Catalytic Degradability of p-Nitrophenol Using Ecofriendly Silver Nanoparticles
Abstract
1. Introduction
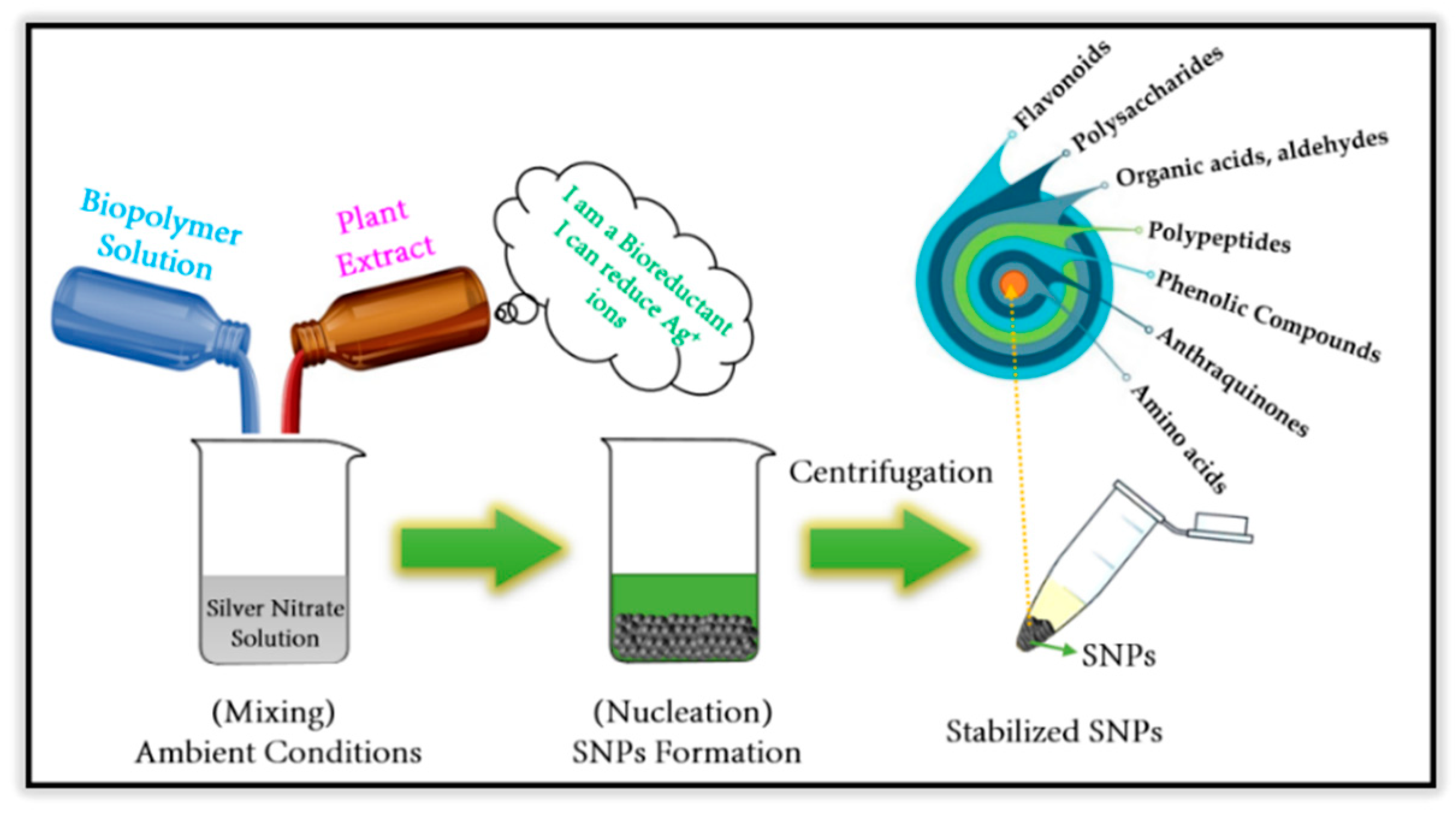
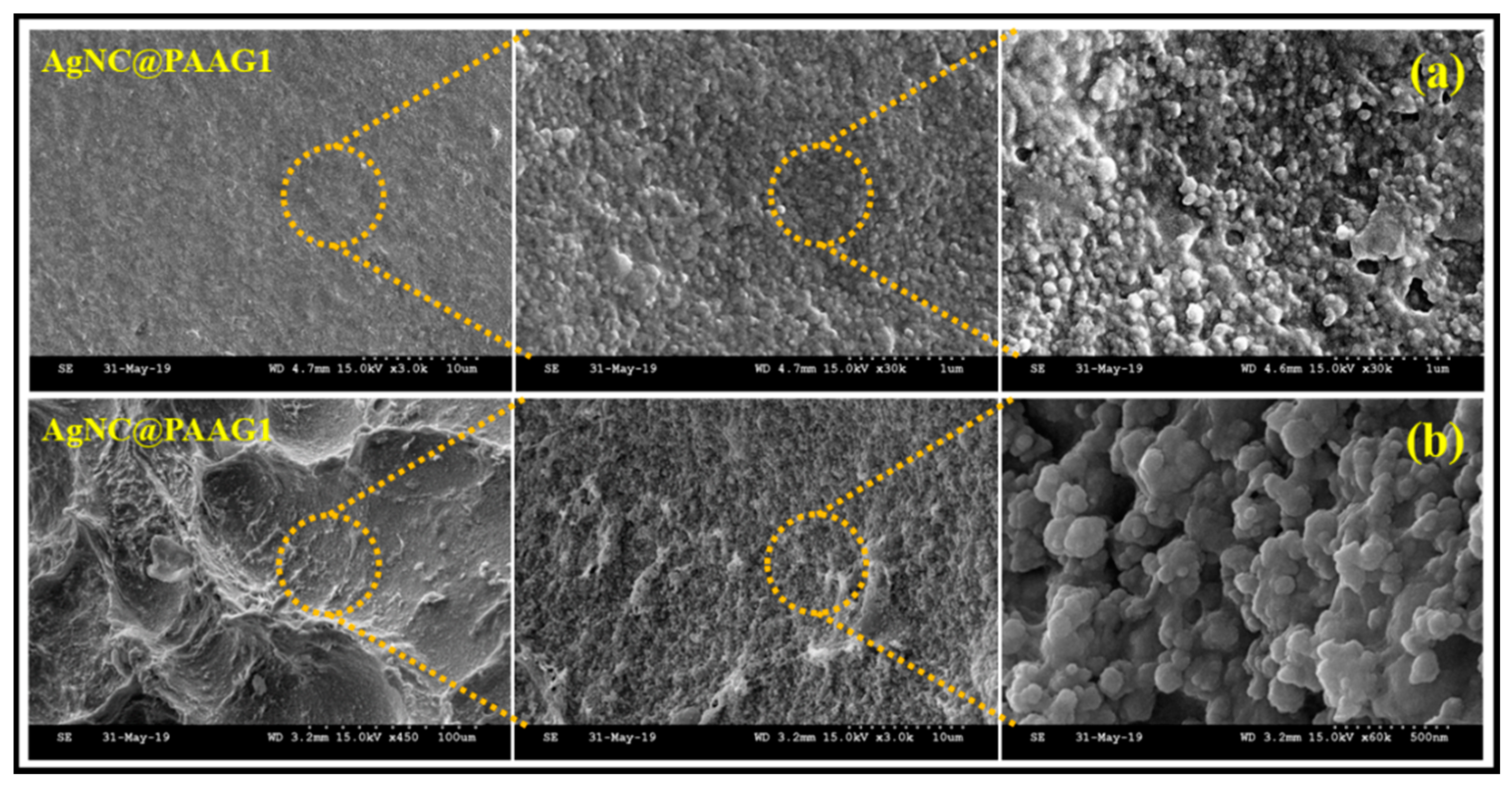
2. Phyto-Synthesis of Silver Nanoparticles and Its Biopolymer Nanocomposites
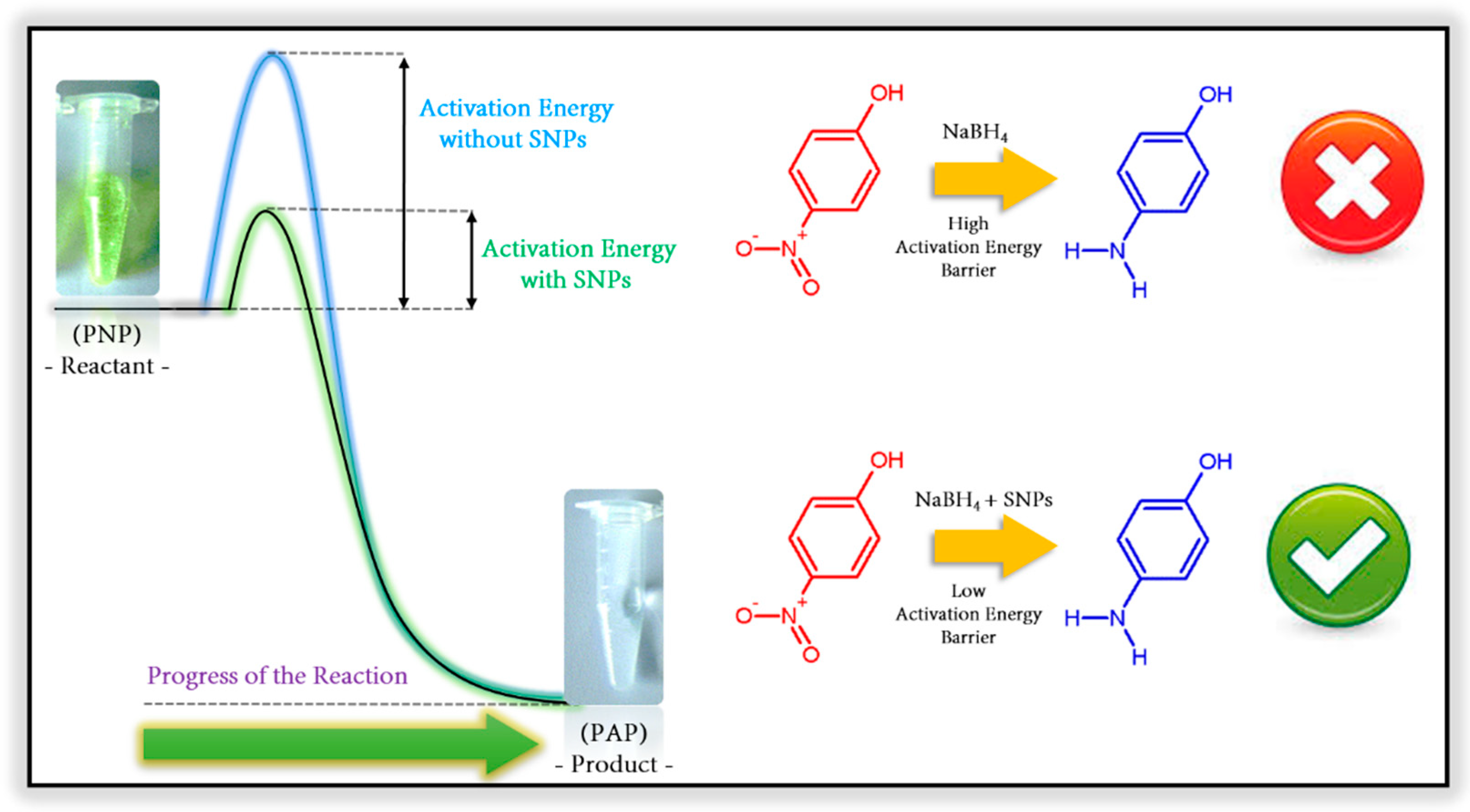
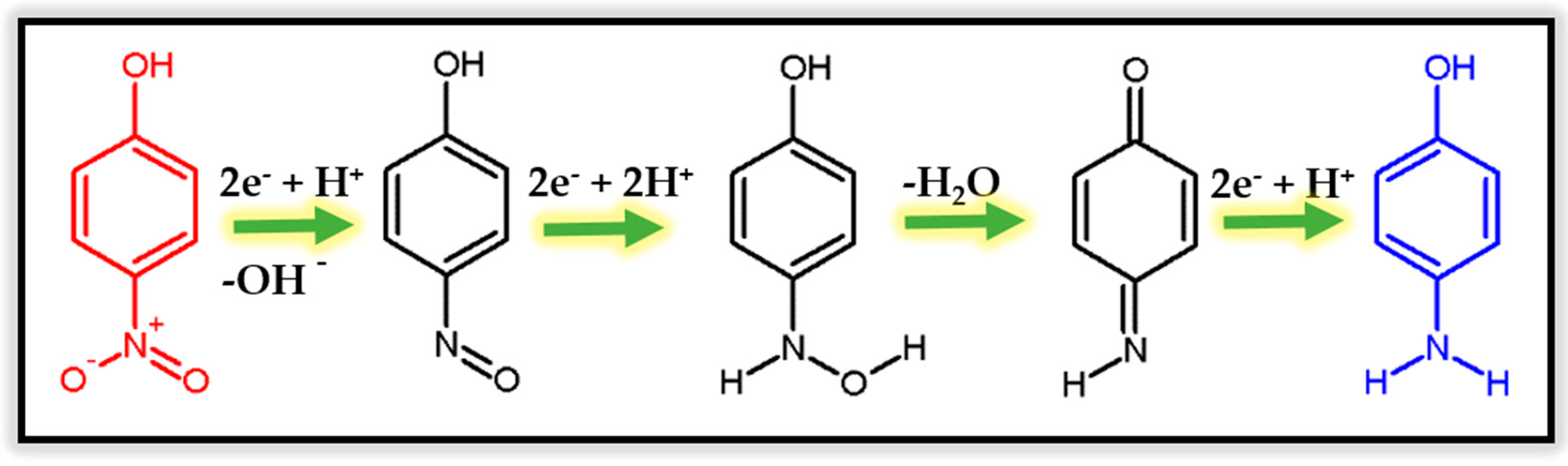
3. Catalytic Degradation of p-Nitrophenol Using Silver Nanoparticles
4. Conclusive Remarks
- A series of well-stabilized SNPs can be achieved with tunable size distribution using plant-mediated protocols.
- Biodegradable and non-toxic polymers in combination with ecofriendly SNPs always play an important role in medicinal and food-based industries.
- Chemical synthesis of SNPs involve the usage of toxic reducing agents and are the subject of environmental concern, so it should be avoided.
- Dynamic tunability of antimicrobial activity of plant-mediated SNPs toward various bacterial strains and several human viral pathogens were observed.
- Ecofriendly SNPs demonstrate extraordinary and unique optical, thermal, and electrical properties of SNPs attracted researchers to utilize in diverse technical fields from photovoltaics to chemical sensors.
- Fabrication of ecofriendly SNPs and its non-toxic biopolymer composites with multi-functional properties are owing to superior catalytic degradability of PNP and wide range of applications in nanocatalysis.