Abstract
The current study focusses on the phase composition, solidification path, and microstructure evaluation of gravity cast Al-4Mg-0.5Si-xLa aluminum alloy, where x = 0, 0.1, 0.25, 0.5, 0.75, and 1 wt.% La. A computational CalPhaD approach implemented in Thermo-Calc software and scanning electron microscopy technique equipped with electron microprobe analysis (EMPA) was employed to assess its above-mentioned characteristics. The thermodynamic analysis showed that the equilibrium solidification path of La-containing Al-Mg-Si alloys consists of only binary phases LaSi2 and Mg2Si precipitation along with α-Al from the liquid and further solid-state transformation of this mixture into α-Al + Al11La3 + Mg2Si + Al3Mg2 composition. Scheil–Gulliver simulation showed a similar solidification pathway but was accompanied by an increase in the solidification range (from ~55 °C to 210 °C). Furthermore, microstructural observations were congruent with the calculated fraction of phases at 560 °C and related to α-Al + LaSi2 + Mg2Si three-phase region in terms of formation of La-rich phase having both eliminating effect on the eutectic Mg2Si phase. Quantitative EMPA analysis and elemental mapping revealed that the La-rich phase included Al, La, and Si and may be described as Al2LaSi2 phase. This phase shows a visible modifying effect on the eutectic Mg2Si phase, likely due to absorbing on the liquid/solid interface.
1. Introduction
Recently, many studies showed that micro-alloying with rare earth (RE) elements may have remarkable effects related to refining of the as-cast structure, thus making them efficient modification agents for aluminum alloys [1,2,3,4,5]. Among the Light Rare-Earth Elements (LREE), also known as the cerium group (Sc, La, Ce, Pr, Nd, Pm, Sm, Eu, and Gd), lanthanum and cerium are the most abundant in the Earth’s crust (31 ppm and 63 ppm, respectively) and the least expensive [6]. According to binary Al-La and Al-Ce systems [7], these elements both provide a formation of eutectic reactions, L→α-Al + Al11La3 (11.7 wt.% La, 640 °C) and L→α-Al + Al11Ce3 (12.2 wt.% Ce, 621 °C). In this respect, several previous studies related to the design of alternative cast alloys for high-temperature applications are published [8,9,10]. Moreover, both La and Ce may show de-gasifying and de-slagging performance, thereby improving the quality of cast products [11]. Despite affinities between La and Ce, the latter caused some contradictory results in terms of modifying effects on the structure. For example, the work [12] on 0.12 wt.% Ce-modified Al-Zn-Mg-Cu alloy reported that nucleation growth of α-Al on one of the crystal faces of the Al11Ce3 in the melt is very efficient upon solidification, also supported by a recent review [11]. On the other hand, on comparing the effect of 0.1–0.2 wt.% of La and Ce on the structure of the 6xxx alloy [13], La showed far better modifying ability compared to Ce, which was inefficient in this respect. Additionally, Ce showed a more detrimental effect in porosity formation because of greater oxidation tendency when compared to La [14]. Meanwhile, La is ubiquitously used as an addition for achieving favorably fine α-Al [15,16], eutectic silicon [1], Fe-bearing phases [5,13], as well as Mg2Si phase [17,18], one of the main structural components in 511 type cast aluminum alloys studied in this paper.
When considering grain refining function, 0.03–0.2 wt.% La suppresses the growth of α-Al grains via enriching upon their solidification front. Moreover, it was observed that La may react with other modifying elements such as Ti and V, thus bonding with Al20(Ti,V)2La inter-metallic and acting as heterogeneous nucleation sites [19]. However, the research data on the modifying effect of La on the eutectic Mg2Si phase is very limited. Most papers are focused on the primary Mg2Si phase playing a reinforcing function in aluminum matrix in-situ composites [18,20,21,22]. Research on Mg-5Si alloy [17] has reported the segregation of La on the growth front of the primary Mg2Si phase, thus changing its surface energy by lattice distortion, poisoning the growth steps, and suppressing the directional growth of the primary phase. In Al-based alloys, not only the surface activity of La was confirmed [18], but also it was shown that La atom clusters may act as effective nuclei due to the similarity in the crystal structure of La and Mg2Si phase.
The above-stated research shows that RE La may serve as a multi-modifying agent for α-Al and intermetallics. However, regarding commercial cast Mg-rich Al-Mg-Si alloys, the available research does not give an ambiguous explanation of its effect on the solidification path and morphology of the eutectic structure. Due to these limitations, the present work plans to discover microstructure evolution and solidification behavior of the gravity-cast Al-4%Mg-0.5%Si aluminum alloy (511 type) after the addition of the different amounts of La (0, 0.1, 0.25, 0.5, 0.75, and 1 wt.%).
2. Materials and Methods
In terms of chemical composition, the experimental alloys studied in this work corresponded to the standard cast aluminum alloy of 511 grade (Aluminum Association, Arlington, VA, USA) with various additions of lanthanum. Alloys of a nominal composition Al-4Mg-0.5Si-xLa, where x = 0, 0.1, 0.25, 0.5, 0.75, and 1 wt.% La, were prepared from pure materials Al (99.99%), Mg (99.9%), Si (99%), and La (99.95%). Melting was carried out in a 500 g capacity alundum crucible, using a vertical electric resistance furnace in an air atmosphere without the addition of protective gas. The melt temperature was kept at 750 °C for each alloy. After the melting of the base Al-Mg-Si alloy, foil-wrapped lanthanum was added and mixed using a graphite stick down to its completed dissolution. Then, the molten metal was held for 10 min for its homogenization, skimmed, stirred, and poured at the temperature of 720–740 °C into a metal mold of Ø20 mm × 100 mm in size. The cooling conditions provided a dendrite cell size (d) of approximately 20 µm and, hence, the cooling rate (Vc) of about 102 °C/s, as it was estimated by well-known dependency Vc = (A/d)1/n [23]. The chemical composition determined by spectral analysis and the calculated phase composition of the experimental alloys is shown in Table 1.

Table 1.
Chemical and phase composition (at 20 °C) of the experimental alloys.
The solidification paths and phase compositions of the experimental alloys were investigated using the Thermo-Calc software (Version 3.1, TCAl4 Al-based alloy database, Thermo-Calc Software AB, Stockholm, Sweden) [24]. Single point equilibrium, phase diagram, property diagram, and Scheil–Gulliver solidification simulation options were used.
The microstructure was examined by optical microscopy (OM, Axio Observer MAT, Carl Zeiss Microscopy GmbH, Oberkochen, Germany), scanning electron microscopy (SEM, TESCAN VEGA3, Tescan Orsay Holding, Brno, Czech Republic) with an electron microprobe analysis system (EMPA, Oxford Instruments plc, Abingdon, UK), and the Aztec software (Version 3.0, Oxford Instruments plc, Abingdon, UK). The metallographic samples were ground with SiC abrasive paper and polished with 1 μm diamond suspension. A 1% hydrogen fluoride (HF) water solution was used for etching.
