Modification of Alumina Inclusions in SWRS82B Steel by Adding Rare Earth Cerium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Procedures
2.2. Sample Processing
3. Results and Discussion
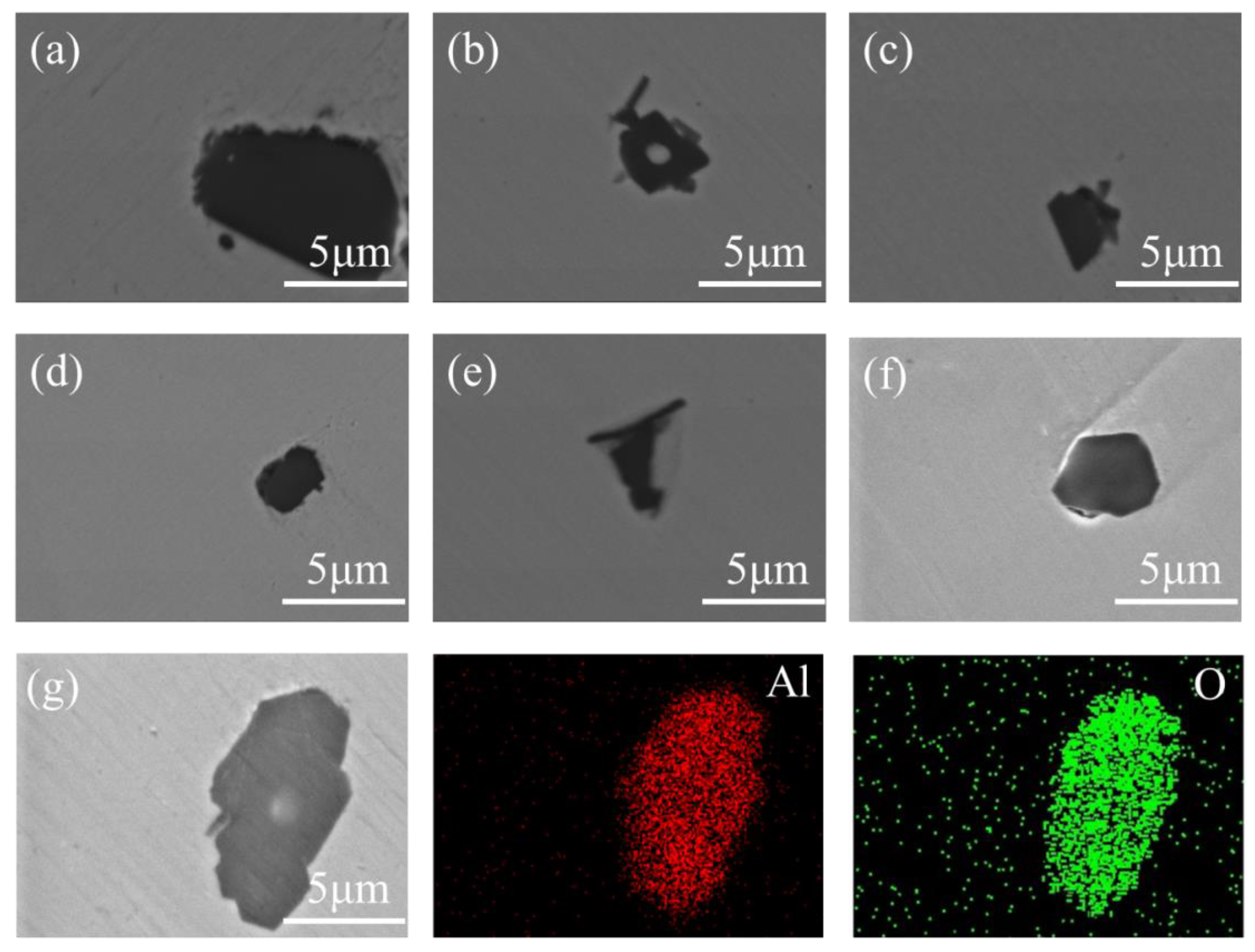
3.1. Change in Composition of Inclusions
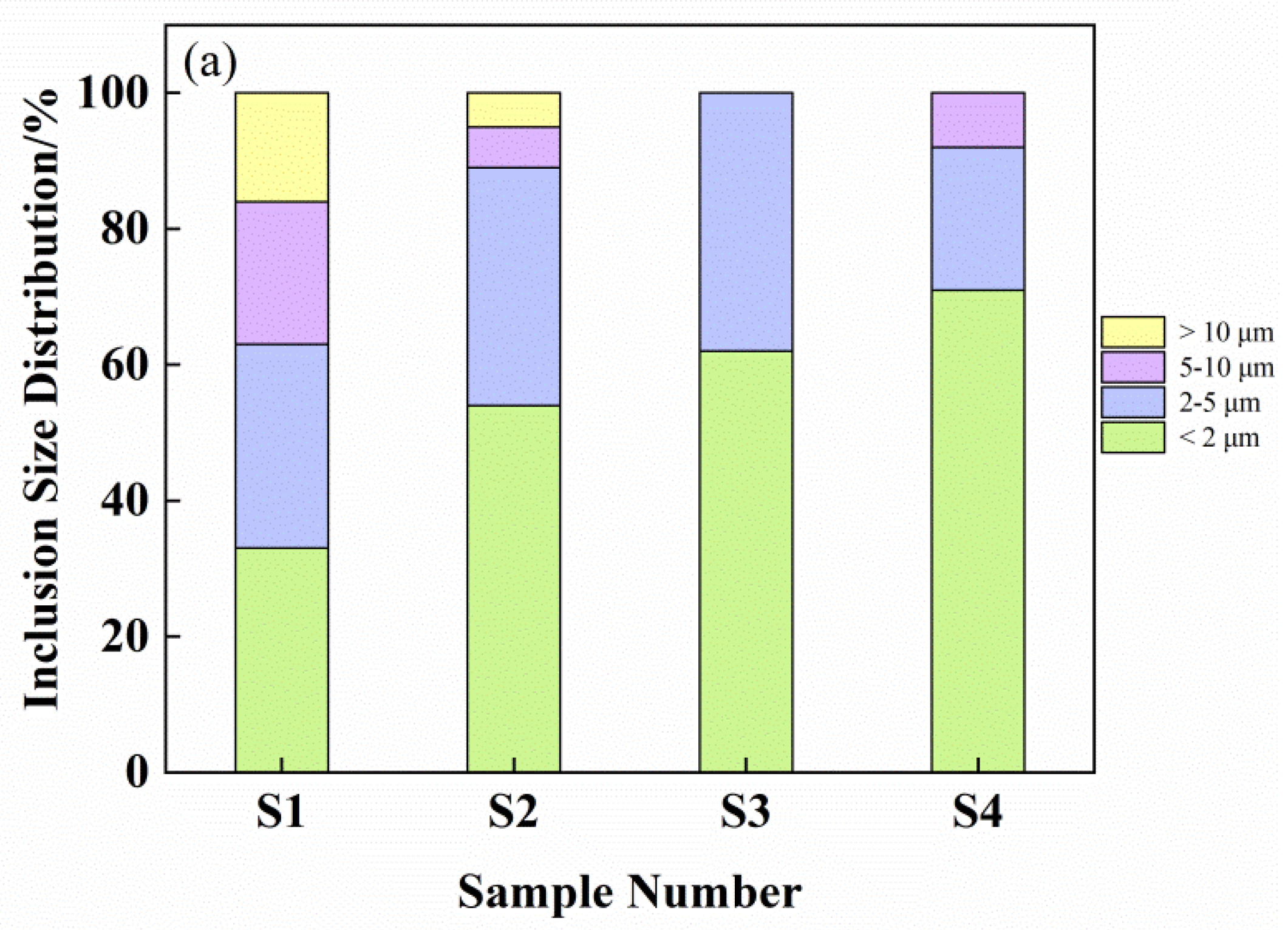
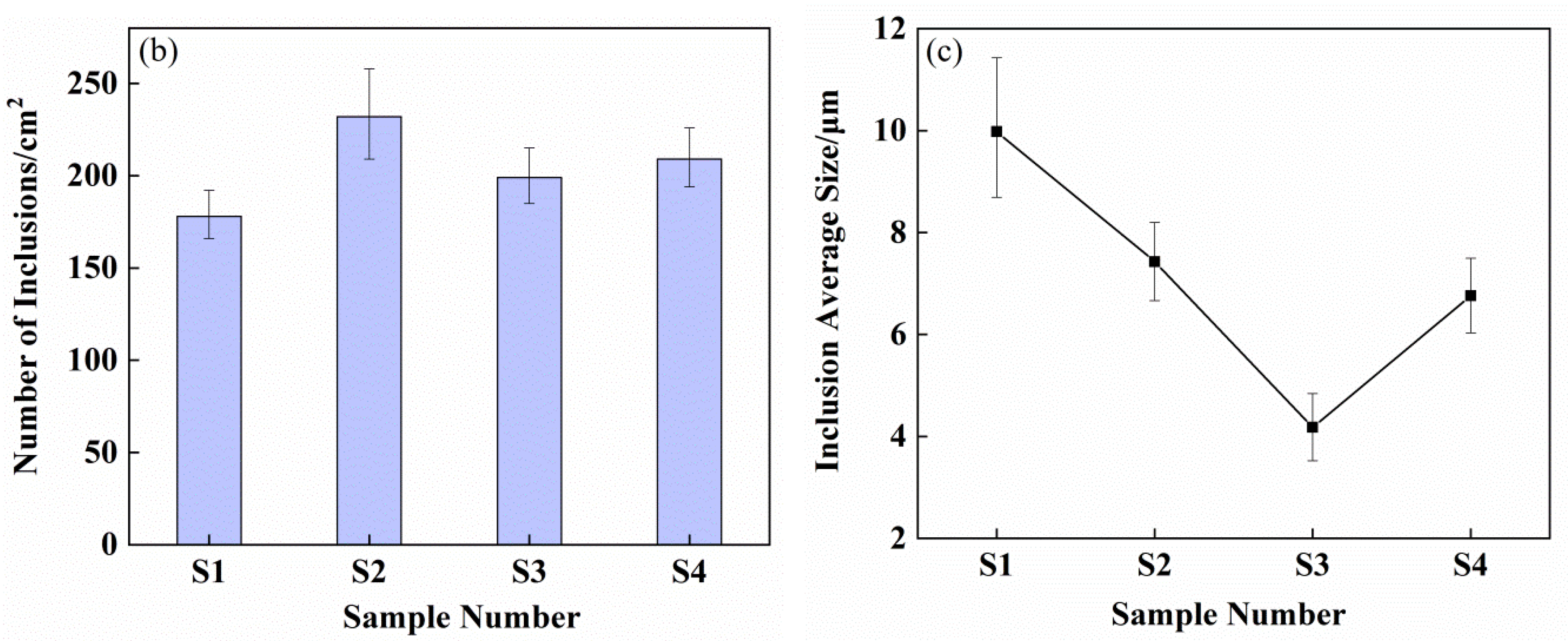
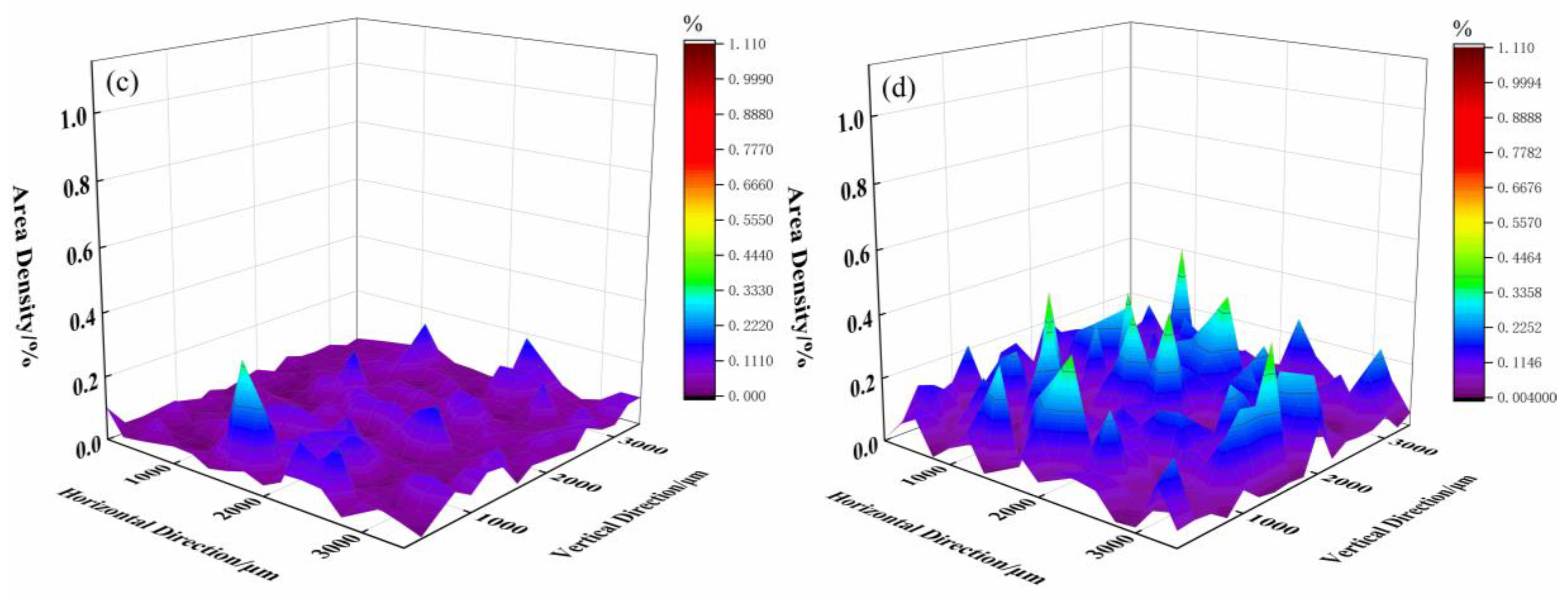
3.2. Number, Size, and Area Density of Inclusions
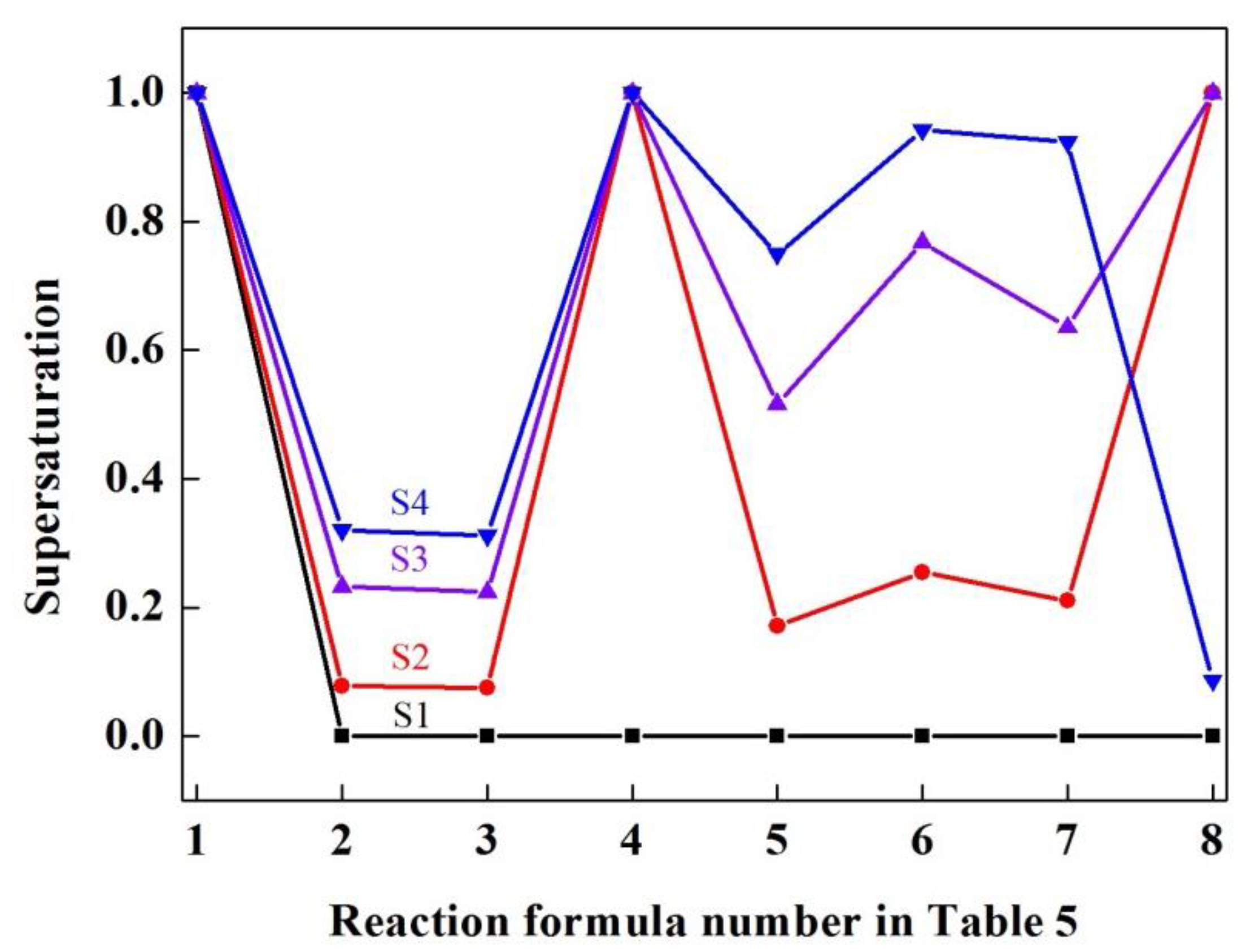
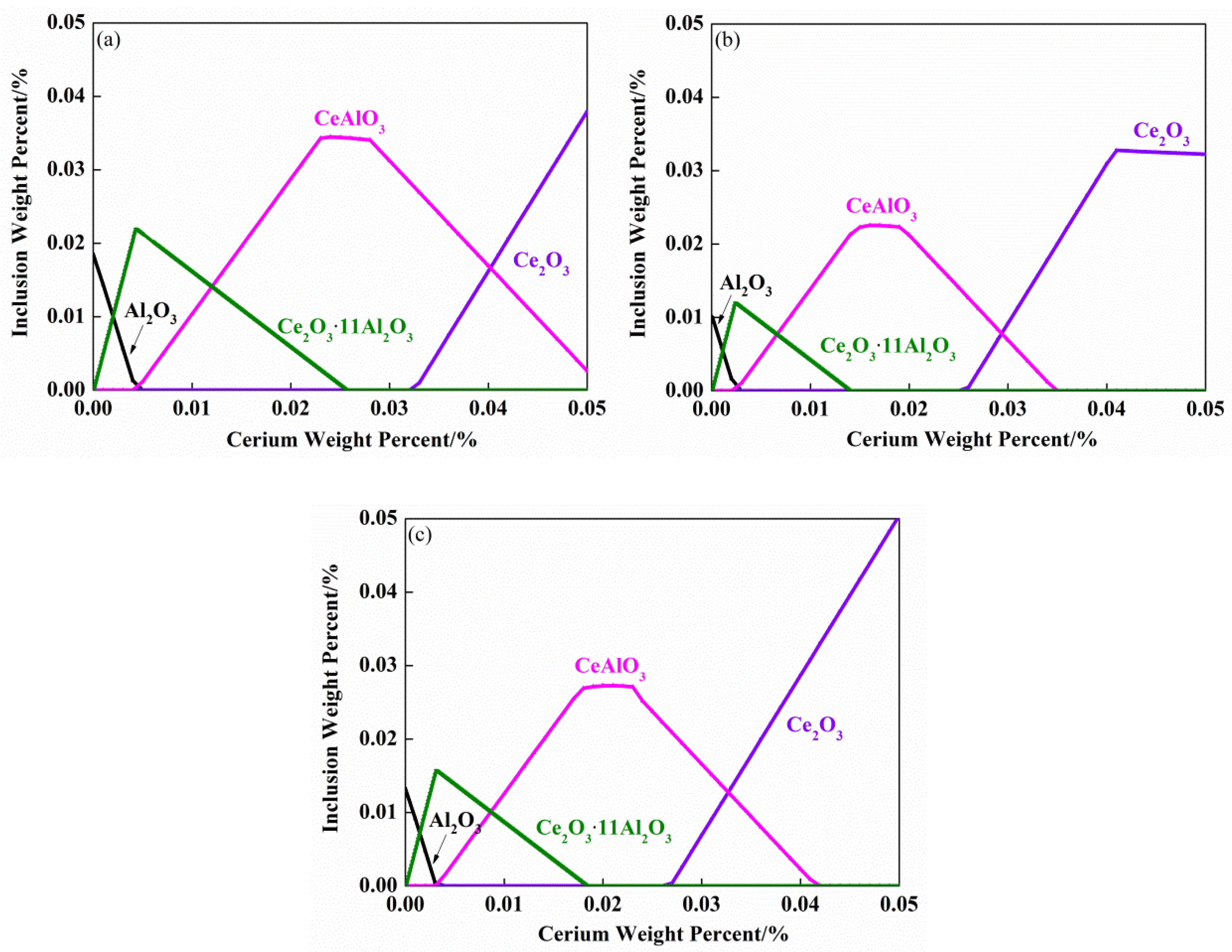
3.3. Thermodynamic Calculation of Inclusions
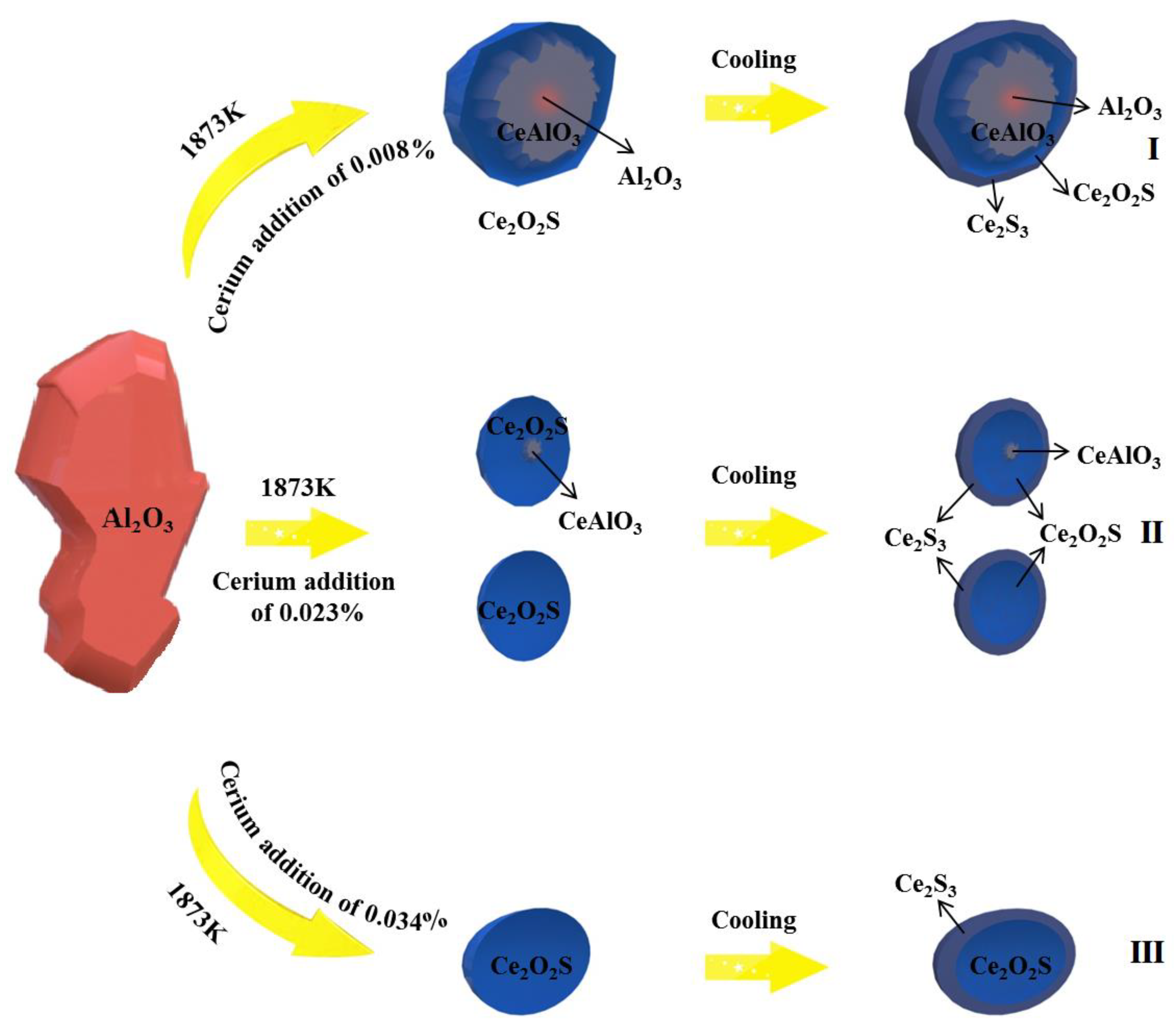
3.4. Transformation of Inclusions during Cooling and Solidification
3.5. Inclusion Evolution Model
4. Conclusions
- (1)
- Before the addition of cerium, the average size of the inclusions is in the range of 8.65 μm–11.32 μm and the shape is irregular. When cerium is added, the inclusions gradually become spheroidized and the average size of the inclusions varies in the range of 3.52 μm–8.19 μm, and if the cerium content exceeds 0.023%, the inclusions will grow excessively.
- (2)
- Compared with Al2O3 inclusions in sample S1, the average size of inclusions produced by adding 0.023% cerium is reduced in the range of 5.13 μm–6.48 μm, and the best characteristics of inclusions in this study were obtained in experimental samples with cerium addition of 0.023%, in which the minimum size of inclusions is in the range of 3.52 μm–4.84 μm and most uniform distribution.
