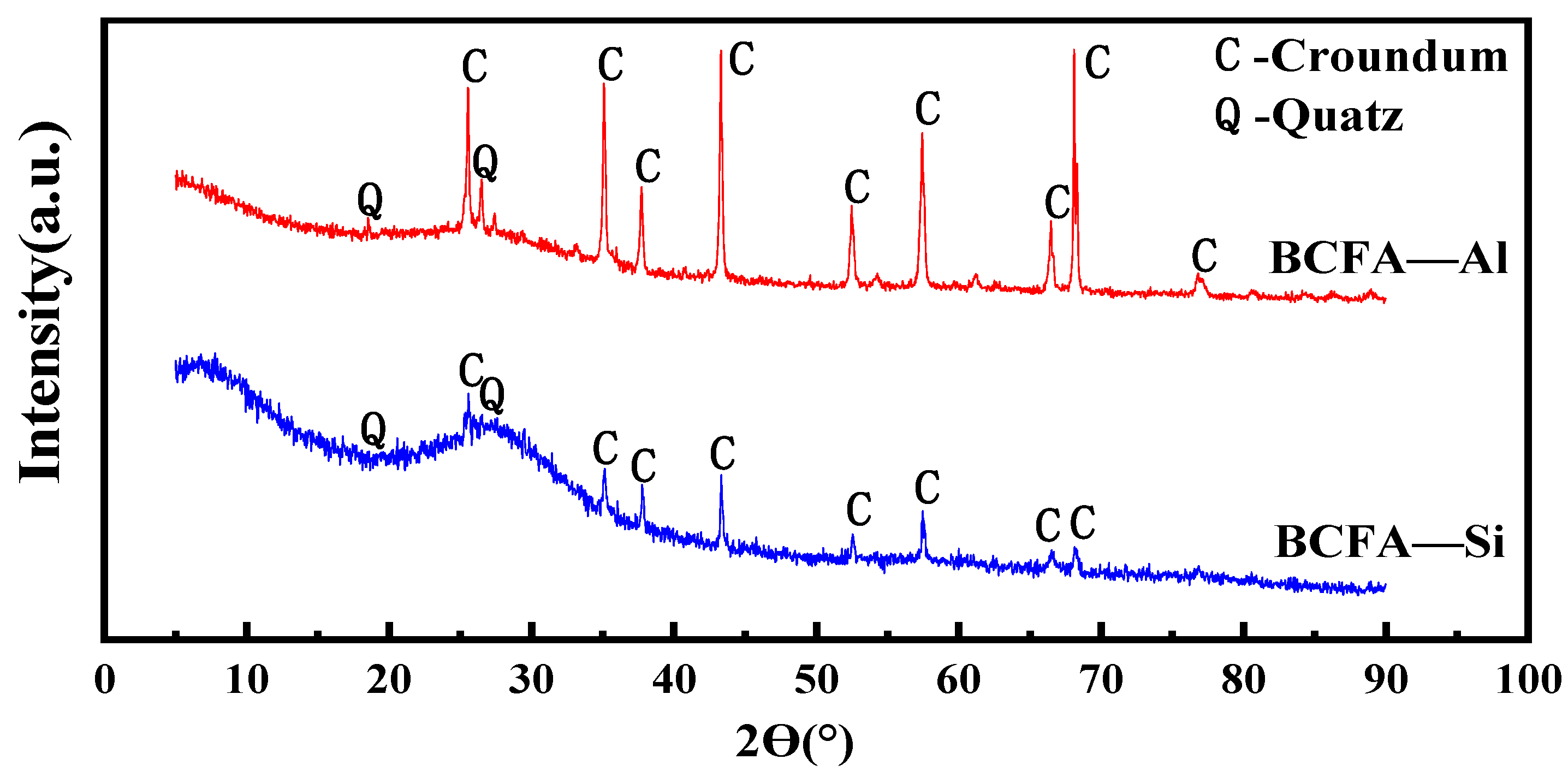
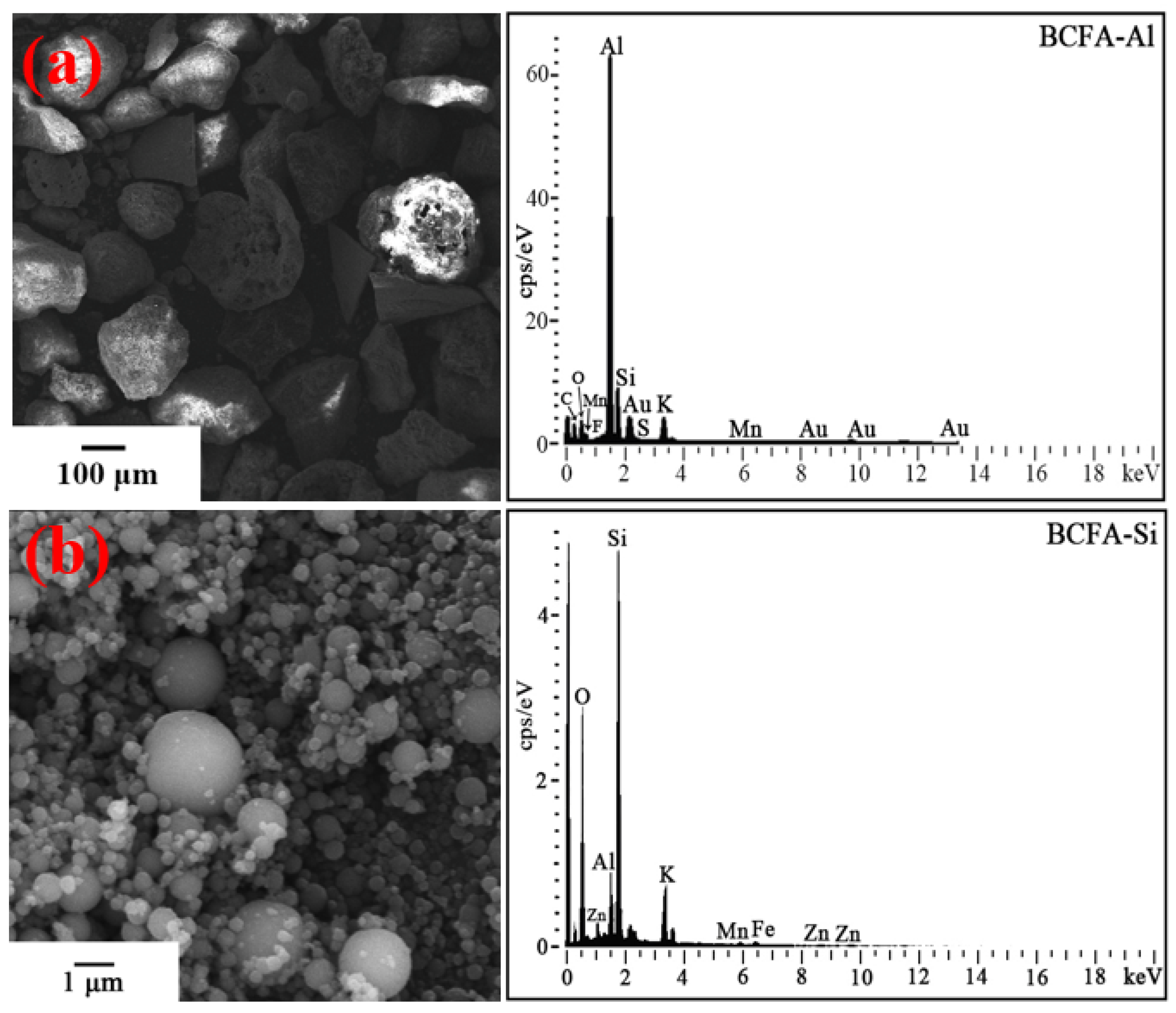
3.3. Separation of the BCFA-Al and BCFA-Si
3.4. Recovery of Potassium Sulfate from BCFA by Water Leaching
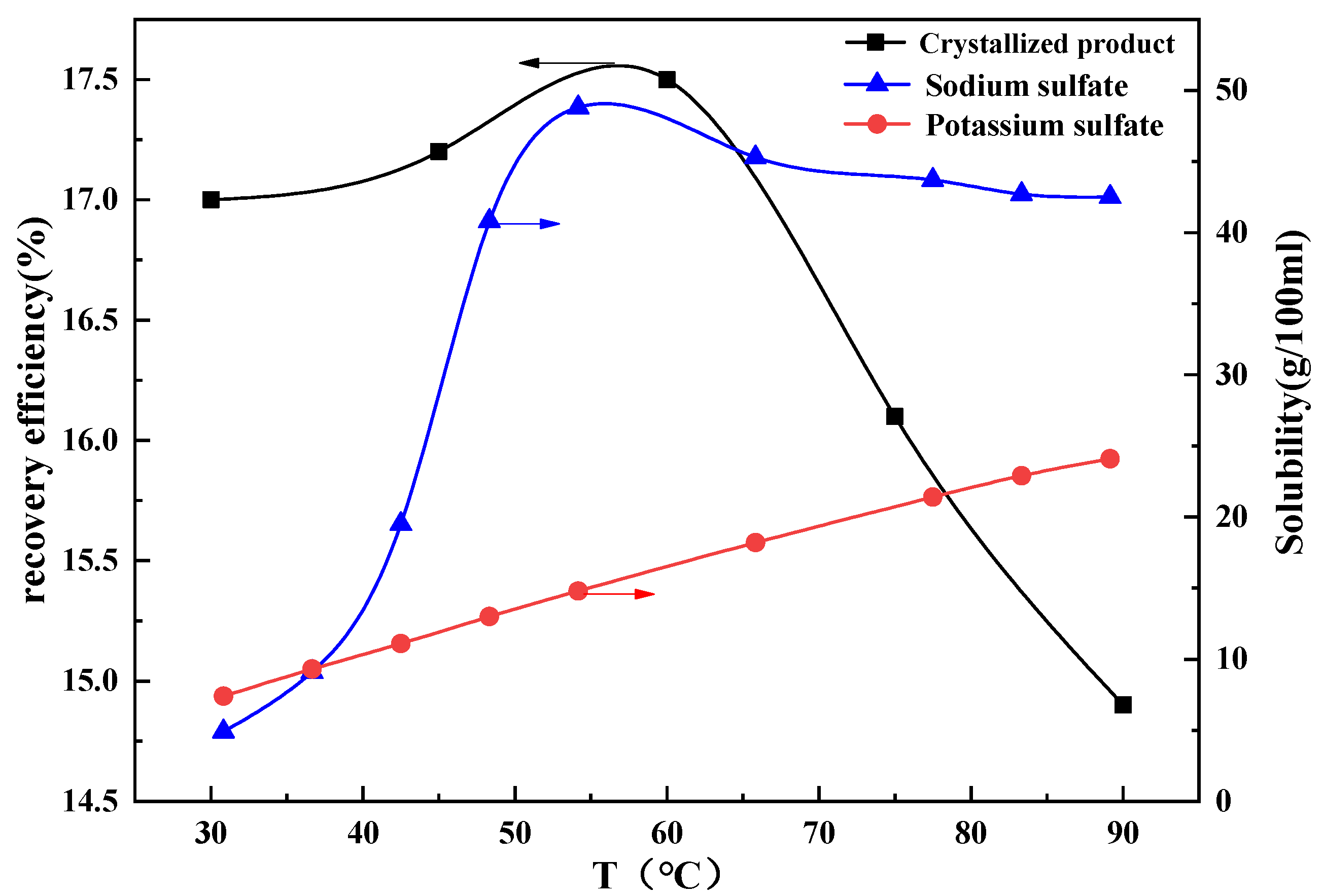
3.4.1. Effect of Temperature on Water Leaching BCFA
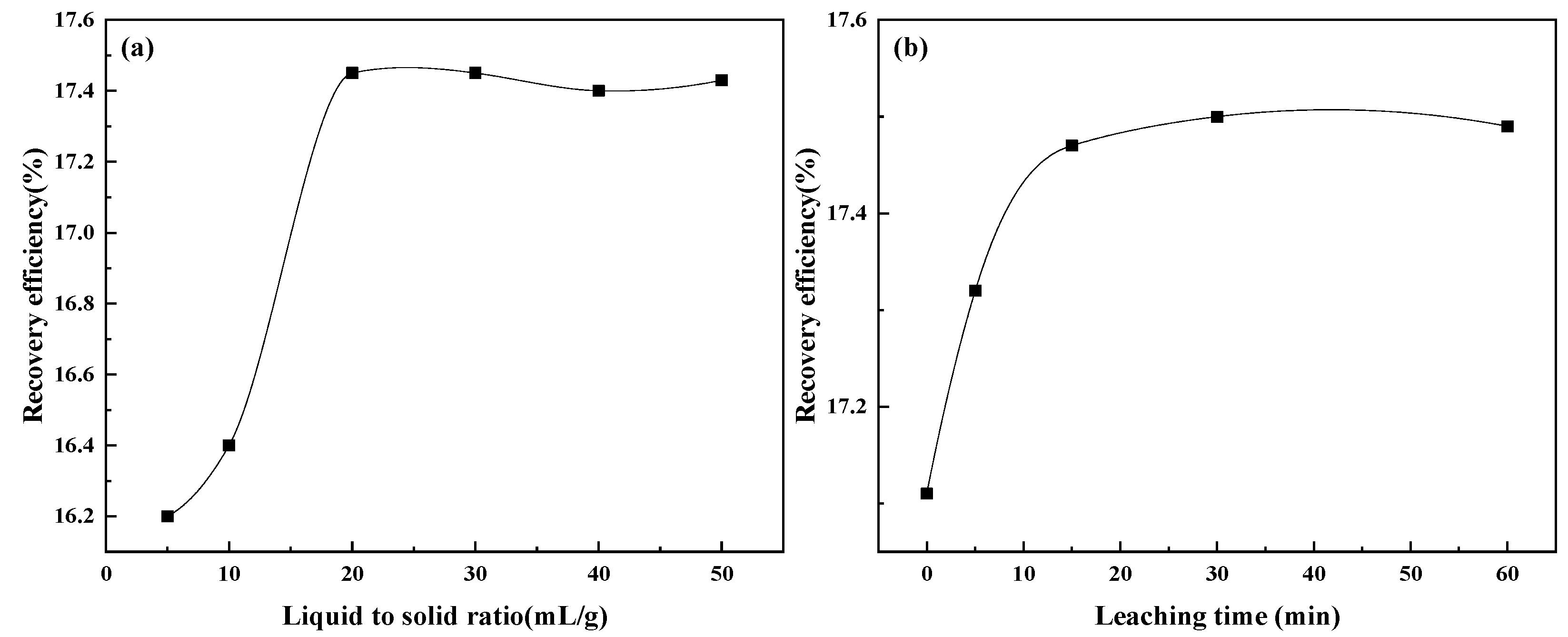
3.4.2. Effect of Liquid to Solid Ratio and Time on Water Leaching BCFA
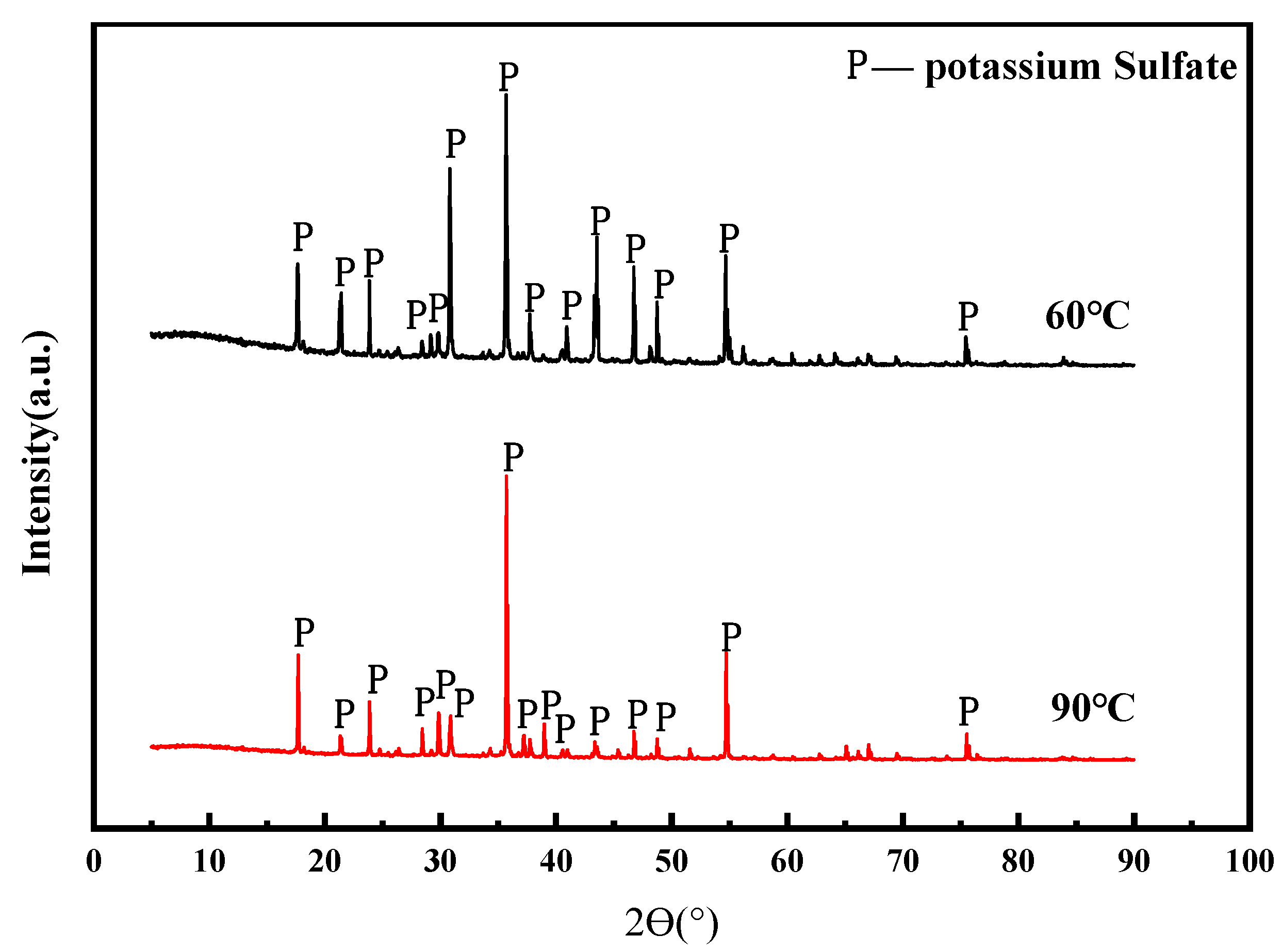
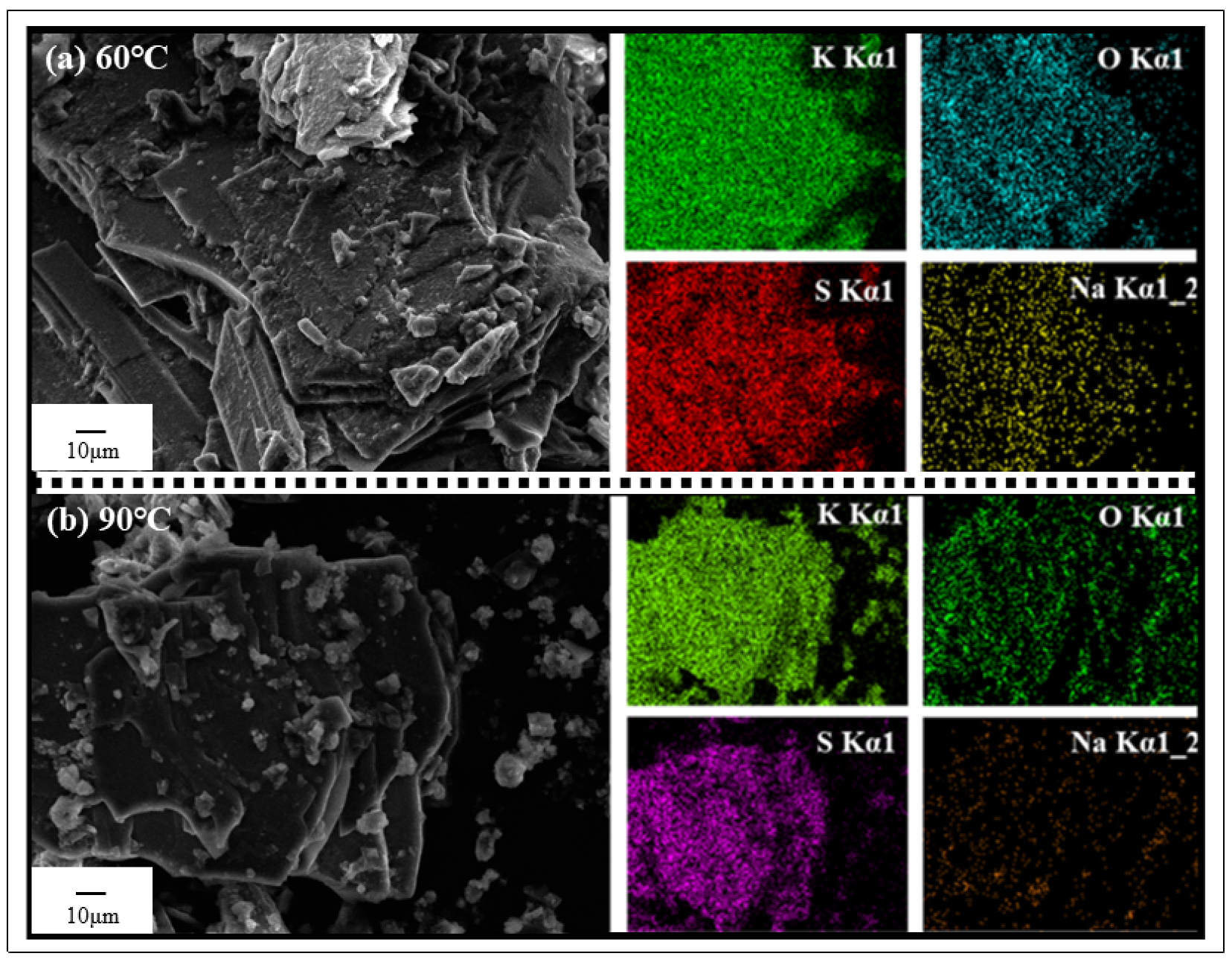
3.4.3. XRF, SEM and EDS Analysis of Crystallized Production
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, J.; Li, H.B.; Duan, Y.H. A facile and reliable preparing route of brown fused alumina from aluminum dross. Key Eng. Mater. 2016, 680, 335–338. [Google Scholar] [CrossRef]
- Karklit, A.K.; Dolgikh, S.G.; Kakhmurov, A.V. Electromelted corundum of northern onega bauxites and refractories based on it. Refract. Ind. Ceram. 1993, 34, 477–485. [Google Scholar] [CrossRef]