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carpenter, D.O. Health effects of persistent organic pollutants: The challenge for the Pacific Basin and for the world. Rev. Env. Health 2011, 26, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Ruzzin, J. Public health concern behind the exposure to persistent organic pollutants and the risk of metabolic diseases. BMC Public Health 2012, 12, 298. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Persistent Organic Pollutants: A Global Issue, a Global Response. Available online: https://www.epa.gov/international-cooperation/persistent-organic-pollutants-global-issue-global-response (accessed on 10 November 2020).
- Bedding, N.D.; McIntyre, A.E.; Perry, R.; Lester, J.N. Organic contaminants in the aquatic environment I. Sources and occurrence. Sci. Total Env. 1982, 25, 143–167. [Google Scholar] [CrossRef]
- Delfino, J.J. Toxic substances in the Great Lakes. Environ. Sci. Technol. 1979, 13, 1462–1468. [Google Scholar] [CrossRef]
- Hjeresen, D.J.; Alamos, L. Green Chemistry: The impact on water quality and supplies (Chapter 2). In Water and Sustainable Development—Opportunities for the Chemical Sciences (A workshop report to the chemical schences roundtable); Norling, P., Wood-Black, F., Masciangioli, T.M., Eds.; The National Academies Press: Washington, DC, USA, 2004; ISBN 0-309-09200-0. [Google Scholar]
- Royal Society of Chemistry. Silver. Available online: https://www.rsc.org/periodic-table/element/47/silver#:~:text=Silver%20is%20used%20to%20make,used%20for%20making%20printed%20circuits (accessed on 10 November 2020).
- Geology News and Information. The Many Uses of Silver. Available online: https://geology.com/articles/uses-of-silver/ (accessed on 10 November 2020).
