Metallurgical Interaction among BNi-9 and Waspaloy, FSX-414 or 304-Type Stainless Steel under TLP Cycle
Abstract
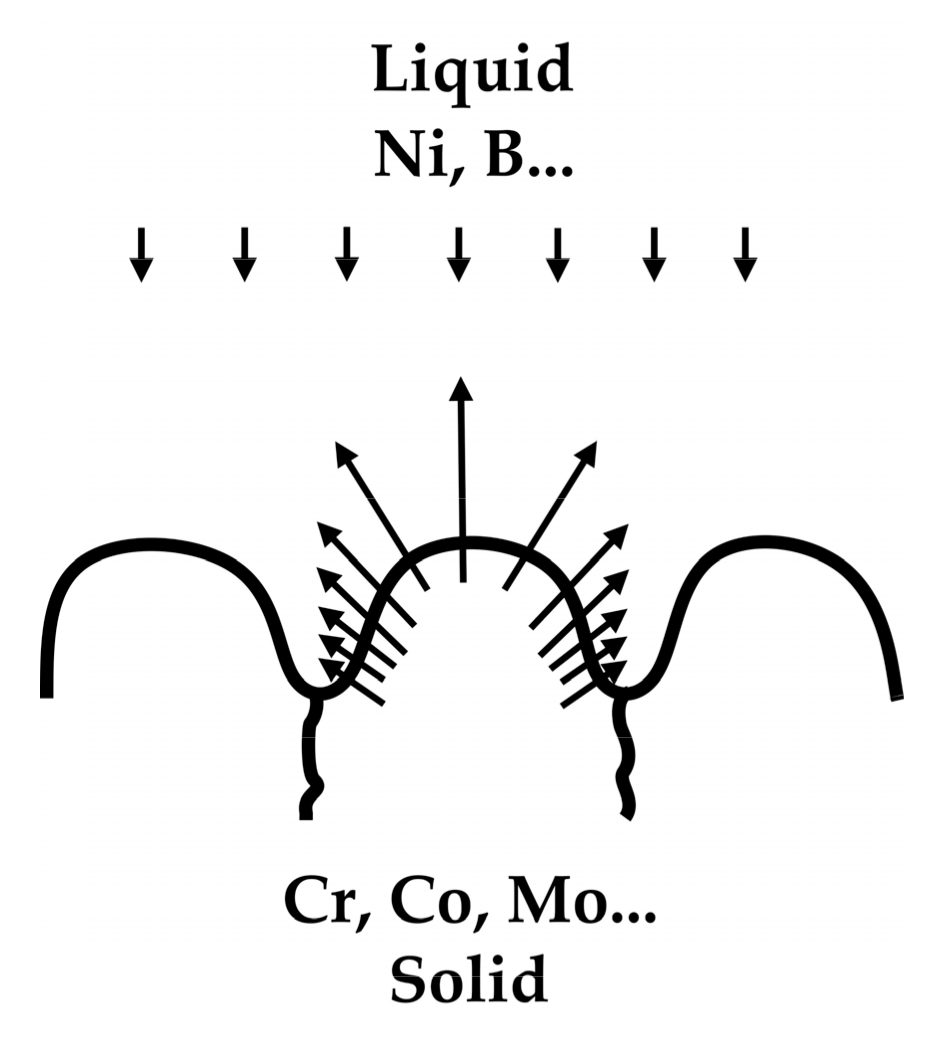
:1. Introduction
2. Materials and Methods
2.1. Base Alloys and the BNi-9 Filler Metal Paste