3. Results and Discussion
3.1. Thermodynamic Prediction
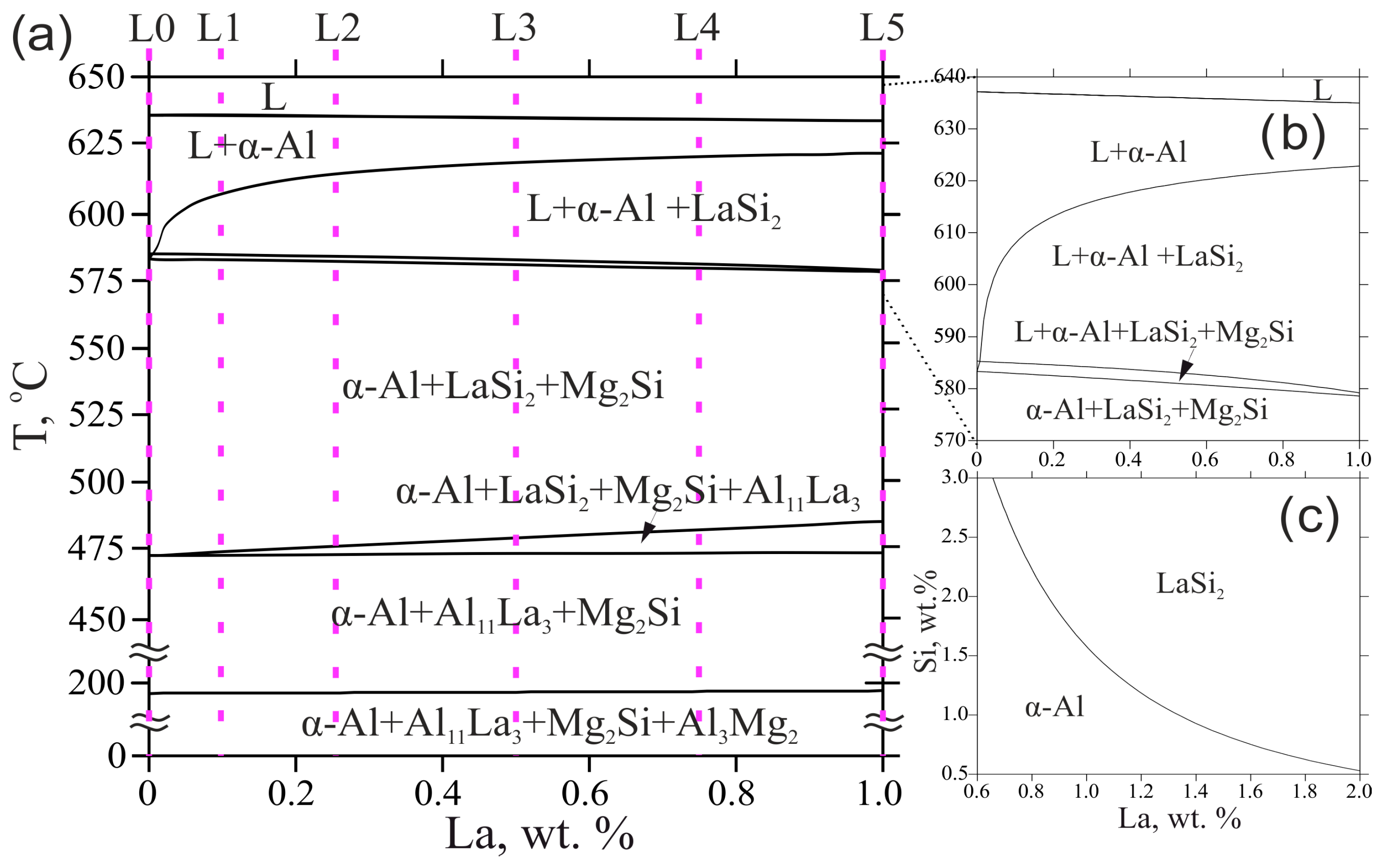
The polythermal section displayed in Figure 1a shows the solidification behavior of the experimental alloys. As can be seen, La had no significant influence on the equilibrium phase composition and transformation temperatures. In this respect, despite the high melting point of the element, alloying with La did not aggravate industrial compatibility of the base alloy since the liquidus temperature, the key factor for energy consumption in melting, was relatively low (635–637 °C), thus starting the solidification from the α-Al phase. Moreover, as can be seen from the magnified section (Figure 1b), the liquidus line goes down slightly along with the area related to the formation of the α-Al phase, likely due to nearness to the eutectic point (4.5 wt.% La, 625 °C) adjoining to the area for LaSi2 primary phase appearance. The liquidus projection shown in Figure 1c reveals that the region for undesirable primary intermetallic crystallization was highly beyond the experimental concentrations of La and Si.
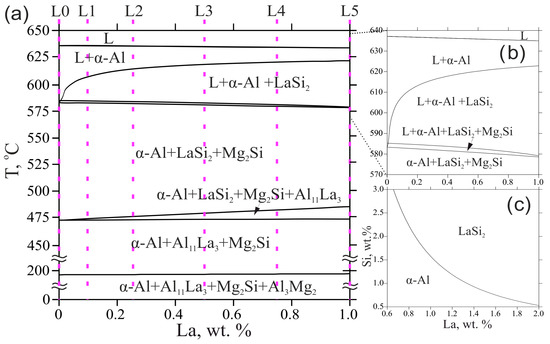
Figure 1.
(a) Polythermal section of the Al-Mg-Si-La system at 4 wt.% Mg and 0.5 wt.% Si; (b) magnified polythermal section showing the change in liquidus and solidus temperatures; (c) liquidus projection of the Al-Mg-Si-La system at 4 wt.% Mg.
The equilibrium solidus decreased with an increase in La concentration but remained relatively high (578–583 °C). It should be noted that the TCAl4 database does not consider solubility of Al in the LaSi2 phase, i.e., the existence of the ternary Al2LaSi2 found in the study [25], though a wide composition range of this phase was reported previously and showed a wide homogeneity in Al content [26]. Since this work does not aim to discover new intermetallics, possible La- and Si-rich phases will be referred to as AlLaSi phase. This may bring some limitations for reliable predicting, but some important conclusions can be deduced due to the depletion of the aluminum melt with Si ultimately being considered. The experimental alloys complete their solidification in the three-phase region α-Al + LaSi2 + Mg2Si. However, the calculation shows that further transformations may proceed in the solid-state with the consistent formation of Al11La3 phase instead of the LaSi2 phase and Al3Mg2 phase. Solid-state diffusion of La in α-Al is negligible, and this transformation can probably be suppressed, which will lead to the formation of as-cast structure α-Al + LaSi2 + Mg2Si (near-equilibrium solidus region).