- Lo, V.K.-Y.; Chan, A.O.-Y.; Che, C.-M. Gold and silver catalysis: From organic transformation to bioconjugation. Org. Biomol. Chem. 2015, 13, 6667–6680. [Google Scholar] [CrossRef]
- Mudarra, A.L.; de Salinas, S.M.; Pérez-Temprano, M.H. Beyond the traditional roles of Ag in catalysis: The transmetalating ability of organosilver(i) species in Pd-catalysed reactions. Org. Biomol. Chem. 2019, 17, 1655–1667. [Google Scholar] [CrossRef] [PubMed]
- Sambale, F.; Wagner, S.; Stahl, F.; Khaydarov, R.R.; Scheper, T.; Bahnemann, D. Investigations of the Toxic Effect of Silver Nanoparticles on Mammalian Cell Lines. J. Nanomater. 2015, 2015, 136765. [Google Scholar] [CrossRef]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef]
- Lekamge, S.; Miranda, A.F.; Abraham, A.; Li, V.; Shukla, R.; Bansal, V.; Nugegoda, D. The Toxicity of Silver Nanoparticles (AgNPs) to Three Freshwater Invertebrates with Different Life Strategies: Hydra vulgaris, Daphnia carinata, and Paratya australiensis. Front. Environ. Sci. 2018, 6, 152. [Google Scholar] [CrossRef]
- Ahmad, S.; Munir, S.; Zeb, N.; Ullah, A.; Khan, B.; Ali, J.; Bilal, M.; Omer, M.; Alamzeb, M.; Salman, S.M.; et al. Green nanotechnology: A review on green synthesis of silver nanoparticles—An ecofriendly approach. Int. J. Nanomed. 2019, 14, 5087–5107. [Google Scholar] [CrossRef]
- Rauwel, P.; Küünal, S.; Ferdov, S.; Rauwel, E. A Review on the Green Synthesis of Silver Nanoparticles and Their Morphologies Studied via TEM. Adv. Mater. Sci. Eng. 2015, 2015, 682749. [Google Scholar] [CrossRef]
- Rafique, M.; Sadaf, I.; Rafique, M.S.; Tahir, M.B. A review on green synthesis of silver nanoparticles and their applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1272–1291. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, K.S.; Husen, A.; Rao, R.A.K. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnol. 2018, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Mahiuddin, M.; Saha, P.; Ochiai, B. Green Synthesis and Catalytic Activity of Silver Nanoparticles Based on Piper chaba Stem Extracts. Nanomaterials 2020, 10, 1777. [Google Scholar] [CrossRef]
- Gangula, A.; Podila, R.; Ramakrishna, M.; Karanam, L.; Janardhana, C.; Rao, A.M. Catalytic Reduction of 4-Nitrophenol using Biogenic Gold and Silver Nanoparticles Derived from Breynia rhamnoides. Langmuir 2011, 27, 15268–15274. [Google Scholar] [CrossRef]
- Capeness, M.; Echavarri-Bravo, V.; Horsfall, L.E. Production of Biogenic Nanoparticles for the Reduction of 4-Nitrophenol and Oxidative Laccase-Like Reactions. Front. Microbiol. 2019, 10, 997. [Google Scholar] [CrossRef]
- Burlacu, E.; Tanase, C.; Coman, N.-A.; Berta, L. A Review of Bark-Extract-Mediated Green Synthesis of Metallic Nanoparticles and Their Applications. Molecules 2019, 24, 4354. [Google Scholar] [CrossRef]
- Park, Y. A New Paradigm Shift for the Green Synthesis of Antibacterial Silver Nanoparticles Utilizing Plant Extracts. Toxicol. Res. 2014, 30, 169–178. [Google Scholar] [CrossRef]
- Kulandaivelu, B.; Gothandam, K.M. Cytotoxic Effect on Cancerous Cell Lines by Biologically Synthesized Silver Nanoparticles. Braz. Arch. Biol. Technol. 2016, 59, e16150529. [Google Scholar] [CrossRef]
- Suganya, S.; Dhanalakshmi, B.; Kumar, S.D.; Santhanam, P. Cytotoxic Effect of Silver Nanoparticles Synthesized from Sargassum wightii on Cervical Cancer Cell Line. Proc. Natl. Acad. Sci. USA India Sect. B Biol. Sci. 2020, 90, 811–818. [Google Scholar] [CrossRef]
- Selvi, B.C.G.; Madhavan, J.; Santhanam, A. Cytotoxic effect of silver nanoparticles synthesized from Padina tetrastromatica on breast cancer cell line. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 035015. [Google Scholar] [CrossRef]
- Stephen, A.; Seethalakshmi, S. Phytochemical Synthesis and Preliminary Characterization of Silver Nanoparticles Using Hesperidin. J. Nanosci. 2013, 2013, 126564. [Google Scholar] [CrossRef]
- Narchin, F.; Larijani, K.; Rustaiyan, A.; Ebrahimi, S.N.; Tafvizi, F. Phytochemical Synthesis of Silver Nanoparticles by Two Techniques Using Saturaja rechengri Jamzad Extract: Identifying and Comparing in Vitro Anti-Proliferative Activities. Adv. Pharm. Bull. 2018, 8, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Azizian-Shermeh, O.; Valizadeh, M.; Taherizadeh, M.; Beigomi, M. Phytochemical investigation and phytosynthesis of eco-friendly stable bioactive gold and silver nanoparticles using petal extract of saffron (Crocus sativus L.) and study of their antimicrobial activities. Appl. Nanosci. 2020, 10, 2907–2920. [Google Scholar] [CrossRef]
- Ratan, Z.A.; Haidere, M.F.; Nurunnabi, M.; Shahriar, S.M.; Shahriar, A.J.S.; Shim, Y.Y.; Reaney, M.J.T.; Cho, J.Y. Green Chemistry Synthesis of Silver Nanoparticles and Their Potential Anticancer Effects. Cancers 2020, 12, 855. [Google Scholar] [CrossRef] [PubMed]
- Mallikarjuna, K.; Sushma, N.J.; Narasimha, G.; Manoj, L.; Raju, B.D.P. Phytochemical fabrication and characterization of silver nanoparticles by using Pepper leaf broth. Arab. J. Chem. 2014, 7, 1099–1103. [Google Scholar] [CrossRef]
- Khorrami, S.; Khorrami, A.; Khorrami, A. Green synthesis of silver nanoparticles at low temperature in a fast pace with unique DPPH radical scavenging and selective cytotoxicity against MCF-7 and BT-20 tumor cell lines. Biotechnol. Rep. 2019, 24, e00393. [Google Scholar] [CrossRef]
- Kim, J.; Kwon, S.; Jeong, S. Preparation of biodegradable polymer/silver nanoparticles composite and its antibacterial efficacy. J. Nanosci. Nanotechnol. 2009, 9, 1098–1102. [Google Scholar] [CrossRef]
- Kraśniewska, K.; Galus, S.; Gniewosz, M. Biopolymers-Based Materials Containing Silver Nanoparticles as Active Packaging for Food Applications—A Review. Int. J. Mol. Sci. 2020, 21, 698. [Google Scholar] [CrossRef]
- Carbone, M.; Donia, D.M.; Sabbatella, G.; Antiochia, R. Silver nanoparticles in polymeric matrices for fresh food packaging. J. King Saud Univ. Sci. 2016, 28, 273–279. [Google Scholar] [CrossRef]
- Safari, J.; Najafabadi, A.E.; Zarnegar, Z.; Masoule, S.F. Catalytic performance in 4-nitrophenol reduction by Ag nanoparticles stabilized on biodegradable amphiphilic copolymers. Green Chem. Lett. Rev. 2016, 9, 20–26. [Google Scholar] [CrossRef]
- Rodrigues, T.S.; da Silva, A.G.M.; Camargo, P.H.C. Nanocatalysis by noble metal nanoparticles: Controlled synthesis for the optimization and understanding of activities. J. Mater. Chem. A 2019, 7, 5857–5874. [Google Scholar] [CrossRef]
- Pandey, S.; Do, J.Y.; Kim, J.; Kang, M. Fast and highly efficient catalytic degradation of dyes using κ-carrageenan stabilized silver nanoparticles nanocatalysts. Carbohydr. Polym. 2020, 230, 115597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Suh, J.M.; Choi, J.-W.; Jang, H.W.; Shokouhimehr, M.; Varma, R.S. Recent Advances in the Nanocatalyst-Assisted NaBH4 Reduction of Nitroaromatics in Water. ACS Omega 2019, 4, 483–495. [Google Scholar] [CrossRef]
- Salam, N.; Banerjee, B.; Roy, A.S.; Mondal, P.; Roy, S.; Bhaumik, A.; Islam, S.K. Silver nanoparticles embedded over mesoporous organic polymer as highly efficient and reusable nanocatalyst for the reduction of nitroarenes and aerobic oxidative esterification of alcohols. Appl. Catal. A Gen. 2014, 477, 184–194. [Google Scholar] [CrossRef]
- Hazlet, S.E.; Dornfeld, C.A. The Reduction of Aromatic Nitro Compounds with Activated Iron. J. Am. Chem. Soc. 1944, 66, 1781–1782. [Google Scholar] [CrossRef]
- Zhu, K.; Shaver, M.P.; Thomas, S.P. Chemoselective nitro reduction and hydroamination using a single iron catalyst. Chem. Sci. 2016, 7, 3031–3035. [Google Scholar] [CrossRef]
- Agrawal, A.; Tratnyek, P.G. Reduction of Nitro Aromatic Compounds by Zero-Valent Iron Metal. Environ. Sci. Technol. 1995, 30, 153–160. [Google Scholar] [CrossRef]
- Chemistry—Stack Exchange. Preference for Tin or Iron in the Reduction of Nitrobenzene. Available online: https://chemistry.stackexchange.com/questions/110602/preference-for-tin-or-iron-in-the-reduction-of-nitrobenzene (accessed on 10 November 2020).