- (3)
- The classical thermodynamic calculation results are basically consistent with the experimental composition results, and the transition route of inclusions in SWRS82B steel at 1873 K is as follows: Al2O3 → Ce2O2S + CeAlO3 + Al2O3 → CeAlO3 + Ce2O2S/Ce2O2S → Ce2O2S.
- (4)
- Ce2S3 precipitated during solidification. The modification route of Al2O3 inclusions in SWRS82B steel by increased cerium additions is as follows: Al2O3 → Ce2S3 + CeAlO3 + Ce2O2S + Al2O3 → Ce2S3 + CeAlO3 + Ce2O2S/Ce2S3 + Ce2O2S → Ce2S3 + Ce2O2S.
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, J.Q.; Liang, Y.L.; Xiang, S.; Yang, X.D.; Yang, M. Effect of Heat Treatment Process on Microstructure and Mechanical Properties of SWRS82B Wire Rod. Adv. Mater. Res. 2010, 97, 752–755. [Google Scholar] [CrossRef]
- Amouyal, Y.; Mao, Z.; Seidman, D.N. Combined atom probe tomography and first-principles calculations for studying atomistic interactions between tungsten and tantalum in nickel-based alloys. Acta Mater. 2014, 74, 296–308. [Google Scholar] [CrossRef]