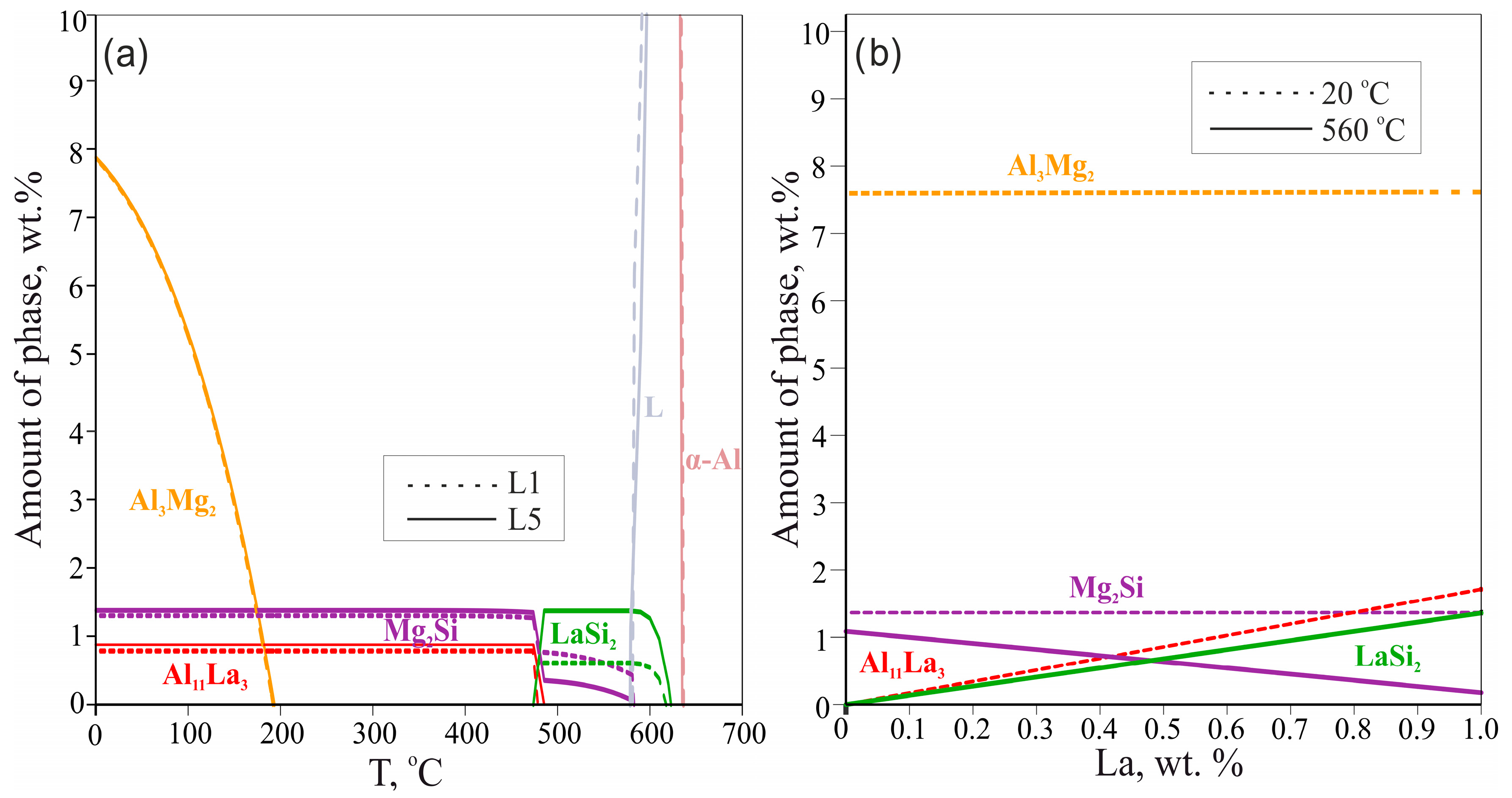
The equilibrium phase mass fractions in the L1 (0.1 wt.% La) and L5 (1 wt.% La) alloys are plotted in Figure 2a. Generally, in the temperature range 0 to 485 °C, La does not influence the amount of Mg2Si and Al3Mg2 phases (1.36 and 7.6 wt.% respectively) but sufficiently changes the amount of the Al11La3 phase (from 0.17 wt.% to 1.71 wt.%, respectively). It can be assumed that the fractions of phases in the as-cast state may correspond to the three-phase region α-Al + LaSi2 + Mg2Si due to low solubility of La in α-Al during the suppressed transformation of α-Al + LaSi2 + Mg2Si into α-Al + Al11La3 + Mg2Si. It is profoundly visible from Figure 2b that in comparison to the plot at 20 °C, at 560 °C (corresponded to α-Al + LaSi2 + Mg2Si region near-equilibrium solidus) the higher La content leads to the higher fraction of the LaSi2 phase and the lower fraction of the Mg2Si phase. This relationship achieves equality (0.5 wt.%) at about 0.48 wt.% La after which the ternary phase is the dominating phase (e.g., 1.2 wt.% LaSi2 vs. 0.1 wt.% Mg2Si in L5 alloy).
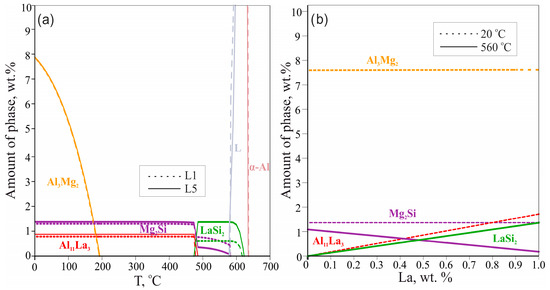
Figure 2.
Calculated phase fractions for L1 and L5 alloys as a function of temperature (a); calculated phase fractions in Al—4 wt.% Mg—0.5 wt.% Si alloy for 20 °C and 560 °C as a function of La content (b).
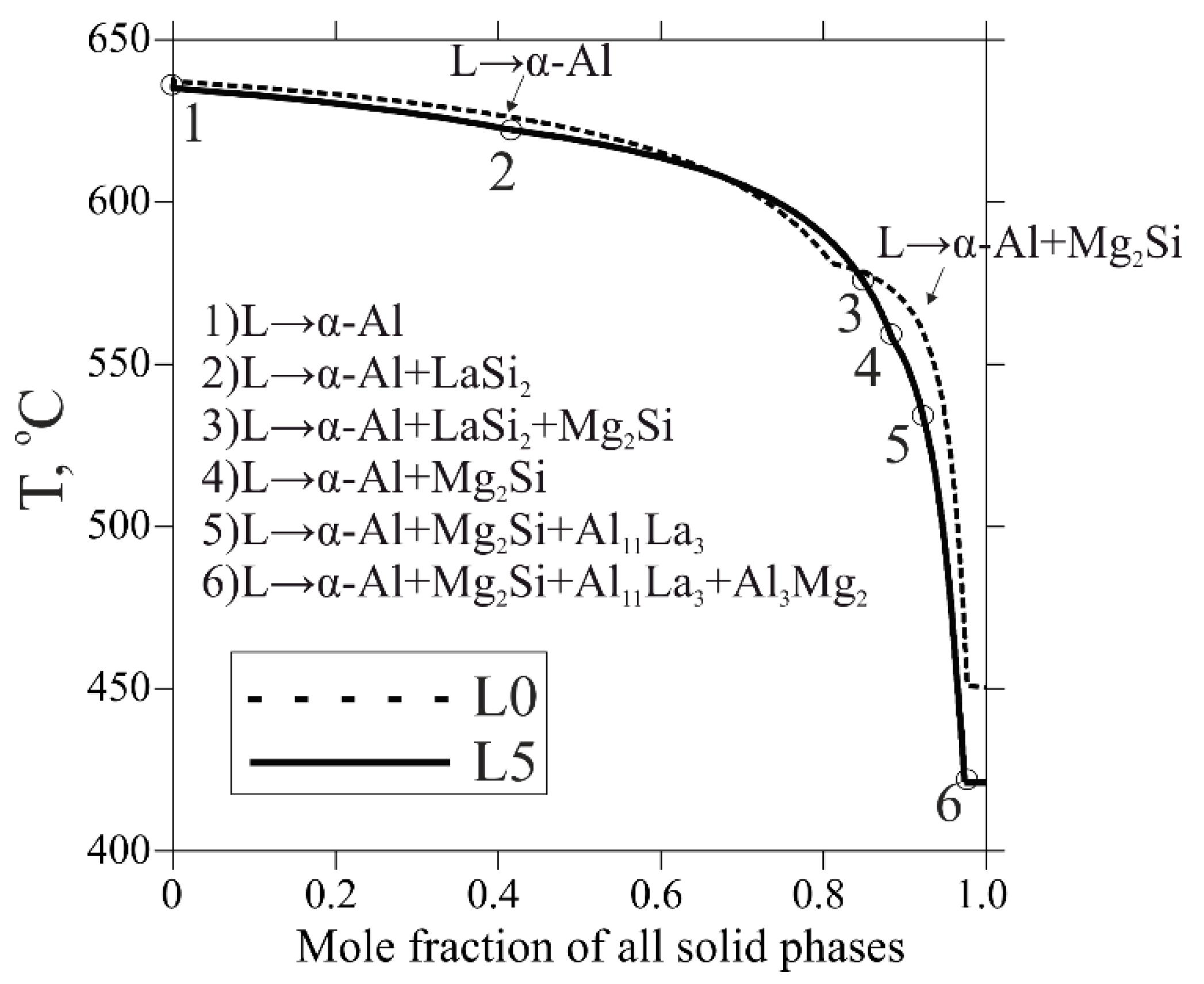
The considered equilibrium solidification analysis is very helpful for predicting of phase composition of the alloys. However, the actual solidification always occurs in non-equilibrium conditions, implying suppressed diffusion and termination after precipitation of the lowest melt-point phase. The non-equilibrium solidification path was plotted according to the Scheil–Gulliver model (DLiquid = ∞, DSolid = 0, where D is a diffusion coefficient). The non-equilibrium solidification terminates in a phase region α-Al + Al11La3 + Mg2Si + Al3Mg2, where the breakup of the LaSi2 phase is also considered (Figure 3). It has already been stated that La slightly decreases liquidus temperature, but it was also observed to have a quite significant influence on the non-equilibrium solidus as well. In comparison to the equilibrium solidification range, the non-equilibrium one for all alloys was approximately fourfold higher. Since the formation of the LaSi2 phase in alloys L1–L5 promotes a reduction in Mg2Si and increase in free Mg, it bonds with Al into Al3Mg2 phase, which solidifies at a relatively low temperature (450 °C for the base L0 alloy vs. 424 °C for other ones, see in Table 2). Accordingly, this may aggravate casting properties due to the wider solidification range. Moreover, the curves show a reduction in a region for primary α-Al formation as a result of La addition (down to halvation at 1 wt.% La), as well as strong domination of La-containing phases. On the one hand, this result seems to be promising due to the increase in eutectic fraction, possibly providing better casting properties and possible heterogeneous nucleation of α-Al on the eutectic particles’ surfaces. Nevertheless, on the other hand, this may cause inadequate growth of the excessive brittle intermetallics, especially the LaSi2 phase nucleating before other ones, adversing ductility, and fracture toughness. To gain further insight into the La effect on the structure formation, the as-cast state samples will be studied in the next sections.
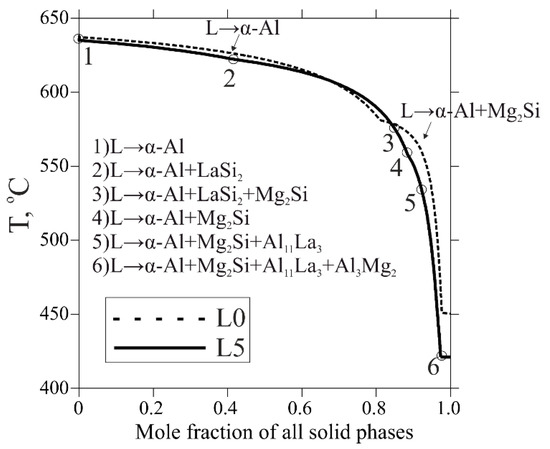
Figure 3.
Calculated non-equilibrium solidification curves of the L0 and L5 alloys according to Scheil–Gulliver simulation.