- Popat, V.; Padhiyar, N. Kinetic Study of Bechamp Process for P-Nitrotoluene Reduction to P-Toluidine. Int. J. Chem. Eng. Appl. 2013, 4, 401–405. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.-Y.; Huang, J.; Chen, C.-Y.; Wang, Z.-X.; Xie, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef]
- Castillo-Henríquez, L.; Alfaro-Aguilar, K.; Ugalde-Álvarez, J.; Vega-Fernández, L.; de Oca-Vásquez, G.M.; Vega-Baudrit, J.R. Green Synthesis of Gold and Silver Nanoparticles from Plant Extracts and Their Possible Applications as Antimicrobial Agents in the Agricultural Area. Nanomaterials 2020, 10, 1763. [Google Scholar] [CrossRef] [PubMed]
- Hanan, N.A.; Chiu, H.I.; Ramachandran, M.R.; Tung, W.H.; Zain, N.N.M.; Yahaya, N.; Lim, V. Cytotoxicity of Plant-Mediated Synthesis of Metallic Nanoparticles: A Systematic Review. Int. J. Mol. Sci. 2018, 19, 1725. [Google Scholar] [CrossRef]
- Fayez, H.; El-Motaleb, M.A.; Selim, A.A. Synergistic Cytotoxicity of Shikonin-Silver Nanoparticles as an Opportunity for Lung Cancer. J. Label. Compd. Radiopharm. 2020, 63, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S. Plant gums for sustainable and eco-friendly synthesis of nanoparticles: Recent advances. Inorg. Nano-Met. Chem. 2020, 50, 469–488. [Google Scholar] [CrossRef]
- Siddiqui, M.Z.; Chowdhury, A.R.; Singh, B.R.; Maurya, S.; Prasad, N. Synthesis, Characterization and Antimicrobial Evaluation of Piyar Gum-Induced Silver Nanoparticles. Natl. Acad. Sci. Lett. 2020. [Google Scholar] [CrossRef]
- Anwar, A.; Masri, A.; Rao, K.; Rajendran, K.; Khan, N.A.; Shah, M.R.; Siddiqui, R. Antimicrobial activities of green synthesized gums-stabilized nanoparticles loaded with flavonoids. Sci. Rep. 2019, 9, 3122. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Pandey, S.; Mewada, A.; Shah, R.; Oza, G.; Sharon, M. Understanding the stability of silver nanoparticles bio-fabricated using Acacia arabica (Babool gum) and its hostile effect on microorganisms. Spectrochim. Acta A 2013, 109, 344–347. [Google Scholar] [CrossRef]
- Gengan, R.M.; Anand, K.; Phulukdaree, A.; Chuturgoon, A. A549 lung cell line activity of biosynthesized silver nanoparticles using Albizia adianthifolia leaf. Colloids Surf. B 2013, 105, 87–91. [Google Scholar] [CrossRef]
- Niraimathi, K.L.; Sudha, V.; Lavanya, R.; Brindha, P. Biosynthesis of silver nanoparticles using Alternanthera sessilis (Linn.) extract and their antimicrobial, antioxidant activities. Colloids Surf. B 2013, 102, 288–291. [Google Scholar] [CrossRef]
- Das, G.; Patra, J.K.; Debnath, T.; Ansari, A.; Shin, H.S. Investigation of antioxidant, antibacterial, antidiabetic, and cytotoxicity potential of silver nanoparticles synthesized using the outer peel extract of Ananas comosus (L.). PLoS ONE 2019, 14, e0220950. [Google Scholar] [CrossRef]
- Vivek, R.; Thangam, R.; Muthuchelian, K.; Gunasekaran, P.; Kaveri, K.; Kannan, S. Green biosynthesis of silver nanoparticles from Annona squamosa leaf extract and its in vitro cytotoxic effect on MCF-7 cells. Process Biochem. 2012, 47, 2405–2410. [Google Scholar] [CrossRef]
- Velusamy, P.; Das, J.; Pachaiappan, R.; Vaseeharan, B.; Pandian, K. Greener approach for synthesis of antibacterial silver nanoparticles using aqueous solution of neem gum (Azadirachta indica L.). Ind. Crop. Prod. 2015, 66, 103–109. [Google Scholar] [CrossRef]
- Nazeruddin, G.M.; Prasad, N.R.; Waghmare, S.R.; Garadkar, K.M.; Mulla, I.S. Extracellular biosynthesis of silver nanoparticle using Azadirachta indica leaf extract and its anti-microbial activity. J. Alloys Compd. 2014, 583, 272–277. [Google Scholar] [CrossRef]
- Vijay Kumar, P.P.N.; Pammi, S.V.N.; Kollu, P.; Satyanarayana, K.V.V.; Shameem, U. Green synthesis and characterization of silver nanoparticles using Boerhaavia diffusa plant extract and their antibacterial activity. Ind. Crop. Prod. 2014, 52, 562–566. [Google Scholar] [CrossRef]
- Ansar, S.; Tabassum, H.; Aladwan1, N.S.M.; Ali, M.N.; Almaarik, B.; AlMahrouqi, S.; Abudawood, M.; Banu, N.; Alsubki, R. Eco friendly silver nanoparticles synthesis by Brassica oleracea and its antibacterial, anticancer and antioxidant properties. Sci. Rep. 2020, 10, 18564. [Google Scholar] [CrossRef]
- Moteriya, P.; Chanda, S. Green Synthesis of Silver Nanoparticles from Caesalpinia pulcherrima Leaf Extract and Evaluation of Their Antimicrobial, Cytotoxic and Genotoxic Potential (3-in-1 System). J. Inorg. Organomet. Polym. 2020, 30, 3920–3932. [Google Scholar] [CrossRef]
- Vinay, S.P.; Chandrasekhar, N. Green Synthesis and Characterization of Silver Nanoparticles using Cassia auriculata Leaves Extract and Its Efficacy as A Potential Antibacterial and Cytotoxic Effect. Adv. Mater. Lett. 2019, 10, 844–849. [Google Scholar] [CrossRef]
- Balashanmugam, P.; Balakumaran, M.D.; Murugan, R.; Dhanapal, K.; Kalaichelvan, P.T. Phytogenic synthesis of silver nanoparticles, optimization andevaluation of in vitro antifungal activity against human and plant pathogens. Microbiol. Res. 2016, 192, 52–64. [Google Scholar] [CrossRef]
- Dhand, V.; Soumya, L.; Bharadwaj, S.; Chakra, S.; Bhatt, D.; Sreedhar, B. Green synthesis of silver nanoparticles using Coffea arabica seed extract and its antibacterial activity. Mater. Sci. Eng. C 2016, 58, 36–43. [Google Scholar] [CrossRef]
- Alwhibi, M.S.; Soliman, D.A.; Khaldy, H.; Alonaizan, A.; Marraiki, N.A.; El-Zaidy, M.; AlSubeie, M.S. Green biosynthesis of silver nanoparticle using Commiphora myrrh extract and evaluation of their antimicrobial activity and colon cancer cells viability. J. King Saud Univ. Sci. 2020, 32, 3372–3379. [Google Scholar] [CrossRef]
- Ahmad, A.; Wei, Y.; Syed, F.; Tahir, K.; Rehman, A.U.; Khan, A.; Ullah, S.; Yuan, Q. The effects of bacteria-nanoparticles interface on the antibacterial activity of green synthesized silver nanoparticles. Microb. Pathog. 2017, 102, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Hemlata; Meena, P.R.; Singh, A.P.; Tejavath, K.K. Biosynthesis of Silver Nanoparticles Using Cucumis prophetarum Aqueous Leaf Extract and Their Antibacterial and Antiproliferative Activity Against Cancer Cell Lines. ACS Omega 2020, 5, 5520–5528. [Google Scholar] [CrossRef] [PubMed]
- Gajendran, B.; Chinnasamy, A.; Durai, P.; Raman, J.; Ramar, M. Biosynthesis and characterization of silver nanoparticles from Datura inoxia and its apoptotic effect on human breast cancer cell line MCF7. Mater. Lett. 2014, 122, 98–102. [Google Scholar] [CrossRef]
- Suresh, G.; Gunasekar, P.H.; Kokila, D.; Prabhu, D.; Dinesh, D.; Ravichandran, N.; Ramesh, B.; Koodalingam, A.; Siva, G.V. Green synthesis of silver nanoparticles using Delphinium denudatum root extract exhibits antibacterial and mosquito larvicidal activities. Spectrochim. Acta A 2014, 127, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, S.; Shojaosadati, S.A. Rapid and green synthesis of silver nanoparticles using Diospyros lotus extract: Evaluation of their biological and catalytic activities. Polyhedron 2019, 171, 172–180. [Google Scholar] [CrossRef]
- Ramesh, P.S.; Kokila, T.; Geetha, D. Plant mediated green synthesis and antibacterial activity of silver nanoparticles using Emblica officinalis fruit extract. Spectrochim. Acta A 2015, 142, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Rathi Sre, P.R.; Reka, M.; Poovazhagi, R.; Arul Kumar, M.; Murugesan, K. Antibacterial and cytotoxic effect of biologically synthesized silver nanoparticles using aqueous root extract of Erythrina indica lam. Spectrochim. Acta A 2015, 135, 1137–1144. [Google Scholar] [CrossRef]
- Huang, L.; Sun, Y.; Mahmud, S.; Liu, H. Biological and Environmental Applications of Silver Nanoparticles Synthesized Using the Aqueous Extract of Ginkgo biloba Leaf. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1653–1668. [Google Scholar] [CrossRef]
- Xu, Z.; Feng, Q.; Wang, M.; Zhao, H.; Lin, Y.; Zhou, S. Green Biosynthesized Silver Nanoparticles With Aqueous Extracts of Ginkgo Biloba Induce Apoptosis via Mitochondrial Pathway in Cervical Cancer Cells. Front. Oncol. 2020, 10, 575415. [Google Scholar] [CrossRef]
- Sana, S.S.; Badineni, V.R.; Arla, S.K.; Boya, V.K.N. Eco-friendly synthesis of silver nanoparticles using leaf extract of Grewia flaviscences and study of their antimicrobial activity. Mater. Lett. 2015, 145, 347–350. [Google Scholar] [CrossRef]