- Yang, S.B.; Song, G.L.; Na, Y.J.; Qi, X.B.; Yang, Z. Experimental study on the recovery of sodium in high sodium fly ash from thermochemical conversion of Zhundong coal. Fuel 2018, 229, 22–33. [Google Scholar] [CrossRef]
- Jahromy, S.; Jordan, C.; Azam, M.; Werner, A.; Harasek, M.; Winter, F. Fly ash from municipal solid waste incineration as a potential thermochemical energy storage. Energy Fuels 2019, 33, 5810–5819. [Google Scholar] [CrossRef]
- Yang, S.B.; Feng, C.Q.; Zhang, X.R.; Shang, X.J. Study on recovery and Utilization of brown fused alumina dust collecting powder (in Chinese). Fire Resist. Lime. 2019, 44, 20–25. [Google Scholar]
- Li, H.W.; Wang, N.; Chen, Y.C.; Tian, Y.J. Environmental significance and mineralogical study of brown fused alumina dust recovery (in Chinese). J. Rock Mineral. 1999, 18, 348–356. [Google Scholar]
- Li, H.W.; Hou, H.Q.; Tian, Y.J. The study of component of brown corundum ash. Guizhou Geol. 1999, 16, 57–65. [Google Scholar]
- Li, H.W.; Wang, N.; Chen, Y.C.; Tian, Y.J. The environmental significance of the removal of corundum dusts and the mineralogical study of such dusts (in Chinese). Acta Petrrologica Mineral. 1999, 18, 348–356. [Google Scholar]
- Zhou, Y.F. Treatment and utilization of corundum dust powder (in Chinese). Refract. Mater. 2007, 41, 319–320. [Google Scholar]
- Zhu, Q. Study on the preparation of foam glass from fused brown corundum dust-removing powder (in Chinese). Doctoral Dissertation, Wuhan University of Science and Technology, Wuhan, China, 2011. [Google Scholar]
- Xue, W.D.; Li, Y.; Tong, J. Effect of brown corundum dust powder on the sintering performance of corundum-silicon nitride castable. Adv. Mater. Res. 2011, 194, 2131–2134. [Google Scholar] [CrossRef]
- Wei, B.; Tan, H.; Wang, Y. Investigation of characteristics and formation mechanisms of deposits on different positions in full-scale boiler burning high alkali coal. Appl. Therm. Eng. 2017, 119, 449–458. [Google Scholar] [CrossRef]