Table 2.
Calculated critical solidification temperatures of the experimental alloys.
3.2. Microstructural Evolution
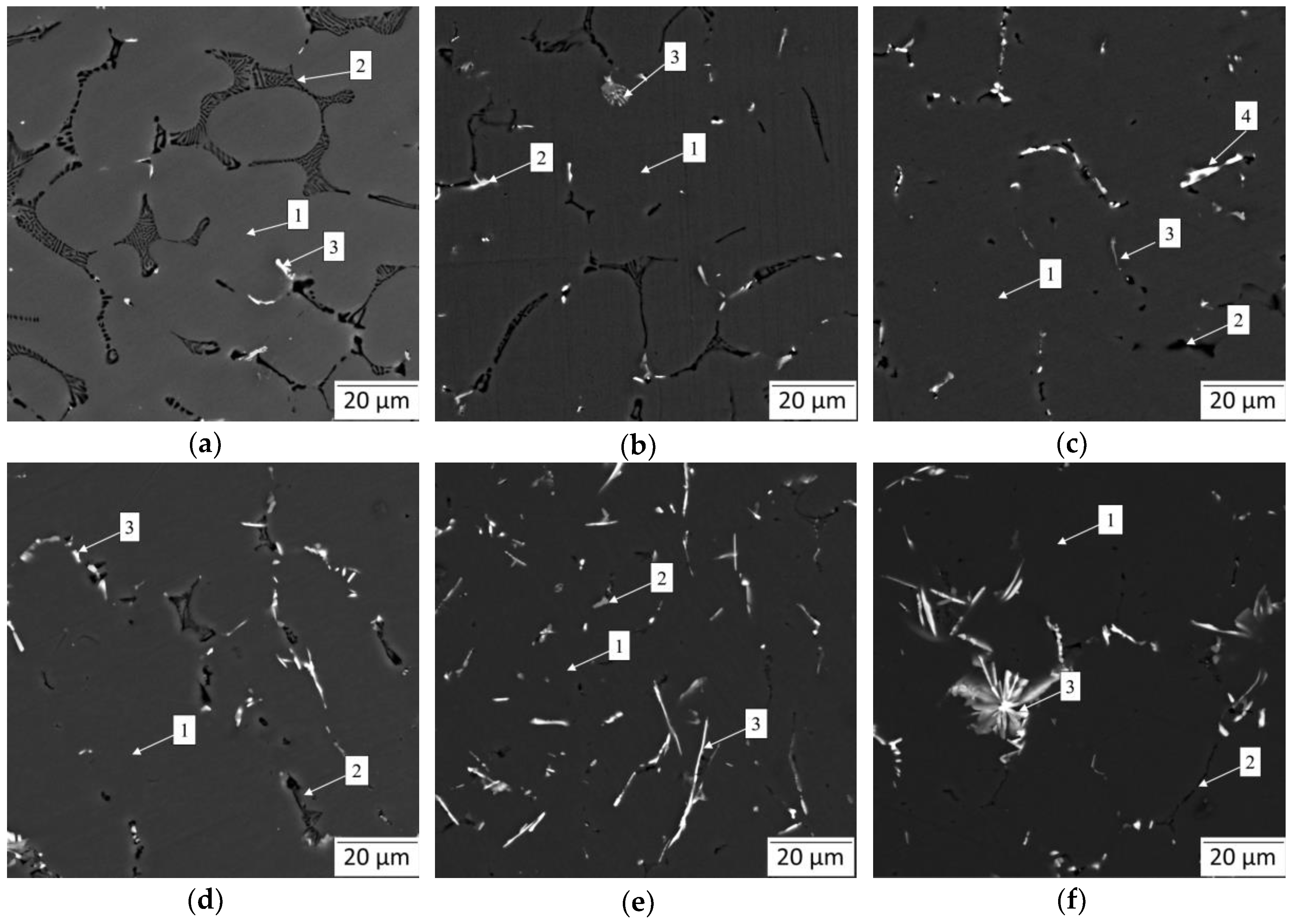
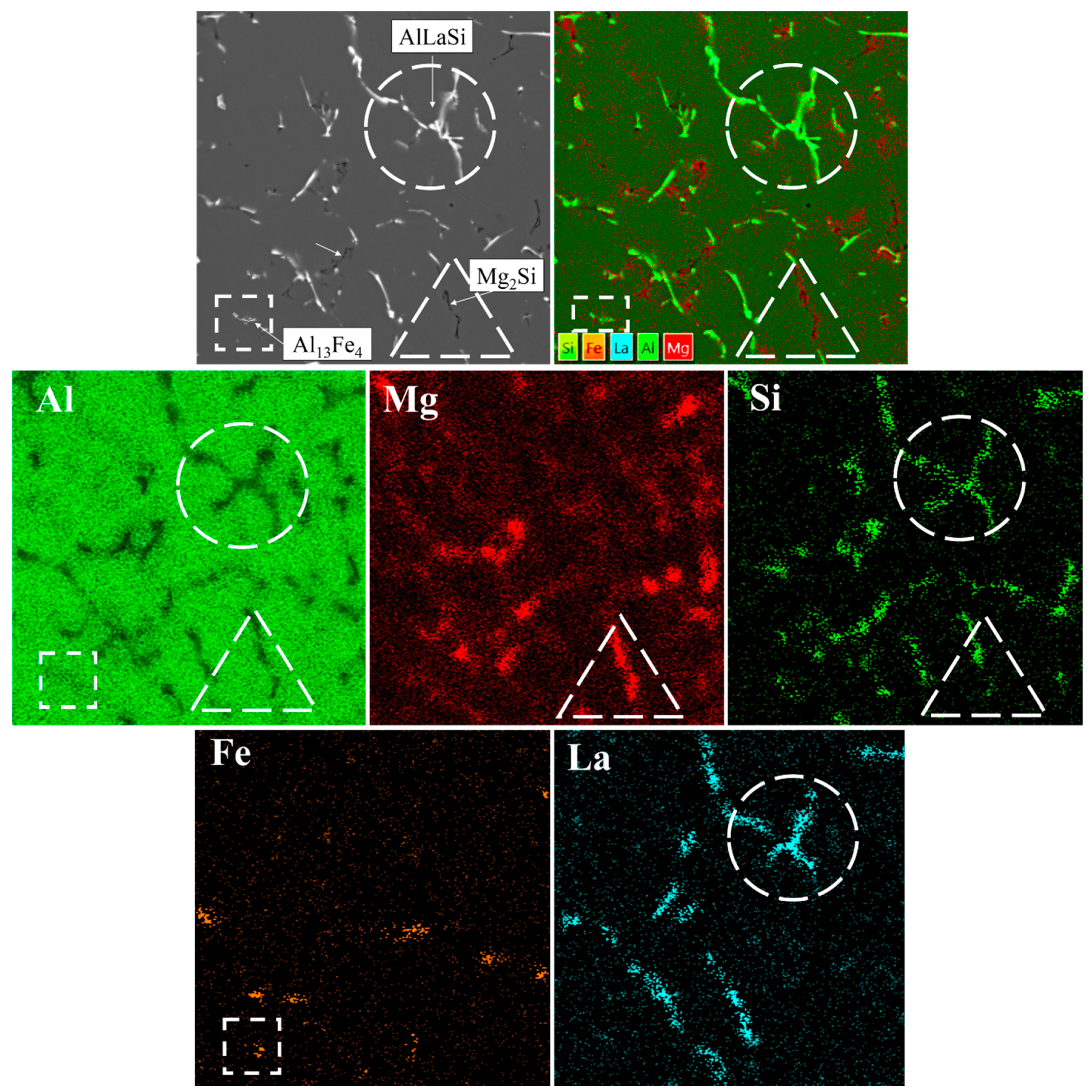
To understand the microstructure and phases of the Al-Mg-Si-xLa alloys the backscattered SEM images are presented in Figure 4. Overall, the microstructures include gray α-Al dendrite arms and intermetallic particles of various shapes and colors in the surroundings. The Mg2Si phase, appeared in black color and was quite visible in all the images. Other phases mostly contained La and some traces of Fe impurity, elements with a high atomic number, which were presented as bright inclusions. The composition of different phase-referred areas, determined by EMPA, are summarized in Table 3. As is shown, there was no appearance of Al3Mg2 in La-rich alloys due to dissolution of Mg in α-Al.
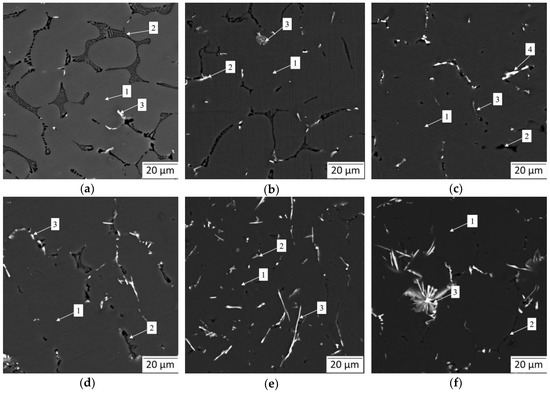
Figure 4.
Backscattered SEM micrographs showing as-cast microstructural evolution of Al—4 wt.% Mg—0.5 wt.% Si alloy with increasing in La content: (a) L0 alloy; (b) L1 alloy; (c) L2 alloy; (d) L3 alloy; (e) L4 alloy; (f) L5 alloy.

Table 3.
Chemical composition of the microstructural components in experimental alloys according to electron microprobe analysis (EMPA) 1.
Following the base L0 alloy as a reference (Figure 4a), a relatively high volume of lamellar and needle-like Mg2Si phase, and bright small inclusions of Fe-containing phase (likely, Al13Fe4 phase, according to calculation) were recorded. The latter was very fine and of particular low proportion compared to the Mg-rich phase. Apart from the formation of the Fe-rich phase, it is remarkable how precisely the microstructure agrees with Scheil–Gulliver prediction.