- Netala, V.R.; Bukke, S.; Domdi, L.; Soneya, S.; Reddy, S.G.; Bethu, M.S.; Kotakdi, V.S.; Saritha, K.V.; Tartte, V. Biogenesis of silver nanoparticles using leaf extract of Indigofera hirsuta L. and their potential biomedical applications (3-in-1 system). Artif. Cells Nanomed. Biotechnol. 2018, 46, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Basumatary, K.; Daimary, P.; Das, S.K.; Thapa, M.; Singh, M.; Mukherjee, A.; Kumar, S. Lagerstroemia speciosa fruit-mediated synthesis of silver nanoparticles and its application as filler in agar based nanocomposite films for antimicrobial food packaging. Food Packag. Shelf Life 2018, 17, 99–106. [Google Scholar] [CrossRef]
- Sekhar, E.C.; Rao, K.S.V.K.; Rao, K.M.; Kumar, S.P. A green approach to synthesize controllable silver nanostructures from Limonia acidissima for inactivation of pathogenic bacteria. Cogent Chem. 2016, 2, 1144296. [Google Scholar] [CrossRef]
- Lokina, S.; Stephen, A.; Kaviyarasan, V.; Arulvasu, C.; Narayanan, V. Cytotoxicity and antimicrobial activities of green synthesized silver Nanoparticles. Eur. J. Med. Chem. 2014, 76, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Kamaraj, C.; Rajakumar, G.; Rahuman, A.A.; Velayutham, K.; Bagavan, A.; Zahir, A.A.; Elango, G. Feeding deterrent activity of synthesized silver nanoparticles using Manilkara zapota leaf extract against the house fly, Musca domestica (Diptera: Muscidae). Parasitol. Res. 2012, 111, 2439–2448. [Google Scholar] [CrossRef]
- Sukirtha, R.; Priyanka, K.M.; Antony, J.J.; Kamalakkannan, S.; Thangam, R.; Gunasekaran, P.; Krishnan, M.; Achiraman, S. Cytotoxic effect of Green synthesized silver nanoparticles using Melia azedarach against in vitro HeLa cell lines and lymphoma mice model. Process Biochem. 2012, 47, 273–279. [Google Scholar] [CrossRef]
- Suman, T.Y.; Rajasree, S.R.R.; Kanchana, A.; Elizabeth, S.B. Biosynthesis, characterization and cytotoxic effect of plant mediated silver nanoparticles using Morinda citrifolia root extract. Colloids Surf. B 2013, 106, 74–78. [Google Scholar] [CrossRef]
- Sankar, R.; Karthik, A.; Prabu, A.; Karthik, S.; Shivashangari, K.S.; Ravikumar, V. Origanum vulgare mediated biosynthesis of silver nanoparticles for its antibacterial and anticancer activity. Colloids Surf. B Biointerfaces 2013, 108, 80–84. [Google Scholar] [CrossRef]
- Mohammed, A.E.; Al-Qahtani, A.; Al-Mutairi, A.; Al-Shamri, B.; Aabed, K. Antibacterial and Cytotoxic Potential of Biosynthesized Silver Nanoparticles by Some Plant Extracts. Nanomaterials 2018, 8, 382. [Google Scholar] [CrossRef]
- Jacob, S.J.P.; Finub, J.S.; Narayanan, A. Synthesis of silver nanoparticles using Piper longum leaf extracts and its cytotoxic activity against Hep-2 cell line. Colloids Surf. B 2012, 91, 212–214. [Google Scholar] [CrossRef]
- Ajitha, B.; Reddy, Y.A.K.; Reddy, P.S. Biosynthesis of silver nanoparticles using Plectranthus amboinicus leaf extract and its antimicrobial activity. Spectrochim. Acta A 2014, 128, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.K.; Tripathy, D.; Choudhary, A.; Aili, P.K.; Chatterjee, A.; Singh, I.P.; Banerjee, U.C. Bio-synthesis of silver nanoparticles using Potentilla fulgens Wall. exHook. and its therapeutic evaluation as anticancer and antimicrobial agent. Mater. Sci. Eng. C 2015, 53, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Raja, K.; Saravanakumar, A.; Vijayakumar, R. Efficient synthesis of silver nanoparticles from Prosopis juliflora leaf extract and its antimicrobial activity using sewage. Spectrochim. Acta A 2012, 97, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Devanesan, S.; AlSalhi, M.S.; Balaji, R.V.; Ranjitsingh, A.J.A.; Ahamed, A.; Alfuraydi, A.A.; AlQahtani, F.Y.; Aleanizy, F.S.; Othman, A.H. Antimicrobial and Cytotoxicity Effects of Synthesized Silver Nanoparticles from Punica granatum Peel Extract. Nanoscale Res. Lett. 2018, 13, 315. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.R.; Ganesh, S.D.; Saha, N.; Zandraa, O.; Sáha, P. Ecofriendly Synthesis of Silver Nanoparticles from Garden Rhubarb (Rheum rhabarbarum). J. Nanotechnol. 2016, 2016, 4964752. [Google Scholar] [CrossRef]
- Palem, R.R.; Ganesh, S.D.; Kronekova, Z.; Slavikova, M.; Saha, N.; Saha, P. Green synthesis of silver nanoparticles and biopolymer nanocomposites: A comparative study on physico-chemical, antimicrobial and anticancer activity. Bull. Mater. Sci. 2018, 41, 55. [Google Scholar] [CrossRef]
- Palem, R.R.; Saha, N.; Shimoga, G.D.; Kronekova, Z.; Slavikova, M.; Saha, P. Chitosan–silver nanocomposites: New functional biomaterial for health-care applications. Int. J. Polym. Mater. 2018, 67, 1–10. [Google Scholar] [CrossRef]
- Dobrucka, R.; Kaczmarek, M.; Dlugaszewska, J. Cytotoxic and antimicrobial effect of biosynthesized silver nanoparticles using the fruit extract of Ribes nigrum. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 025015. [Google Scholar] [CrossRef]
- Daghestani, M.; Al Rashed, S.A.; Bukhari, W.; Al-Ojayan, B.; Ibrahim, E.M.; Al-Qahtani, A.M.; Merghani, N.M.; Ramadan, R.; Bhat, R.S. Bactericidal and cytotoxic properties of green synthesized nanosilver using Rosmarinus officinalis leaves. Green Process. Synth. 2020, 9, 230–236. [Google Scholar] [CrossRef]
- Dayanidhi, K.; Vadivel, P.; Jothi, S.; Eusuff, N.S. Facile synthesis of Silver@Eggshell nanocomposite: A heterogeneous catalyst for the removal of heavy metal ions, toxic dyes and microbial contaminants from water. J. Environ. Manag. 2020, 271, 110962. [Google Scholar] [CrossRef]
- Palanisamy, S.; Rajasekar, P.; Vijayaprasath, G.; Ravi, G.; Manikandan, R.; Prabhu, N.M. A green route to synthesis silver nanoparticles using Sargassum polycystum and its antioxidant and cytotoxic effects: An in vitro analysis. Mater. Lett. 2017, 189, 196–200. [Google Scholar] [CrossRef]
- Ramar, M.; Manikandan, B.; Marimuthu, P.N.; Raman, T.; Mahalingam, A.; Subramanian, P.; Karthick, S.; Munusamy, A. Synthesis of silver nanoparticles using Solanum trilobatum fruits extract and its antibacterial, cytotoxic activity against human breast cancer cell line MCF 7. Spectrochim. Acta A 2015, 140, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, K.; Rather, H.A.; Rajagopal, K.; Shanthi, M.P.; Sheriff, K.; Illiyas, M.; Rather, R.A.; Manikandan, E.; Uvarajan, S.; Bhaskar, M.; et al. Synthesis of silver nanoparticles (Ag NPs) for anticancer activities (MCF 7 breast and A549 lung cell lines) of the crude extract of Syzygium aromaticum. J. Photochem. Photobiol. B Biol. 2017, 167, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Espenti, C.S.; Rao, K.S.V.K.; Rao, K.M. Bio-synthesis and characterization of silver nanoparticles using Terminalia chebula leaf extract and evaluation of its antimicrobial potential. Mater. Lett. 2016, 174, 129–133. [Google Scholar] [CrossRef]
- Sharma, V.; Kaushik, S.; Pandit, P.; Dhull, D.; Yadav, J.P.; Kaushik, S. Green synthesis of silver nanoparticles from medicinal plants and evaluation of their antiviral potential against chikungunya virus. Appl. Microbiol. Biotechnol. 2019, 103, 881–891. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mimohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar]
- Hamouda, R.A.; Hussein, M.H.; Abo-elmagd, R.A.; Bawazir, S.S. Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Sci. Rep. 2019, 9, 13071. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8858–8874. [Google Scholar] [CrossRef]
- Sarsar, V.; Selwal, K.K.; Selwal, M.K. Nanosilver: Potent antimicrobial agent and its biosynthesis. Afr. J. Biotechnol. 2014, 13, 546–554. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Bharath, L.V. Mechanism of plant-mediated synthesis of silver nanoparticles—A review on biomolecules involved, characterisation and antibacterial activity. Chem. Biol. Interact. 2017, 273, 219–227. [Google Scholar] [CrossRef]
- Lade, B.D.; Shanware, A.S. Phytonanofabrication: Methodology and Factors Affecting Biosynthesis of Nanoparticles; IntechOpen, 2020. Available online: https://www.intechopen.com/books/smart-nanosystems-for-biomedicine-optoelectronics-and-catalysis/phytonanofabrication-methodology-and-factors-affecting-biosynthesis-of-nanoparticles (accessed on 9 December 2020). [CrossRef]
- National Ocean Service. Contaminants in the Environment. Available online: https://oceanservice.noaa.gov/observations/contam/#:~:text=Most%20contaminants%20enter%20the%20environment,treatment%20plants%20and%20sewage%20systems (accessed on 11 November 2020).