- Tanaka, Y.; Pahlevani, F.; Moon, S.C.; Dippenaar, R.; Sahajwalla, V. In situ characterisation of MnS precipitation in high carbon steel. Sci. Rep. 2019, 9, 1798–1810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.Y.; Luan, Y.K.; Li, D.Z.; Li, Y.Y. Effects of rare earth elements on inclusions and impact toughness of high-carbon chromium bearing steel. J. Mater. Sci. Technol. 2019, 35, 1298–1308. [Google Scholar] [CrossRef]
- Wang, H.P.; Xiong, L.; Zhang, L.; Wang, Y.; Shu, Y.Y.; Zhou, Y.H. Investigation of RE-O-S-As Inclusions in High Carbon Steels. Metall. Mater. Trans. B 2017, 48, 2849–2858. [Google Scholar] [CrossRef]
- Khurana, B.; Spooner, S.; Rao, M.B.V.; Roy, G.G.; Srirangam, P. In situ observation of calcium oxide treatment of inclusions in molten steel by confocal microscopy. Metall. Mater. Trans. B 2017, 48, 1409–1415. [Google Scholar] [CrossRef] [Green Version]
- Wilson, W.G.; Heaslip, L.J.; Sommerville, I.D. Rare Earth Additions in Continuously Cast Steel. JOM 1985, 37, 36–41. [Google Scholar] [CrossRef]
- Ye, G.; Jonsson, P.; Lund, T. Thermodynamics and Kinetics of the Modification of Al2O3 Inclusions. ISIJ Int. 1996, 36, S105–S108. [Google Scholar] [CrossRef]
- Tugrul, E.T. Ladle deoxidation, desulphurisation and inclusions in steel-Part II: Observations in practice. Archiv. Dassenhuttenwesen 1983, 54, 45–52. [Google Scholar]
- Fruehan, R.J. The free energy of formation of Ce2O2S and the nonstoichiometry of cerium oxides. Metall. Mater. Trans. B 1979, 10, 143–148. [Google Scholar] [CrossRef]