Observations of the La-containing alloys show significant changes in intermetallic shapes. The addition of 0.1 wt.% La resulted in thinning of the Mg2Si phase branches, though they retained lamellar morphology. Meanwhile, some traces of La-rich phase resembled the AlLaSi compound due to the prominent presence of Si and some amount of Mg possibly captured during spectral emission (Table 3). Besides, having exhibited the Al13Fe4 phase, it was complicated to distinguish two binary intermetallics (Fe- and La-rich phases). However, the AlLaSi phase was far more differentiated, while the Al13Fe4 phase was mostly of a small blocky shape. The appearance of the AlLaSi phase becomes more evident with an increase in La due to thicker branches of intermetallics and less capturing of Mg-rich α-Al phase coupled by more evident Si content. Strikingly, the addition of 0.25 wt.% La almost eliminated the presence of the Mg2Si phase. As is seen from the microstructure of the L2 alloy (Figure 4c), the AlLaSi phase is located in the vicinity of a very fine eutectic Mg2Si phase. This partially confirms the absorbing effect of the La-rich phase. This effect may have suppressed the anisotropic growth of the Mg2Si phase inhibiting its shape factor. On the other hand, since the amount of the Mg-rich phase is visibly low, the thermodynamically predicted relationship between the volume of AlLaSi and Mg2Si is confirmed by the presence of a higher amount of a AlLaSi phase and a lower amount of the Mg2Si phase. On further investigation of different alloys, the incredible growth of the AlLaSi phase and evolution of the Mg2Si phase down to submicron in thickness, accompanied by an increase in Mg solubility in α-Al (e.g., from 1.83 wt.% in the L0 alloy vs. 5.36 wt.% in the L4 alloy) was noted. Under the further increase in La content, the shape of the AlLaSi phase progresses dramatically from acicular in the L3 alloy (Figure 4d) up to needle-like the L4 alloy (Figure 4e) and to coarse star-like shape in L5 alloy intrinsic for Al13Fe4 phase in Fe-rich aluminum alloys. This advancement is accompanied by a prominent change in morphology of the Mg2Si phase, which became both slender and vermicular in the L5 alloy microstructure.
In addition, the microstructural analysis revealed that almost all the microstructures included Fe-rich intermetallics (Al13Fe4). Generally, they must have a commonly adverse needle-like morphology in most alloys, even at a low concentration of Fe. It is of particular note that in the experimental alloys, Al13Fe4 shows a short flake-like shape at a relatively high Fe content (up to 0.12 wt.%). Indeed, it was reported that La may hamper the orientation growth of the Al13Fe4 phase in a similar manner as of Mg2Si phase [5]. However, La content of more than 0.25 wt.% brings degradation and formation of very coarse acicular intermetallics related to the AlLaSi phase, which may act as stress raisers, thus being detrimental to mechanical properties.
It should be taken into account that quantitative EPMA analysis produces a misleading backlighting effect due to the capturing elemental emissions from nearby areas. However, an EMPA mapping of the representative L4 alloy (Figure 5) is strongly consistent with previous thermodynamic predictions and microstructural investigations. The existence of three types of microstructural components was noted, and the corresponding elemental correlations provide a reliable qualitative explanation of their chemical composition. It was found that Mg (red-color in the maps) is distributed mostly in the region related to the α-Al phase, advocating its dissolution. Its presence in regions adjacent to La-bearing phases (turquoise-color in the maps) may also be observed, which is probably related to Mg micro-segregation enriching to the edges of the α-Al phase dendrite cells because of non-equilibrium solidification conditions [23]. Besides, Mg along with Si (deep-green-color in the maps) are incorporated into the Mg2Si phase of worm-like shape. Ultimately, while Fe is bonded into unevenly distributed fine particles of less than 3 µm in size, the most dominating microstructural component is a very coarse AlLaSi phase revealed by correlation among Al, Si, and La enriched regions.
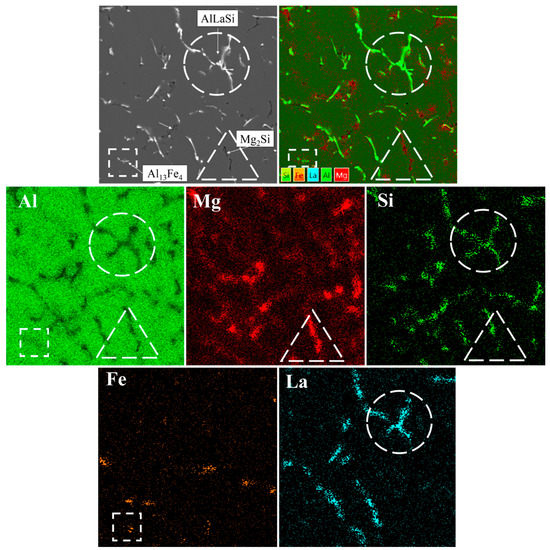
Figure 5.
EMPA mapping (Al, Mg, Si, Fe, La) of the phases presented in the microstructure of the L4 alloy.
4. Discussion
Firstly, for explaining the absence of thermodynamically predicted formation of the Al3Mg2 phase and uncompromised formation of the Mg2Si phase, the solidification behavior of ternary Al-Mg-Si alloys will be considered. According to the binary Al–Mg phase diagram, the solubility of Mg in solid aluminum is about 17%. According to [27], the binary alloys with less than 3.5 wt.%Mg exhibit a single-phase microstructure containing only α-Al. However, the presence of the Al8Mg5 phase (also known and notified in this study as Al3Mg2) may be inhibited at a Mg content of more than 5 wt.% as a result of an increase in cooling rate due to deceleration of Mg enrichment in the melt during the final solidification stage [28]. Furthermore, the addition of silicon greatly reduced the solid solubility of Mg in α-Al. Conversely, the solid solubility of Si is reduced under the appearance of Mg (e.g., 1.17 wt.% Mg and 0.68 wt.% Si at the quasibinary line in Al-Mg-Si diagram [29]). Under these conditions, they both segregate to the front of solid/liquid interface, concentrating together in the final solidification zone, thus promoting the formation of the Mg2Si phase without alternative.
Secondly, for considering La-containing alloys, it was substantiated that they complete solidification in the α-Al + AlLaSi + Mg2Si region that, in turn, contradicts with a thermodynamic calculation showing the formation of only binary phases. Whilst the inhibition of the Al3Mg2 phase seems to be clear, the formation of the AlLaSi phase is under discussion. However, the latter has received a lot of attention recently by the discovery of the new phases (AlSi2La, Al2Si2La, and (Al1-xSix)2La (0.075 ≤ x ≤ 0.18) in Al-Si-La system [26], the results are still not implemented in describing the solidification of commercial Al-Mg-Si alloys modified with La. Actually, the TCAl4 database, implemented into Thermo-Calc software, likely uses thermodynamic data regarding Al-La, La-Si, and Mg-La due to the lack of thermodynamic optimization of the Al-Mg-Si-La quaternary diagram even for the novel advanced TCAl7 database [30]. The formation of aluminides was observed in this study. The interaction between Mg and La in La-modified Al-4%Mg-0.5%Si alloys may not be anticipated. Not only the binary LaMg phase but also the ternary (Al,Mg)3La was observed in Mg-Al-La alloys [31]. However, since we showed a substantial Mg-enrichment of α-Al out of solidification and the clear presence of Mg2Si phases at up to 1% La, the Mg content is negligible for providing the formation of Mg-La phases. Studies also supported this and revealed such phases only in Mg alloys or Al alloys with more than 30% Mg content [32]. Besides, the study [33] reported an influence of isomorphic La and Ce on Al-3%Mg alloy and found only Al11La3 and Al11Ce3 phases.