- Song, G.; Qi, X.; Song, W. Slagging behaviors of high alkali Zhundong coal during circulating fluidized bed gasification. Fuel 2016, 186, 140–149. [Google Scholar] [CrossRef]
- Merwe, E.M.V.D.; Gray, C.; Castleman, B.A. Ammonium sulphate and/or ammonium bicolpate as extracting agents for the recovery of aluminum from ultrafine coal fly ash. Hydrometallurgy 2017, 171, 185–190. [Google Scholar] [CrossRef]
- Yao, Z.T.; Xia, M.S.; Sarker, P.K. A review of the alumina recovery from coal fly ash, with a focus in China. Fuel 2014, 120, 74–85. [Google Scholar] [CrossRef]
- Mahinroosta, M.; Allahverdi, A. Hazardous aluminum dross characterization and recycling strategies: A critical review. J. Environ. Manag. 2018, 223, 452–468. [Google Scholar] [CrossRef]
- Mahinroosta, M.; Allahverdi, A. Enhanced alumina recovery from secondary aluminum dross for high purity nanostructured γ-alumina powder production: Kinetic study. J. Environ. Manag. 2018, 212, 278–291. [Google Scholar] [CrossRef]
- Hu, Y.; Bakker, M.C.M.; Heij, P.G.D. Recovery and distribution of incinerated aluminum packaging waste. Waste Manag. 2011, 31, 2430. [Google Scholar] [CrossRef]
- Lin, L.Y.; Bai, H. Efficient method for recycling silica materials from waste powder of the photonic industry. Environ. Sci. Technol. 2013, 47, 4636–4643. [Google Scholar] [CrossRef]
- Li, F.; Liu, X.D. Preparation of glass ceramic made from fly ash. Adv. Mater. Res. 2011, 295, 487–490. [Google Scholar] [CrossRef]
- Bai, G.; Teng, W.; Wang, X. Processing and kinetics studies on the alumina enrichment of coal fly ash by fractionating silicon dioxide as nano particles. Fuel Process. Technol. 2010, 91, 175–184. [Google Scholar] [CrossRef]
- Zhang, J.B.; Li, S.P.; Li, H.Q. Acid activation for pre-desilicated high-alumina fly ash. Fuel Process. Technol. 2016, 151, 64–71. [Google Scholar] [CrossRef]
- Ma, N.; Houser, J.B. Recycling of steelmaking slag fines by weak magnetic separation coupled with selective particle size screening. J. Clean. Prod. 2014, 82, 221–231. [Google Scholar] [CrossRef]