- Nicholson, A.; Howarth, D.J.; Turton, J. Metallurgical Development of Ultra Clean and Low Sulphur Steel at BSC River Don Works. Ironmak. Steelmak. 1983, 10, 185–192. [Google Scholar]
- Hirata, H.; Isobe, K. Steel Having Finely Dispersed Inclusions. U.S. Patent US20060157162 A1, 20 July 2006. [Google Scholar]
- Yang, X.H.; Wu, P.F.; Cheng, G.G. Behavior of rare earth on modifying inclusion in special steel. J. Chin. Rare Earth Soc. 2010, 28, 612. [Google Scholar]
- Gao, S.; Wang, M.; Guo, J.L.; Wang, H.; Zhi, J.G.; Bao, Y.P. Characterization Transformation of Inclusions Using Rare Earth Ce Treatment on Al-Killed Titanium Alloyed Interstitial Free Steel. Steel Res. Int. 2019, 90, 1900194. [Google Scholar] [CrossRef]
- Wen, B.; Song, B. In Situ Observation of the Evolution of Intragranular Acicular Ferrite at Ce-Containing Inclusions in 16Mn Steel. Steel Res. Int. 2012, 83, 487–495. [Google Scholar]
- Li, H.; Yu, Y.C.; Ren, X.; Zhang, S.H.; Wang, S.B. Evolution of Al2O3 inclusions by cerium treatment in low carbon high manganese steel. J. Iron Steel Res. Int. 2017, 24, 925–934. [Google Scholar] [CrossRef]