For an explanation of intermetallic formation, we discovered very extensive recent efforts on Si-alternative eutectic-forming elements (Ce, Ni, Ca, La, etc.). Meanwhile, there are similar compounds, Al2CeSi2 [1,2,3] and Al2CaSi2 [34], observed in Al-Si alloys that show a needle-like shape similar to the AlLaSi phase in the present work. Based on this, it is most likely that the composition of the observed AlLaSi phase is the same as of Al2LaSi2, corresponding to the same space group P3m1 as the ternary Al2CeSi2 and Al2CaSi2 [26]. In regards to RE Ce, current investigations on phase equilibria are focused on Si-rich Al-Mg-Si alloys solidifying under quaternary eutectic reaction L→α-Al + (Si) + Al2LaSi2 + Mg2Si [35]. Either way, there is no appropriate research on the solidification of Mg-rich Al-Mg-Si with REM. In contrast, the efforts [36] toward the quaternary Al-Ca-Mg-Si diagram show a very similar phase composition at room temperature (Al3Mg2 + Al4Ca + Mg2Si) but also consider a formation of hexagonal Al2CaSi2 phase. It was also reported that peritectic reaction L + Al2CaSi2→α-Al + Al4Ca + Mg2Si is suppressed. In this respect, RE La may act as an alternative to Si because it is a common eutectic-forming element. It promotes the reduction of both in equilibrium liquidus and solidus, whilst the non-equilibrium solidus remains constant under an increase in La content. Therefore, we assumed that equilibrium invariant peritectic reaction L + AlLaSi→α-Al + Al11La3 + Mg2Si likely exists supported by Al depletion of the melt due to its solubility in AlLaSi phase and negligible in fraction (see point 3 in Figure 3) transition area L→α-Al + LaSi2(or AlLaSi) + Mg2Si. This reaction is likely to be suppressed, leading to the formation of the AlLaSi in the as-cast structure along with phases α-Al and Mg2Si.
Finally, a substantial effect of La on the eutectic Mg2Si phase was shown, notwithstanding the adverse degradation of the La-rich intermetallics at more than 0.5 wt.% La. Generally, the modifying effect may be described by heterogeneous nucleation and surface absorption. A study [18] reported the modification of the primary Mg2Si phase, referring to small (3.72%) lattice misfit between the La and Mg2Si phase causing heterogeneous nucleation of the Mg2Si particles on the La clusters during the early stage of the solidification. However, in our study, the Mg2Si phase precipitates according to the eutectic reaction along with AlLaSi (or Al2LaSi2) phase. The difference between two phases Mg2Si and Al2LaSi2 in lattice parameters and lattice-type is enormous (cubic lattice, Fm3m space group, 12 atoms/cell, a = 6.35 Å for Mg2Si [36] and hexagonal lattice, P-3m1 space group, a = 4.23 Å for Al2LaSi2 [26]). The possibility of the AlLaSi compound being a nucleus is minor based on the before mentioned observation. On the other hand, as it is shown in the section related to microstructural observation, the Mg2Si phase precipitates in the vicinity of the AlLaSi phase, showing the latter increased around and may act as a surfactant for the Mg2Si eutectic phase. This means that after the formation of the α-Al phase, the AlLaSi compound is further segregated at the solid-liquid interface of the Mg2Si phase. A quite similar effect is shown by La in modifying eutectic Si particles, which, as shown in [37], have a great similarity with the Mg2Si phase in terms of properties and solidification behavior.
The presented results are promising steps toward the development of the currently static research area on modification of eutectic Mg2Si in cast 5xx aluminum alloys. While the behavior of the intermetallics formation has been comprehended, the detailed mechanism of the modification process of eutectic Mg2Si with La remains debatable and additional investigations to find out will be performed in further works.
5. Conclusions
In summary, the influence of La at concentrations up to 1 wt.% on the phase composition and intermetallics formation during solidification of the nominal Al-4Mg-0.5Si aluminum alloy (AA 511 grade) has been investigated. Based on the results obtained, the main conclusion are as follows:
- (1)
- Thermodynamic prediction using the CalPhaD approach is limited due to the lack of optimized databases resulting in the formation of only binary Mg2Si, LaSi2, Al11La3, and Al3Mg2 intermetallics of eutectic origin. However, the tendency toward change in intermetallics fraction and critical temperature may be considered as reliable. The addition of La causes an increase in LaSi2 along with a sharp decrease in the Mg2Si phase at the temperature under solidus. Moreover, the calculated equilibrium solidification ranges of the experimental alloys are near 55 °C that is increased fourfold in non-equilibrium conditions based on calculations using the Scheil–Gulliver model.
- (2)
- In contradiction to calculation, the microstructures of the La-containing alloys exhibited dominating ternary AlLaSi phase that is likely to be Al2LaSi2 compound. An increase in La content leads to the growth of the ternary phase. This growth is particularly enormous at over 0.5 wt.% La when the intermetallics evolved as slender needles (at 0.75 wt.% La) and stars (at 1 wt.% La).
- (3)
- A remarkable modifying effect of La on the eutectic Mg2Si phase was observed. The latter’s shape changed slightly after the addition of 0.1 wt.% La but evolved incredibly at a higher concentration from the lamellar down to slender flakes. This effect was referred to as adsorption of AlLaSi (Al2LaSi2) phase in the solid/liquid interface inhibiting the growth of the Mg2Si.
Author Contributions
Conceptualization, V.D., E.P. and P.S.; methodology, X.C.; formal analysis, V.D. and S.K.; investigation, V.D., E.P., S.S. and P.S.; writing—original draft preparation, V.D. and P.S.; writing—review and editing, E.P., E.R. and S.K.; project administration, S.K.; funding acquisition, V.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation (project No. 20-19-00687).