- Dalun, Y. Manual of Thermodynamic Data for Practical Inorganic Materials; Metallurgical Industry Press: Beijing, China, 1981. [Google Scholar]
- Zhan, G.; Guo, Z. Basic properties of sintering dust from iron and steel plant and potassium recovery. J. Environ. Sci. 2013, 6, 176–184. [Google Scholar] [CrossRef]
- Zhu, Z.Q.; Wang, K. The improved methanol salting out method was used to separate potassium and sodium mixed salt solution to prepare potassium sulfate (in Chinese). Inorg. Salt Ind. 2013, 7, 18–20. [Google Scholar]

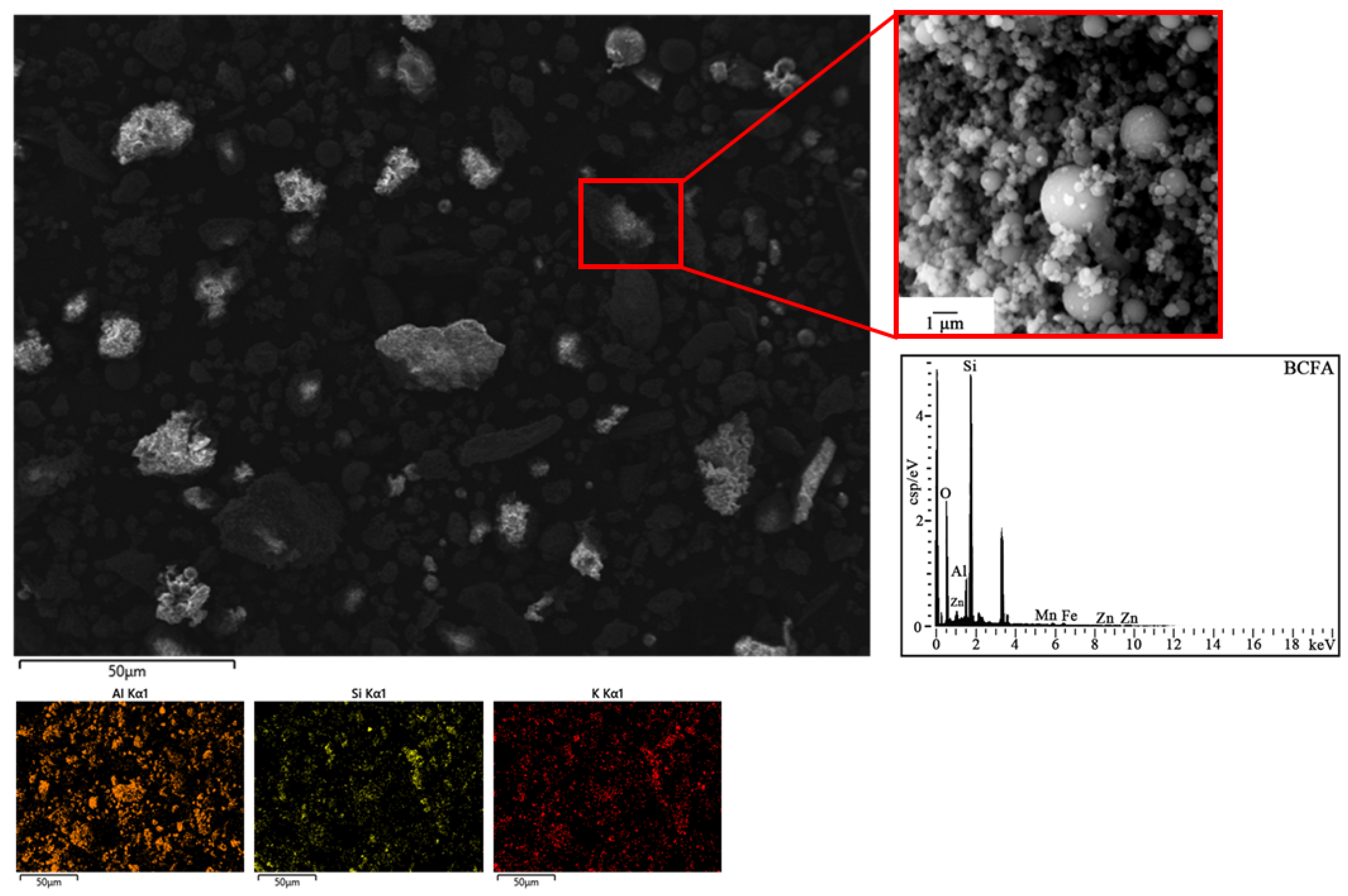
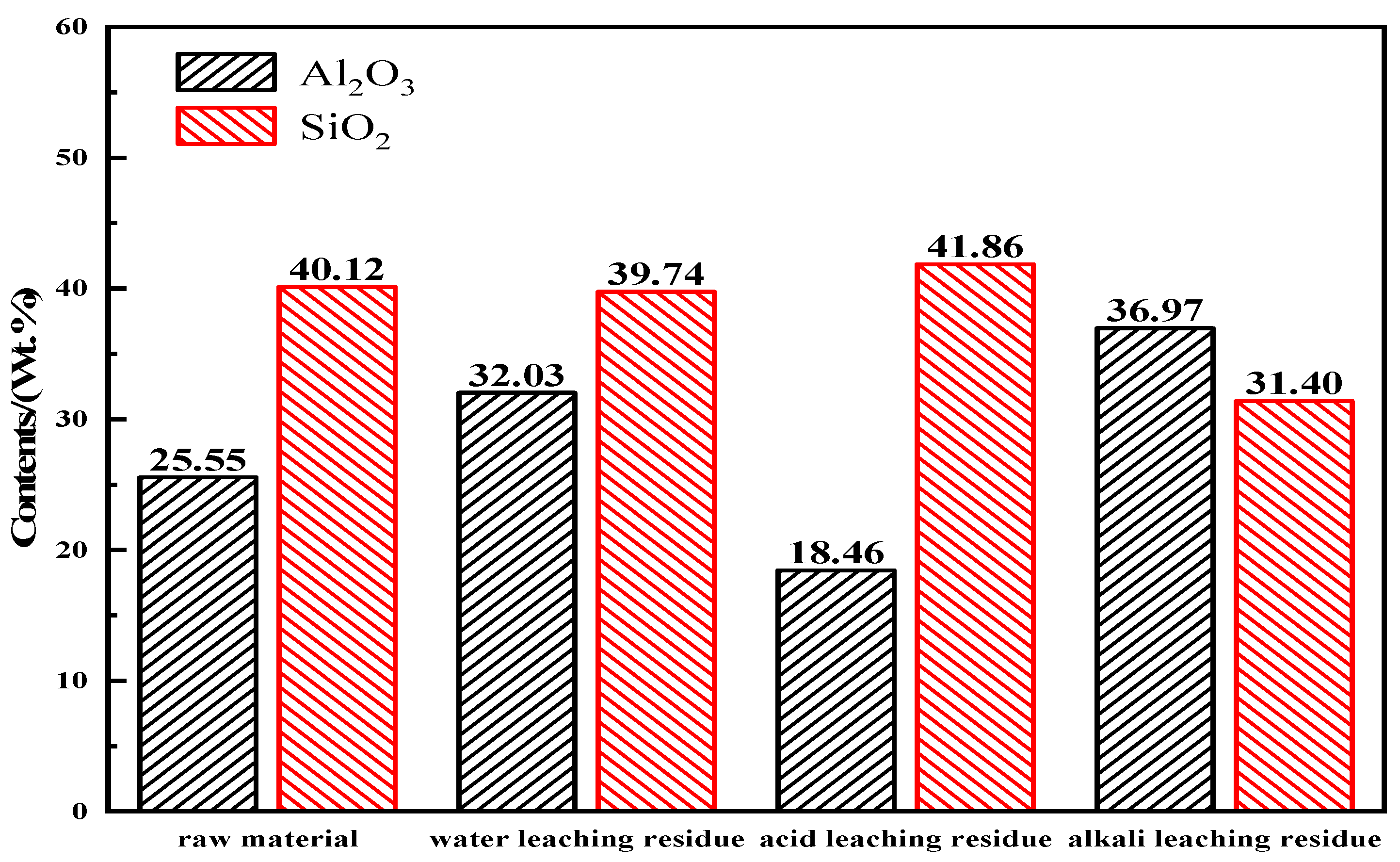
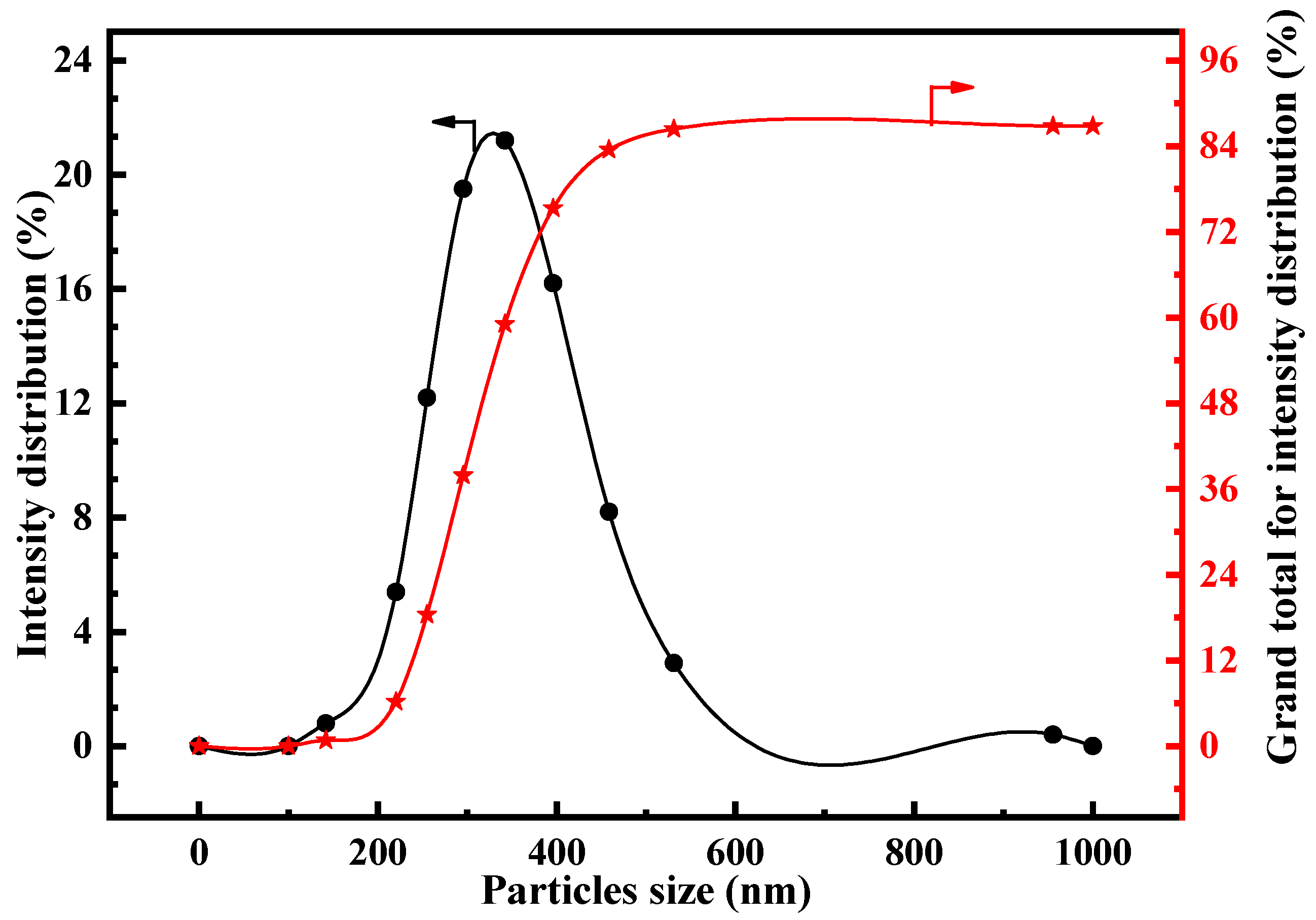
| Al2O3 | SiO2 | TiO2 | K2O | CaO | Fe2O3 | Na2O | ST | F | Cl | A/S |
|---|---|---|---|---|---|---|---|---|---|---|
| 25.55 | 40.12 | 0.92 | 16.55 | 0.32 | 4.88 | 1.21 | 3.85 | 0.51 | 0.36 | 0.64 |
| Leaching Method | Soluble | Insoluble |
|---|---|---|
| Water | 17.50 | 82.50 |
| Acid (1 N HCl) | 40.80 | 59.20 |
| Alkali (Na2O concentration = 110 g/L) | 63.15 | 36.85 |