- Thompson, L.A.; Darwish, W.S. Environmental Chemical Contaminants in Food: Review of a Global Problem. J. Toxicol. 2019, 2019, 2345283. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, P. Environmental Toxicants and Hazardous Contaminants: Recent Advances in Technologies for Sustainable Development. J. Hazard. Toxic Radioact. Waste 2017, 21, 02017001. [Google Scholar] [CrossRef]
- Rout, P.R.; Bhunia, P. Assessing Possible Applications of Waste Organic Solid Substances as Carbon Sources and Biofilm Substrates for Elimination of Nitrate Toxicity from Wastewater. J. Hazard. Toxic Radioact. Waste 2017, 21, 04016027. [Google Scholar] [CrossRef]
- The World Bank. What You Need to Know about Toxic Pollution: A Conversation with Richard Fuller. Available online: https://www.worldbank.org/en/news/feature/2015/04/21/what-you-need-to-know-about-toxic-pollution-a-conversation-with-richard-fuller (accessed on 11 November 2020).
- Uberoi, V.; Bhattacharya, S.K. Toxicity and Degradability of Nitrophenols in Anaerobic Systems. Water Environ. Res. 1997, 69, 146–156. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Nitrophenols: 2-Nitrophenol, 4-Nitrophenol. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp50.pdf (accessed on 11 November 2020).
- Subashchandrabose, S.R.; Megharaj, M.; Venkateshwarlu, K.; Naidu, R. p-Nitrophenol toxicity to and its removal by three select soil isolates of microalgae: The role of antioxidants. Environ. Chem. 2012, 31, 1980–1988. [Google Scholar] [CrossRef]
- Biodegradation. Research Watch: Nitrophenol toxicity. Environ. Sci. Technol. 1997, 31, 259A. [CrossRef]
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Nitrobenzene. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp140.pdf (accessed on 11 November 2020).
- Material Safety Data Sheet. 4-Nitrophenol. Available online: https://datasheets.scbt.com/sc-206922.pdf (accessed on 11 November 2020).
- Duda, J.M.W. Phenols—Sources and Toxicity. Pol. J. Environ. Stud. 2007, 16, 347–362. Available online: http://www.pjoes.com/pdf-87995-21854?filename=Phenols%20_%20Sources%20and.pdf (accessed on 11 November 2020).
- Šljukić, B.; Santos, D.M.; Sequeira, C.A.C.; Banks, C.E. Analytical monitoring of sodium borohydride. Anal. Methods 2013, 5, 829–839. [Google Scholar] [CrossRef]
- Davis, R.E.; Swain, C.G. General acid catalysis of the hydrolysis of sodium borohydride. J. Am. Chem. Soc. 1960, 82, 5949–5950. [Google Scholar] [CrossRef]
- Schlesinger, H.I.; Brown, H.C.; Finholt, A.E.; Gilbreath, J.R.; Hoekstra, H.R.; Hyde, E.K. Sodium Borohydride, Its Hydrolysis and its Use as a Reducing Agent and in the Generation of Hydrogen. J. Am. Chem. Soc. 1953, 75, 215–219. [Google Scholar] [CrossRef]
- Kong, X.; Zhu, H.; Chen, C.; Huang, G.; Chen, Q. Insights into the reduction of 4-nitrophenol to 4-aminophenol on catalysts. Chem. Phys. Lett. 2017, 684, 148–152. [Google Scholar] [CrossRef]
- Kästner, C.; Thünemann, A.F. Catalytic Reduction of 4-Nitrophenol Using Silver Nanoparticles with Adjustable Activity. Langmuir 2016, 32, 7383–7391. [Google Scholar] [CrossRef] [PubMed]
- Thawarkar, S.R.; Thombare, B.; Munde, B.S.; Khupse, N.D. Kinetic investigation for the catalytic reduction of nitrophenol using ionic liquid stabilized gold nanoparticles. RSC Adv. 2018, 8, 38384–38390. [Google Scholar] [CrossRef]
- Ayad, A.I.; Luart, D.; Dris, A.O.; Guénin, E. Kinetic Analysis of 4-Nitrophenol Reduction by “Water-Soluble” Palladium Nanoparticles. Nanomaterials 2020, 10, 1169. [Google Scholar] [CrossRef] [PubMed]
- Bhaduri, B.; Polubesova, T. Facile synthesis of carbon-supported silver nanoparticles as an efficient reduction catalyst for aqueous 2-methyl-p-nitrophenol. Material Letters 2020, 267, 127546. [Google Scholar] [CrossRef]
- Priya, D.B.; Asharani, I.V. Size Dependent Catalytic Activity of Actinodaphne madraspatana Bedd Leaves Mediated Silver Nanoparticles. J. Clust. Sci. 2017, 28, 1837–1856. [Google Scholar] [CrossRef]
- Ismail, M.; Khan, M.I.; Khan, S.B.; Akhtar, K.; Khan, M.A.; Asiri, A.M. Catalytic reduction of picric acid, nitrophenols and organic azo dyes via green synthesized plant supported Ag nanoparticles. J. Mol. Liq. 2018, 268, 87–101. [Google Scholar] [CrossRef]
- Ko, J.W.; Ko, W.B. Catalytic Activity for Reduction of 4-Nitrophenol with [C60] Fullerene Nanowhisker-Silver Nanoparticle Composites. Mater. Trans. 2016, 57, 2122–2126. [Google Scholar] [CrossRef]
- Corbet, J.F. Pseudo first-order kinetics. J. Chem. Educ. 1972, 49, 663. [Google Scholar] [CrossRef]
- Kalantari, K.; Afifi, A.B.M.; Bayat, S.; Shameli, K.; Yousefi, S.; Mokhtar, N.; Kalantari, A. Heterogeneous catalysis in 4-nitrophenol degradation and antioxidant activities of silver nanoparticles embedded in Tapioca starch. Arab. J. Chem. 2019, 8, 5243–5252. [Google Scholar] [CrossRef]
- Chemistry—LibreTexts. Pseudo-1st-Order Reactions. Available online: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Kinetics/02%3A_Reaction_Rates/2.08%3A_Second-Order_Reactions/2.8.01%3A_Pseudo-1st-order_reactions (accessed on 11 November 2020).
- Irvine, W.M. Langmuir-Hinshelwood Mechanism. In Encyclopedia of Astrobiology; Gargaud, M., Amils, R., Quintanilla, C., Cleaves, H.J., Irvine, W.M., Pinti, D., Viso, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Gavade, S.J.M.; Nikam, G.H.; Sabale, S.R.; Tamhankar, B.V. Green synthesis of fluorescent silver nanoparticles using Acacia nilotica gum extract for kinetic studies of 4-nitrophenol reduction. Mater. Today Proc. 2016, 3, 4109–4114. [Google Scholar] [CrossRef]
- Shah, Z.; Hassan, S.; Shaheen, K.; Khan, S.A.; Gul, T.; Anwar, Y.; Al-shaeri, M.A.; Khan, M.; Khan, R.; Haleem, M.A.; et al. Synthesis of AgNPs coated with secondary metabolites of Acacia nilotica: An efficient antimicrobial and detoxification agent for environmental toxic organic pollutants. Mater. Sci. Eng. C 2020, 111, 110829. [Google Scholar] [CrossRef] [PubMed]
- Manjari, G.; Saran, S.; Arun, T.; Devipriya, S.P.; Rao, A.V.B. Facile Aglaia elaeagnoidea Mediated Synthesis of Silver and Gold Nanoparticles: Antioxidant and Catalysis Properties. J. Clust. Sci. 2017, 28, 2041–2056. [Google Scholar] [CrossRef]
- Gangarapu, M.; Sarangapany, S.; Veerabhali, K.K.; Devipriya, S.P.; Arava, V.B.R. A High-Performance Catalytic and Recyclability of Phyto-Synthesized Silver Nanoparticles Embedded in Natural Polymer. J. Clust. Sci. 2017, 28, 3127–3138. [Google Scholar] [CrossRef]
- Khoshnamvand, M.; Huo, C.; Liu, J. Silver nanoparticles synthesized using Allium ampeloprasum L. leaf extract: Characterization and performance in catalytic reduction of 4-nitrophenol and antioxidant activity. J. Mol. Struct. 2019, 1175, 90–96. [Google Scholar] [CrossRef]
- Nguyen, T.T.N.; Vo, T.T.; Nguyen, B.N.H.; Nguyen, D.T.; Dang, V.S.; Dang, C.H.; Nguyen, T.D. Silver and gold nanoparticles biosynthesized by aqueous extract of burdock root, Arctium lappa as antimicrobial agent and catalyst for degradation of pollutants. Environ. Sci. Pollut. Res. 2018, 25, 34247–34261. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Issaabadi, Z.; Sajadi, S.M. Green synthesis of the Ag/Al2O3 nanoparticles using Bryonia alba leaf extract and their catalytic application for the degradation of organic pollutants. J. Mater. Sci. Mater. Electron. 2019, 30, 3847–3859. [Google Scholar] [CrossRef]