- Wang, L.J.; Liu, Y.Q.; Wang, Q.; Chou, K.C. Evolution Mechanisms of MgO center dot Al2O3 Inclusions by Cerium in Spring Steel Used in Fasteners of High-speed Railway. ISIJ Int. 2015, 55, 970–975. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.D.; Zhang, T.S.; Liu, C.J.; Jiang, M.F. Thermodynamic and Experimental Studies on Al Addition of 253MA Steel. Metals 2019, 9, 433. [Google Scholar] [CrossRef] [Green Version]
- Šuler, B.; Burja, J.; Medved, J. Modification of non-metallic inclusions with rare-earth metals in 50CrMoV13-1 steel. Mater. Tehnol. 2019, 53, 441–447. [Google Scholar] [CrossRef]
- Yan, H.H.; Hu, Y.; Zhao, D.W. Analysis of the microstructure and properties of AISI 1026 steel containing rare earth elements. Mater. Res. Express 2019, 6, 106–115. [Google Scholar] [CrossRef]
- Li, Y.D.; Liu, C.J.; Zhang, T.S.; Jiang, M.F.; Peng, C. Liquid Inclusions in Heat-Resistant Steel Containing Rare Earth Elements. Metall. Mater. Trans. B 2016, 48, 956–965. [Google Scholar] [CrossRef]
- Gundersen, H.J.G. Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William, R. Thompson. J. Microsc. 1986, 143, 3–45. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Shimasaki, S.I.; Taniguchi, S.J.; Uesugi, K.; Narita, S.S. Stereological Analysis of Nonspherical Particles in Solid Metal. Metall. Mater. Trans. B 2013, 44, 750–761. [Google Scholar] [CrossRef]
- Zhang, D.; Shen, P.; Xie, J.B.; An, J.M.; Huang, Z.Z.; Fu, J.X. A method for observing tridimensional morphology of sulfide inclusions by non-aqueous solution electrolytic etching. J. Iron Steel Res. Int. 2018, 26, 275–284. [Google Scholar] [CrossRef]