| Mesh | BCFA-Si | BCFA-Al | ||||
|---|---|---|---|---|---|---|
| ˂1 μm | 1–6.5 μm | 6.5–28 μm | 28–48 μm | 48–109 μm | >109 μm | |
| Percentage (%) | 85.12 | 5.30 | 2.67 | 0.55 | 4.11 | 2.25 |
| Grand total (%) | 85.12 | 90.42 | 93.09 | 93.64 | 97.75 | 100 |
| Compositions | ˂1 μm | >1 μm | >6.5 μm | >13 μm | >18 μm | >28 μm | >48 μm | >109 μm |
|---|---|---|---|---|---|---|---|---|
| Al2O3 | 17.19 | 54.65 | 49.42 | 49.63 | 49.80 | 48.53 | 51.70 | 58.58 |
| SiO2 | 57.57 | 11.04 | 12.40 | 13.60 | 13.99 | 14.70 | 13.26 | 15.62 |
| A/S | 0.30 | 4.95 | 3.99 | 3.65 | 3.56 | 3.30 | 3.87 | 3.75 |
| Al2O3 | SiO2 | Fe2O3 | TiO2 | CaO | MgO | K2O | Na2O | SO3 | F | Cl | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BCFA-Al | 54.65 | 11.04 | 16.21 | 3.95 | 2.58 | 0.57 | 2.04 | 0.57 | 0.72 | 0.83 | 0.25 |
| BCFA-Si | 17.19 | 57.57 | 2.56 | 0.36 | 0.35 | 0.81 | 3.05 | 0.53 | 0.19 | 0.04 | 0.00 |