- Aboelfetoh, E.F.; El-Shenody, R.A.; Ghobara, M.M. Eco-friendly synthesis of silver nanoparticles using green algae (Caulerpa serrulata): Reaction optimization, catalytic and antibacterial activities. Environ. Monit. Assess. 2017, 189, 349. [Google Scholar] [CrossRef]
- Eze, F.N.; Tola, A.J.; Nwabor, O.F.; Jayeoye, T.J. Centella asiatica phenolic extract-mediated biofabrication of silver nanoparticles: Characterization, reduction of industrially relevant dyes in water and antimicrobial activities against foodborne pathogens. RSC Adv. 2019, 9, 37957. [Google Scholar] [CrossRef]
- Arya, G.; Sharma, N.; Ahmed, J.; Gupta, N.; Kumar, A.; Chandra, R.; Nimesh, S. Degradation of anthropogenic pollutant and organic dyes by biosynthesized silver nano-catalyst from Cicer arietinum leaves. J. Photochem. Photobiol. B Biol. 2017, 174, 90–96. [Google Scholar] [CrossRef]
- Bordbar, M.; Mortazavimanesh, N. Biosynthesis of waste pistachio shell supported silver nanoparticles for the catalytic reduction processes. IET Nanobiotechnol. 2018, 12, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Naraginti, S.; Sivakumar, A. Eco-friendly synthesis of silver and gold nanoparticles with enhanced bactericidal activity and study of silver catalyzed reduction of 4-nitrophenol. Spectrochim. Acta A 2014, 128, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Denrah, S.; Sarkar, M. Design of experiment for optimization of nitrophenol reduction by green synthesized silver nanocatalyst. Chem. Eng. Res. Des. 2019, 114, 494–504. [Google Scholar] [CrossRef]
- Muniyappan, N.; Nagarajan, N.S. Green synthesis of silver nanoparticles with Dalbergia spinosa leaves and their applications in biological and catalytic activities. Process Biochem. 2014, 49, 1054–1061. [Google Scholar] [CrossRef]
- Ismail, M.; Khan, M.I.; Khan, M.A.; Akhtar, K.; Asiri, A.M.; Khan, S.B. Plant-supported silver nanoparticles: Efficient, economically viable and easily recoverable catalyst for the reduction of organic pollutants. Appl. Organomet. Chem. 2019, 33, e4971. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, W.; Tan, X.; Wang, Z.; Li, Y.; Li, W. Extract of Ginkgo biloba leaves mediated biosynthesis of catalytically active and recyclable silver nanoparticles. Colloids Surf. A 2019, 563, 31–36. [Google Scholar] [CrossRef]
- Vartooni, A.R.; Nasrollahzadeh, M.; Alizadeh, M. Green synthesis of perlite supported silver nanoparticles using Hamamelis virginiana leaf extract and investigation of its catalytic activity for the reduction of 4-nitrophenol and Congo red. J. Alloy. Compd. 2016, 680, 309–314. [Google Scholar] [CrossRef]
- Ajitha, B.; Reddy, Y.A.K.; Reddy, P.S.; Suneetha, Y.; Jeon, H.-J.; Ahn, C.W. Instant biosynthesis of silver nanoparticles using Lawsonia inermis leaf extract: Innate catalytic, antimicrobial and antioxidant activities. J. Mol. Liq. 2016, 219, 474–481. [Google Scholar] [CrossRef]
- Rani, P.; Kumar, V.; Singh, P.P.; Matharu, A.S.; Zhang, W.; Kim, K.-H.; Singh, J.; Rawat, M. Highly stable AgNPs prepared via a novel green approach for catalytic and photocatalytic removal of biological and non-biological pollutants. Environ. Int. 2020, 143, 105924. [Google Scholar] [CrossRef]
- Edison, T.J.I.; Sethuraman, M.G. Biogenic robust synthesis of silver nanoparticles using Punica granatum peel and its application as a green catalyst for the reduction of an anthropogenic pollutant 4-nitrophenol. Spectrochim. Acta A 2013, 104, 262–264. [Google Scholar] [CrossRef]
- Muthu, K.; Rajeswari, S.; Akilandaeaswari, B.; Nagasundari, S.M.; Rangasamy, R. Synthesis, characterisation and photocatalytic activity of silver nanoparticles stabilised by Punica granatum seeds extract. Mater. Technol. 2020. [Google Scholar] [CrossRef]
- Palem, R.R.; Shimoga, G.; Kang, T.J.; Lee, S.-H. Fabrication of multifunctional Guar gum-silver nanocomposite hydrogels for biomedical and environmental applications. Int. J. Biol. Macromol. 2020, 159, 474–486. [Google Scholar] [CrossRef] [PubMed]
- Rokade, A.A.; Kim, J.H.; Lim, S.R.; Yoo, S.I.; Jin, Y.E.; Park, S.S. A Novel Green Synthesis of Silver Nanoparticles Using Rubus crataegifolius Bge Fruit Extract. J. Clust. Sci. 2017, 28, 2017–2026. [Google Scholar] [CrossRef]
- Vellaichamy, B.; Periakaruppan, P. Silver-nanospheres as a green catalyst for the decontamination of hazardous pollutants. RSC Adv. 2015, 5, 105917–105924. [Google Scholar] [CrossRef]
- Veisi, H.; Kazemi, S.; Mohammadi, P.; Safarimehr, P.; Hemmati, S. Catalytic reduction of 4-nitrophenol over Ag nanoparticles immobilized on Stachys Lavandulifolia extract-modified multi walled carbon nanotubes. Polyhedron 2019, 157, 232–240. [Google Scholar] [CrossRef]
- Ajitha, B.; Reddy, Y.A.K.; Lee, Y.; Kim, M.J.; Ahn, C.W. Biomimetic synthesis of silver nanoparticles using Syzygium aromaticum (clove) extract: Catalytic and antimicrobial effects. Appl. Organomet. Chem. 2019, 33, e4867. [Google Scholar] [CrossRef]
- Sherin, L.; Sohail, A.; Amjad, U.S.; Mustafa, M.; Jabeen, R.; Ul-Hamid, A. Facile green synthesis of silver nanoparticles using Terminalia bellerica kernel extract for catalytic reduction of anthropogenic water pollutants. Colloids Interface Sci. Commun. 2020, 37, 100276. [Google Scholar] [CrossRef]
- Veisi, H.; Azizi, S.; Mohammadi, P. Green synthesis of the silver nanoparticles mediated by Thymbra spicata extract and its application as a heterogeneous and recyclable nanocatalyst for catalytic reduction of a variety of dyes in water. J. Clean. Prod. 2018, 170, 1536–1543. [Google Scholar] [CrossRef]
- Singh, J.; Mehta, A.; Rawat, M.; Basu, S. Green synthesis of silver nanoparticles using sun dried tulsi leaves and its catalytic application for 4-Nitrophenol reduction. J. Environ. Chem. Eng. 2018, 6, 1468–1474. [Google Scholar] [CrossRef]
- Zayed, M.F.; Eisa, W.H.; Abdel-Moneam, Y.K.; El-kousy, S.M.; Atia, A. Ziziphus spina-christi based bio-synthesis of Ag nanoparticles. J. Ind. Eng. Chem. 2015, 23, 50–56. [Google Scholar] [CrossRef]


| Name of Plant | Source | Size of Silver Nanoparticles (SNPs) | Ultraviolet-Visible Spectroscopy (UV-Vis) RANGE | Antimicrobial Activity | Cytotoxicity Effective on | References |
|---|---|---|---|---|---|---|
| (nm) | (nm) | |||||
| Acacia arabica | Gum | 35.0 | 435.0 | Effective | NR | [52] |
| Albizia adianthifolia | Leaves | 4.0–35.0 | 448.0 | NR | A549 cells | [53] |
| Alternanthera sessilis Linn. | Leaves | 20.0–30.0 | 435.0 | Effective | NR | [54] |
| Ananas comosus L. | Peels | NR | 485.0 | Effective | HepG2 cells | [55] |
| Annona squamosa | Leaves | 20.0–100.0 | 444.0 | NR | MCF-7 cells | [56] |
| Azadirachta indica L. | Gum | <35.0 | 418.0 | Effective | NR | [57] |
| Azadirachta indica | Leaves | 11.5 | 421.0 | Effective | NR | [58] |
| Boerhaavia diffusa | Plant | 25.0 | 418.0 | Effective | NR | [59] |
| Brassica oleracea | Leaves | 20.0 | 415.0 | Effective | MCF-7 cell | [60] |
| Caesalpinia pulcherrima | Leaves | 410.0 | 410.0 | Effective | HeLa cell | [61] |