Acknowledgments
The authors would like to thank Natalia Korotkova for providing SEM investigations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gursoy, O.; Timelli, G. Lanthanides: A focused review of eutectic modification in hypoeutectic Al–Si alloys. J. Mater. Res. Tech. 2020, 9, 8652–8666. [Google Scholar] [CrossRef]
- De Giovanni, M.; Kaduk, J.A.; Srirangam, P. Modification of Al–Si Alloys by Ce or Ce with Sr. JOM 2019, 71, 426–434. [Google Scholar] [CrossRef]
- Abdelaziz, M.H.; Samuel, A.M.; Doty, H.W.; Valtierra, S.; Samuel, F.H. Effect of additives on the microstructure and tensile properties of Al–Si alloys. J. Mater. Res. Technol. 2019, 8, 2255–2268. [Google Scholar] [CrossRef]
- Lin, G.; Li, K.; Feng, D.; Feng, Y.; Song, W.; Xiao, M. Effects of La–Ce addition on microstructure and mechanical properties of Al–18Si–4Cu–0.5Mg alloy. Trans. Nonferr. Met. Soc. China 2019, 29, 1592–1600. [Google Scholar] [CrossRef]
- Jiang, H.; Li, S.; Zheng, Q.; Zhang, L.; He, J.; Song, Y.; Deng, C.; Zhao, J. Effect of minor lanthanum on the microstructures, tensile and electrical properties of Al-Fe alloys. Mater. Des. 2020, 195, 108991. [Google Scholar] [CrossRef]
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Cao, Z.; Kong, G.; Che, C.; Wang, Y.; Peng, H. Experimental investigation of eutectic point in Al-rich Al-La, Al-Ce, Al-Pr and Al-Nd systems. J. Rare Earths 2017, 35, 1022–1028. [Google Scholar] [CrossRef]
- Akopyan, T.K.; Belov, N.A.; Naumova, E.A.; Letyagin, N.V. New in-situ Al matrix composites based on Al-Ni-La eutectic. Mater. Lett. 2019, 245, 110–113. [Google Scholar] [CrossRef]
- Akopyan, T.K.; Letyagin, N.V.; Sviridova, T.A.; Korotkova, N.O.; Prosviryakov, A.S. New casting alloys based on the Al + Al4 (Ca,La) eutectic. JOM 2020. [Google Scholar] [CrossRef]
- Weiss, D. Improved High-Temperature Aluminum Alloys Containing Cerium. J. Mater. Eng. Perform. 2019, 28, 1903–1908. [Google Scholar] [CrossRef]
- Czerwinski, F. Cerium in aluminum alloys. J. Mater. Sci. 2020, 55, 24–72. [Google Scholar] [CrossRef]
- Yu, X.; Sun, J.; Li, Z.; Dai, H.; Fang, H.; Zhao, J.; Yin, D. Solidification behaviour and elimination of undissolved Al2CuMg phase during homogenization in Ce-modified Al–Zn–Mg–Cu alloy. Rare Met. 2020, 39, 1279–1287. [Google Scholar] [CrossRef]
- Hosseinifar, M.; Malakhov, D.V. Effect of Ce and La on microstructure and properties of a 6xxx series type aluminum alloy. J. Mater. Sci. 2008, 43, 7157–7164. [Google Scholar] [CrossRef]
- Mahmoud, M.G.; Elgallad, E.M.; Ibrahim, M.F.; Samuel, F.H. Effect of rare earth metals on porosity formation in A356 alloy. Int. J. Metalcast. 2018, 12, 251–265. Available online: http://dx.doi.org/10.1007/s40962-017-0156-5 (accessed on 3 November 2020). [CrossRef]
- Chen, Y.; Pan, Y.; Lu, T.; Tao, S.; Wu, J. Effects of combinative addition of lanthanum and boron on grain refinement of Al–Si casting alloys. Mater. Des. 2014, 64, 423–426. [Google Scholar] [CrossRef]
- Yao, D.; Xia, Y.; Qiu, F.; Jiang, Q. Effects of La addition on the elevated temperature properties of the casting Al–Cu alloy. Mater. Sci. Eng. A 2011, 528, 1463–1466. [Google Scholar] [CrossRef]
- Liping, W.; Erjun, G.; Baoxia, M. Modification effect of lanthanum on primary phase Mg2Si in Mg-Si alloys. J. Rare Earths 2008, 26, 105–109. [Google Scholar] [CrossRef]
- Bai, G.; Liu, Z.; Lin, J.; Yu, Z.; Hue, Y.; Wen, C. Effects of the addition of lanthanum and ultrasonic stirring on the microstructure and mechanical properties of the in situ Mg2Si/Al composites. Mater. Des. 2016, 90, 424–432. [Google Scholar] [CrossRef]
- Yao, D.; Qiu, F.; Jiang, Q.; Li, Y.; Arnberg, L. Effect of Lanthanum on grain refinement of casting aluminum-copper alloy. Int. J. Metalcast. 2013, 7, 49–54. [Google Scholar] [CrossRef]
- Pramod, S.L.; Bakshi, S.R.; Murty, B.S. Aluminum-Based Cast In Situ Composites: A Review. J. Mater. Eng. Perform. 2015, 24, 2185–2207. [Google Scholar] [CrossRef]
- Sun, Y.; Ahlatci, H. Mechanical and wear behaviors of Al-12Si-XMg composites reinforced with in situ Mg2Si particles. Mater. Des. 2011, 32, 2983–2987. [Google Scholar] [CrossRef]
- Prusov, E.; Deev, V.; Rakhuba, E. Aluminum Matrix In-Situ Composites Reinforced with Mg2Si and Al3Ti. Mater. Today Proc. 2019, 11, 386–391. [Google Scholar] [CrossRef]
- Glazoff, M.; Khvan, A.; Zolotorevsky, V.; Belov, N.; Dinsdale, A. Casting Aluminium Alloys: Their Physical and Mechanical Metallurgy, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Thermo-Calc Software TCAL4—TCS Al-Based Alloy Database, version 4.0; Thermo-Calc Software AB: Stockholm, Sweden, 2015; Available online: http://www.thermocalc.com (accessed on 15 October 2020).
- Hosseinifar, M.; Malakhov, D.V. The sequence of intermetallics formation during the solidification of an Al-Mg-Si alloy containing La. Metall. Mater. Trans. A 2011, 42, 825–833. [Google Scholar] [CrossRef]
- Du, J.; Tu, H.; Peng, H.; Liu, Y.; Wu, C.; Wang, J.; Su, X. Phase equilibria of the Al-Si-La system between 0 and 50 at.% La at 600 and 800 °C. JALCOM 2018, 765, 608–615. [Google Scholar] [CrossRef]
- Liu, Y.L.; Kang, S.B. Solidification and segregation of Al-Mg alloys and influence of alloy composition and cooling rate. Mater. Sci. Technol. 1997, 13, 331–336. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, L.; Han, C.; Ou, L.; Wang, J.; Liu, C. Effect of Fe, Si and cooling rate on the formation of Fe- and Mn-rich intermetallics in Al–5Mg–0.8Mn alloy. J. Mater. Sci. Technol. 2016, 32, 305–312. [Google Scholar] [CrossRef]
- Liu, Y.L.; Kang, S.B. The solidification process of Al–Mg–Si alloys. J. Mater. Sci. 1997, 32, 1443–1447. [Google Scholar] [CrossRef]
- Thermo-Calc Software TCAL7—TCS Al-Based Alloy Database, version 4.0; Thermo-Calc Software AB: Stockholm, Sweden, 2020; Available online: http://www.thermocalc.com (accessed on 15 October 2020).
- Wong, C.; Styles, M.J.; Zhu, S.; Qiu, D.; McDonald, S.D.; Zhu, Y.; Gibson, M.A.; Abbott, T.B.; Easton, M.A. (Al,Mg)3La: A new phase in the Mg–Al–La system. Acta Cryst. 2018, B74, 370–375. [Google Scholar] [CrossRef]
- Gröbner, J.; Kevorkov, D.; Schmid-Fetzer, R. Thermodynamic modelling of Al–Ce–Mg phase equilibria coupled with key experiments. Intermetallics 2002, 10, 415–422. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Zhou, Z.; Xu, J. Influence of rare earth (Ce and La) addition on the performance of Al-3.0 wt%Mg alloy. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2017, 32, 611–618. [Google Scholar] [CrossRef]
- Wang, M.; Xu, W.; Han, Q. Study of Refinement and Morphology Change of AlFeSi Phase in A380 Alloy due to Addition of Ca, Sr/Ca, Mn and Mn, Sr. Mater. Trans. 2016, 57, 1509–1513. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, H.; Jiang, H.; Mi, Z.; Zhang, H. Multi-Refinement Effect of Rare Earth Lanthanum on α-Al and Eutectic Si Phase in Hypoeutectic Al-7Si Alloy. Metals 2020, 10, 621. [Google Scholar] [CrossRef]
- Belov, N.A.; Naumova, E.A.; Akopyan, T.K.; Doroshenko, V.V. Phase Diagram of Al-Ca-Mg-Si System and Its Application for the Design of Aluminum Alloys with High Magnesium Content. Metals 2017, 7, 429. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, Z.; Wang, Y.; Zhou, B. Microstructural development of Al–15wt.% Mg2Si in situ composite with mischmetal addition. Mater. Sci. Eng. A 2000, 281, 104–112. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).