| K2O | SO3 | F | P2O5 | Cl | Na2O | CaO | Al2O3 | SiO2 | MgO | Fe2O3 | MnO |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 52.27 | 23.43 | 5.89 | 0.45 | 1.53 | 1.53 | 0.34 | 2.78 | 9.55 | 0.18 | 0.35 | 0.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, Y.; Li, J.; Long, Q.; Chen, C.; Lan, Y.; Wang, L. Recovery of K2SO4 and Separation of SiO2/Al2O3 from Brown Corundum Fly Ash. Metals 2020, 10, 1603. https://doi.org/10.3390/met10121603
Xiong Y, Li J, Long Q, Chen C, Lan Y, Wang L. Recovery of K2SO4 and Separation of SiO2/Al2O3 from Brown Corundum Fly Ash. Metals. 2020; 10(12):1603. https://doi.org/10.3390/met10121603
Chicago/Turabian StyleXiong, Yuandong, Junqi Li, Qian Long, Chaoyi Chen, Yuanpei Lan, and Linzhu Wang. 2020. "Recovery of K2SO4 and Separation of SiO2/Al2O3 from Brown Corundum Fly Ash" Metals 10, no. 12: 1603. https://doi.org/10.3390/met10121603
APA StyleXiong, Y., Li, J., Long, Q., Chen, C., Lan, Y., & Wang, L. (2020). Recovery of K2SO4 and Separation of SiO2/Al2O3 from Brown Corundum Fly Ash. Metals, 10(12), 1603. https://doi.org/10.3390/met10121603