- Bi, Y.Y.; Karasev, A.; Jönsson, P.G. Three-Dimensional Investigations of Inclusions in Ferroalloys. Steel Res. Int. 2014, 85, 659–669. [Google Scholar] [CrossRef]
- Zhang, X.W.; Zhang, L.F.; Yang, W.; Wang, Y.; Liu, Y.; Dong, Y.C. Characterization of the Three-Dimensional Morphology and Formation Mechanism of Inclusions in Linepipe Steels. Metall. Mater. Trans. B 2017, 48, 701–712. [Google Scholar] [CrossRef]
- Wang, Y.; Karasev, A.; Jönsson, P.G. An Investigation of Non-Metallic Inclusions in Different Ferroalloys using Electrolytic Extraction. Metals 2019, 9, 687. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.H. Principles of Iron and Steel Metallurgy, 3rd ed.; Metallurgical Industry Press: Beijing, China, 1981; p. 426. ISBN 7-5024-2861-5. [Google Scholar]
- Li, X.; Jiang, Z.; Geng, X.; Chen, M.J.; Peng, L.Z. Evolution Mechanism of Inclusions in H13 Steel with Rare Earth Magnesium Alloy Addition. ISIJ Int. 2019, 59, 1552–1561. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Yang, J.C.; Yang, L.; Gao, X.Z. Effect of Ce on Inclusions and Impact Property of 2Cr13 Stainless Steel. J. Iron Steel Res. Int. 2010, 17, 62–67. [Google Scholar] [CrossRef]
- Li, G.; Lan, P.; Zhang, J.Q. Solidification Structure Refinement in TWIP Steel by Ce Inoculation. Acta Metall Sin. 2020, 56, 704–714. [Google Scholar]