| Cassia auriculata | Leaves | 30.0–50.0 | 423.0 | Effective | PC-3 cell | [62] |
| Cassia roxburghii | Leaves | 10.0–30.0 | 435.0 | Effective | NR | [63] |
| Coffea arabica | Seeds | 20.0–30.0 | 445.0–459.0 | Effective | NR | [64] |
| Commiphora myrrha | Plant | 0.5–25.0 | 445.0 | Effective | SW480 cells | [65] |
| Coptis Chinensis + Chitosan | Rhizome | 15.0–20.0 | 428.0 | Effective | J-774 cell | [66] |
| Cucumis prophetarum | Leaves | 30.0−50.0 | 420.0 | Effective | MCF-7, MDA-MB-231, HepG2, & A549 | [67] |
| Datura inoxia | Leaves | 13.0–60.0 | 420.0 | NR | MCF-7 cells | [68] |
| Delphinium denudatum | Roots | <85.0 | 416.0 | Effective | Aedes aegypti | [69] |
| Diospyros lotus | Leaves | 20.0 | 409.0 | Effective | NR | [70] |
| Emblica officinalis | Fruits | 10.0–70.0 | 432.0–436.0 | Effective | NR | [71] |
| Erythrina indica lam | Roots | 20.0–118.0 | 438.0 | Effective | MCF-7 & HEPG2 cell | [72] |
| Ginkgo biloba | Leaves | 8.0–21.0 | 400.0–413.0 | Effective | NR | [73] |
| Ginkgo biloba | Leaves | 20.0–90.0 | 448.0 | NR | HeLa, and SiHa | [74] |
| Grewia flaviscences | Leaves | 60.0 | 380.0–460.0 | Effective | NR | [75] |
| Indigofera hirsuta L. | Leaves | 5.0–10.0 | 436.0 | Effective | B16F10, PC3 & COLO205 | [76] |
| Lagerstroemia speciose + Agar | Fruits | 32.0–62.0 | 412.0 | Effective | NR | [77] |
| Limonia acidissima | Leaves | <30.0 | 425.0 | Effective | NR | [78] |
| Malus domestica | Apples | 20.0 | 420.0 | Effective | MCF-7 | [79] |
| Manilkara zapota | Leaves | 70.0–140.0 | 421.0 | NR | Anopheles subpictus | [80] |
| Melia azedarach | Leaves | 78.0 | 436.0 | NR | HeLa | [81] |
| Morinda citrifolia | Roots | 32.0–55.0 | 413.0 | NR | HeLa | [82] |
| Origanum vulgare | Leaves | 136.0 | 440.0 | NR | A549 cell | [83] |
| Phoenix dactylifera, Ferula asafetida, Acacia nilotica | Fruits | 67.0–156.0 | 420.0–440.0 | Effective | LoVo | [84] |
| Piper longum | Leaves | 17.6–41.0 | 420.0 | NR | HEp-2 cell | [85] |
| Plectranthus amboinicus | Leaves | 18.0 | 428.0 | Effective | NR | [86] |
| Potentilla fulgens | Roots | 10.0–15.0 | 400.0–450.0 | Effective | MCF-7 & U-87 | [87] |
| Prosopis juliflora | Leaves | 11.0–19.0 | 420.0 | Effective | NR | [88] |
| Punica granatum | Peels | 20.0–40.0 | 378.0 | Effective | RKO cells | [89] |
| Rheum rhabarbarum | Stems | 60.0–80.0 | 420.0–460.0 | Effective | HeLa | [90] |
| Rheum rhabarbarum + Chitosan | Stems | 50.0 | 433.0 | Effective | HeLa | [91] |
| Rheum rhabarbarum + Chitosan | Stems | 5.0–50.0 | 430.0–450.0 | Effective | HeLa | [92] |
| Ribes nigrum | Fruits | 5.0–10.0 | 450.0 | Effective | A549 cells | [93] |
| Rosmarinus officinalis | Leaves | 12.0–22.0 | 400.0 | Effective | MDA MB 231 | [94] |
| Sapindus mukorossi | Extract | 35.0 | 420.0 | Effective | NR | [95] |
| Sargassum polycystum | 28.0 | 405.0 | NR | HT-29 cells | [96] | |
| Solanum trilobatum | Fruits | 12.0–41.0 | 432.0 | Effective | MCF 7 | [97] |
| Syzygium aromaticum | Cloves | 5.0–40.0 | 441.0 | NR | MCF 7 & A549 | [98] |
| Terminaliachebula | Leaves | 10.0–30.0 | 421.0 | Effective | NR | [99] |
| Prepared SNPs Catalyst from Plant Source | SNPs Size (nm) | Catalyst Loading | Conversion Time (min) | PNP (mM) | BH4− (mM) | Rate Constant (kapp) | References |
|---|---|---|---|---|---|---|---|
| Acacia nilotica (Gum) | 10.0–40.0 | a 1.5 mg | 12.0 | 4.3 | 100.0 | 0.3606 min−1 | [134] |
| Acacia nilotica (Stem) | <50.0 | 5.0 mg | 10.0 | 0.1 | 0.1 | 0.0806 min−1 | [135] |
| Actinodaphne madraspatana (Leaves) | <60.0 | 5.0 mg | 1.5 | 0.1 | 5.0 | 13.25 × 10−3 s−1 | [127] |
| Aglaia elaeagnoidea (Flowers) | 17.0 | NR | 15.0 | 1.0 | 10.0 | 22.5 × 10−2 min−1 | [136] |
| Aglaia elaeagnoidea (Leaves) + Alginate | 12.0 | 144.8 mg | 5.0 | 1.0 | 10.0 | 0.5054 min−1 | [137] |
| Allium ampeloprasum L. (Leaves) | 2.0–43.0 | NR | 12.0 | 20.0 | 500.0 | 0.2596 min−1 | [138] |
| Arctium lappa (Roots) | 21.3 | 1.0 mg | 12.0 | 0.1 | 1000.0 | 6.77 × 10−3 s−1 | [139] |
| Bryonia alba (Leaves) | <20.0 | 5.0 mg | ≈30.0 b | 2.5 | 250.0 | NR | [140] |
| Caulerpa serrulata (Green Algae) | 10.0 | 0.1 mL | 5.0 | NR | 1.74 | 0.580 min−1 | [141] |
| Centella asiatica (Aerial Parts) | 20.0–25.0 | NR | NR | 21.5 | 21.5 | 3.9 × 10−3 s−1 | [142] |
| Cicer arietinum (Leaves) | 88.8 | 30.0 µg | 40.0 | 2.0 | 30.0 | NR | [143] |
| Cichorium intybus L. (Leaves) + Pistachio shell | 10.0–15.0 | 5.0 mg | 0.51 | 2.5 | 250.0 | NR | [144] |
| Coleus forskohlii (Roots) | 35.0–55.0 | 25.0 µL | 24.0 | 10 | 50.0 | 0.10118 min−1 | [145] |
| Colocasia esculenta (Rhizome) | 68.0 | 3.3 mg | 6.0 | 1.0 | 500 | 5.27 × 10−3 s−1 | [128] |
| Cyperus Rotundus (Rhizome) | 10.0–40.0 | 100.0 µL | 10.0 | 5.0 | 100.0 | 0.293 min−1 | [146] |
| Dalbergia spinosa (Leaves) | 18.0 | 200.0 µL | 40.0 | 0.1 | 0.1 | NR | [147] |
| Ginger (Rhizome) | 25.0 | 2.8 mg | 14.0 | 1.0 | 1.0 | 2.38 × 10−3 s−1 | [148] |
| Ginkgo biloba (Leaves) | 20.0–40.0 | 0.2 mg | 100.0 | 2.5 | 250.0 | 0.0452 min−1 | [149] |
| Hamamelis virginiana (Leaves) | 8.0–25.0 | 0.24 mg | 4.0 | 2.5 | 250.0 | NR | [150] |
| Lawsonia Inermis (Leaves) | 18.0 | 20.0 µL | 15.0 | 1.0 | 1.0 | NR | [151] |
| Phaseolus vulgaris (Beans) | 10.0–20.0 | 1590.0 nM | 15.0 | 50.0 | 200.0 | 1.59 mM/g/h | [152] |
| Punica granatum (Peels) | 30.0 | 10.0 µL | NR | 1.0 | 1.0 mg c | NR | [153] |
| Punica granatum (Seeds) | 10.0–35.0 | 50.0 µL | 7.0 | 5.0 | 1000.0 | 0.1424 min−1 | [154] |
| Rheum rhabarbarum (Stems) + Guar gum | <10.0 | 100.0 mg | 14.0 | 0.6 | 100.0 | 0.1218 min−1 | [155] |
| Rubus crataegifolius (Bge Fruits) | 13.0 | 100.0 µL | 30.0 | 0.1 | 5.0 | NR | [156] |
| Simarouba glauca (Leaves) | 7.0 | 0.01 mg | 6.0 | 0.1 | 10.0 | 18.424 × 10−3 s−1 | [157] |
| Stachys Lavandulifolia + MWCNT | 3.15 | 0.06 mg | 4.0 | 0.2 | 150.0 | 1.92 × 10−2 s−1 | [158] |
| Syzygium aromaticum (Cloves) | 9.0 | 5.0 mg | 30.0 | NR | 100.0 | 0.07494 min−1 | [159] |
| Terminalia bellerica kernel (Fruits) | 29.6 | 0.4 mg | 60.0 | 0.001 | 500.0 | 0.03 min−1 | [160] |
| Thymbra spicata (Leaves) | 7.0 | 0.35 mg | 1.0 | 0.002 | 250.0 | 0.0645 s−1 | [161] |
| Tulsi (Leaves) | 5.0–10.0 | 10.0 µL | 30.0 | 5.0 | 200.0 | 2.048 min−1 | [162] |
| Ziziphus spina-christi (Leaves) | 15.0 | 50.0 µL | 15.0 | 10.0 | 100.0 | 4.4 × 10−3 s−1 | [163] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimoga, G.; Palem, R.R.; Lee, S.-H.; Kim, S.-Y. Catalytic Degradability of p-Nitrophenol Using Ecofriendly Silver Nanoparticles. Metals 2020, 10, 1661. https://doi.org/10.3390/met10121661
Shimoga G, Palem RR, Lee S-H, Kim S-Y. Catalytic Degradability of p-Nitrophenol Using Ecofriendly Silver Nanoparticles. Metals. 2020; 10(12):1661. https://doi.org/10.3390/met10121661
Chicago/Turabian StyleShimoga, Ganesh, Ramasubba Reddy Palem, Soo-Hong Lee, and Sang-Youn Kim. 2020. "Catalytic Degradability of p-Nitrophenol Using Ecofriendly Silver Nanoparticles" Metals 10, no. 12: 1661. https://doi.org/10.3390/met10121661
APA StyleShimoga, G., Palem, R. R., Lee, S.-H., & Kim, S.-Y. (2020). Catalytic Degradability of p-Nitrophenol Using Ecofriendly Silver Nanoparticles. Metals, 10(12), 1661. https://doi.org/10.3390/met10121661