- Straumanis, M.E.; Kim, C.D. Phase extent of gallium arsenide determined by the lattice constant and density method. Acta Cryst. 1965, 19, 256–259. [Google Scholar] [CrossRef]
- Kolitsch, U.; Seifert, H.J.; Ludwig, T.; Aldinger, F. Phase equilibria and crystal chemistry in the Y2O3–Al2O3–SiO2 system. J. Mater. Res 1999, 14, 447–455. [Google Scholar] [CrossRef]




| Element | C | Si | Mn | P | S | Al | O |
|---|---|---|---|---|---|---|---|
| S1 | 0.826 | 0.21 | 0.83 | 0.019 | 0.008 | 0.0254 | 0.015 |
| S2 | 0.823 | 0.21 | 0.82 | 0.018 | 0.007 | 0.0253 | 0.0093 |
| S3 | 0.824 | 0.20 | 0.83 | 0.019 | 0.007 | 0.0254 | 0.0053 |
| S4 | 0.821 | 0.21 | 0.83 | 0.019 | 0.009 | 0.0254 | 0.0068 |
| Sample | Actual Content/%Ce | Actual Yield/% |
|---|---|---|
| S1 | 0 | 0 |
| S2 | 0.008 | 9.34 |
| S3 | 0.023 | 10.07 |
| S4 | 0.034 | 9.53 |
| C | Si | Mn | P | Al | O | S | Ce | |
|---|---|---|---|---|---|---|---|---|
| O | −0.45 | −0.133 | −0.021 | 0.07 | −3.9 | −0.20 | −0.133 | −0.57 |
| S | 0.11 | 0.063 | −0.026 | 0.029 | 0.035 | −0.27 | −0.026 | - |
| Ce | −0.077 | - | 0.13 | 1.77 | −2.58 | −106 | −10.32 | 0.0069 |
| Al | 0.091 | 0.0056 | - | - | 0.045 | −6.6 | 0.03 | - |
| Number | a[O] | a[S] | a[Ce] | a[Al] |
|---|---|---|---|---|
| S1 | 0.00454 | 0.00967 | 0 | 0.02419 |
| S2 | 0.00452 | 0.00964 | 0.00718 | 0.02625 |
| S3 | 0.00450 | 0.00965 | 0.02163 | 0.02801 |
| S4 | 0.00438 | 0.00965 | 0.03142 | 0.02737 |
| Number | Equation | ΔGθ/(J·mol−1) |
|---|---|---|
| 1 | 2[Al] + 3[O] = Al2O3(s) | −1,225,196 + 393.78T |
| 2 | [Ce] + 2[O] = CeO2(s) | −852,720 + 249.96T |
| 3 | [Ce] + 3/2[O] = 1/2Ce2O3(s) | −714,380 + 179.74T |
| 4 | [Ce]+[O] + 1/2[S] = 1/2 Ce2O2S(s) | −675,700 + 165.5T |
| 5 | [Ce] + 3/2[S] = 1/2Ce2S3(s) | −536,420 + 163.86T |
| 6 | [Ce] + 4/3[S] = 1/3Ce3S4(s) | −497,670 + 146.3T |
| 7 | [Ce] + [S] = CeS(s) | −422,100 + 120.38T |
| 8 | [Ce] + 3[O] + [Al] = CeAlO3(s) | −1,366,460 + 364.3T |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, C.; Wang, L.; Xiong, X.; Chen, L.; Zhuang, C. Modification of Alumina Inclusions in SWRS82B Steel by Adding Rare Earth Cerium. Metals 2020, 10, 1696. https://doi.org/10.3390/met10121696
Wang Y, Li C, Wang L, Xiong X, Chen L, Zhuang C. Modification of Alumina Inclusions in SWRS82B Steel by Adding Rare Earth Cerium. Metals. 2020; 10(12):1696. https://doi.org/10.3390/met10121696
Chicago/Turabian StyleWang, Yi, Changrong Li, Linzhu Wang, Xingqiang Xiong, Lu Chen, and Changling Zhuang. 2020. "Modification of Alumina Inclusions in SWRS82B Steel by Adding Rare Earth Cerium" Metals 10, no. 12: 1696. https://doi.org/10.3390/met10121696