2.2. Characterization by Differential Scanning Calorimetry of the BNi-9 Filler Metal Paste
2.3. Aging Heat-Treatment of Waspaloy
2.4. Surface Preparation and Ni Coating
2.4.1. Surface Preparation
2.4.2. Ni Coating
2.5. Preparation of the Wedge and Drop Samples
2.6. Brazing Treatment
2.7. Microstructural Characterization
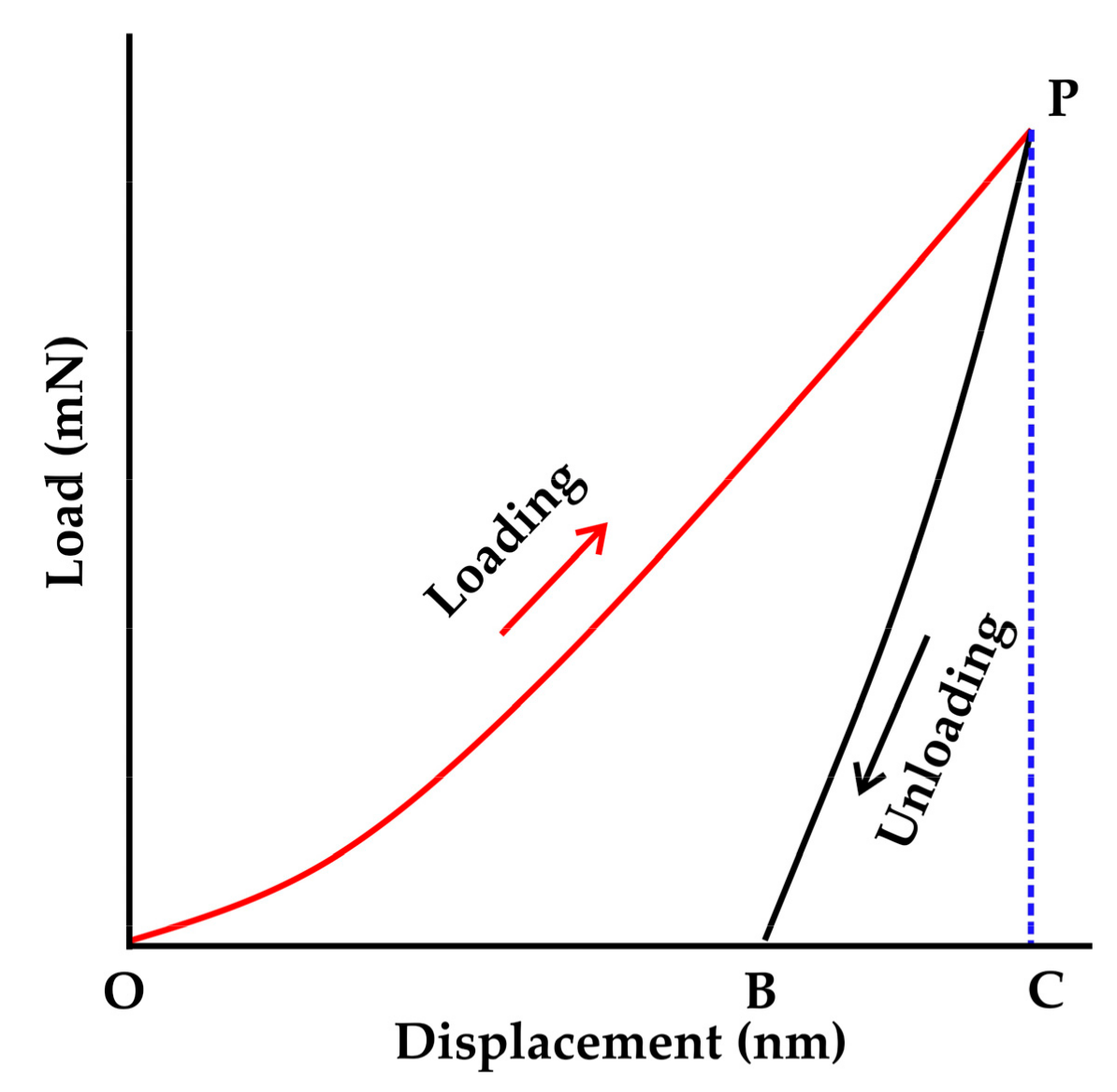
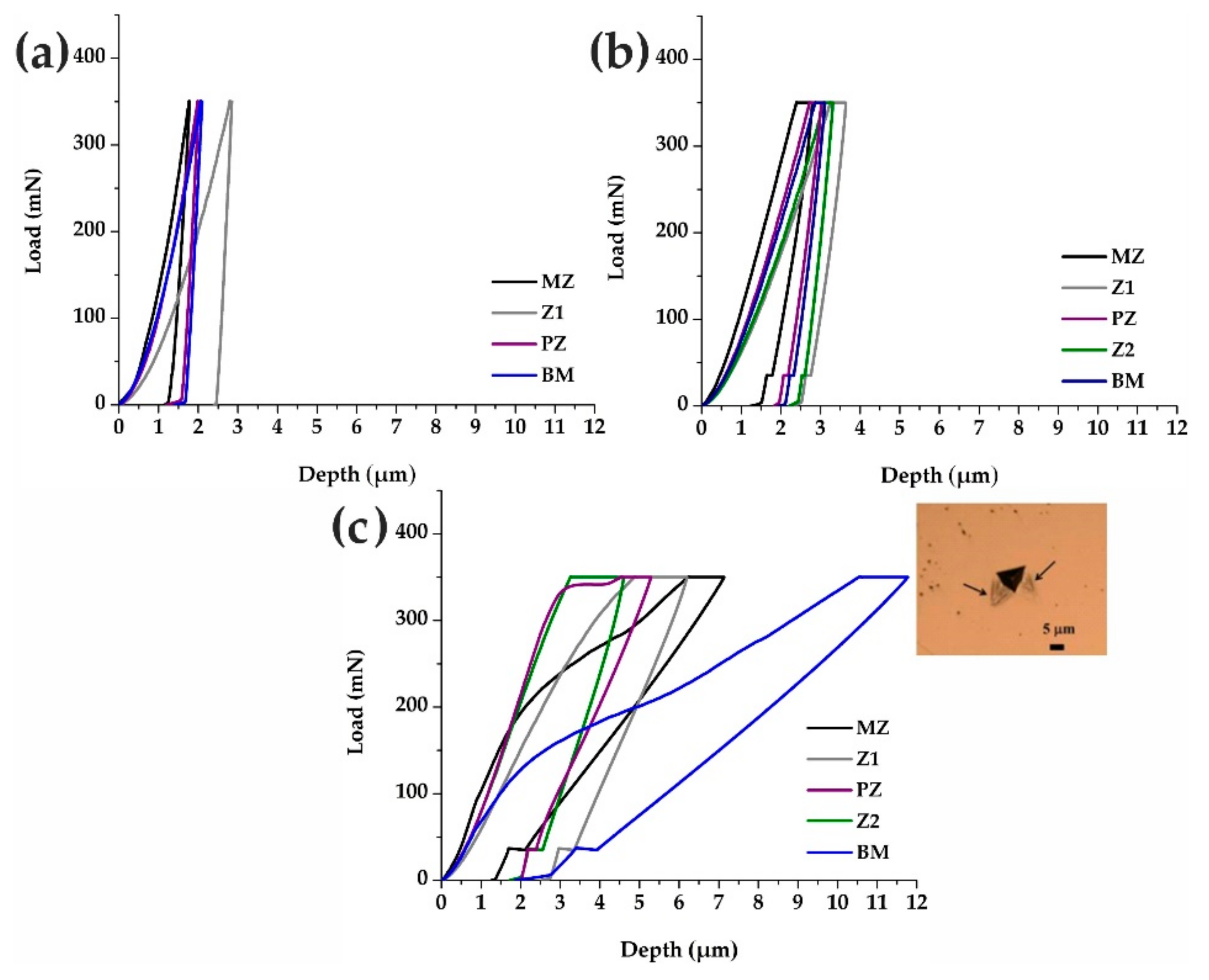
2.8. Evaluation of the Elastic and Plastic Properties by Nanoindentation
3. Results
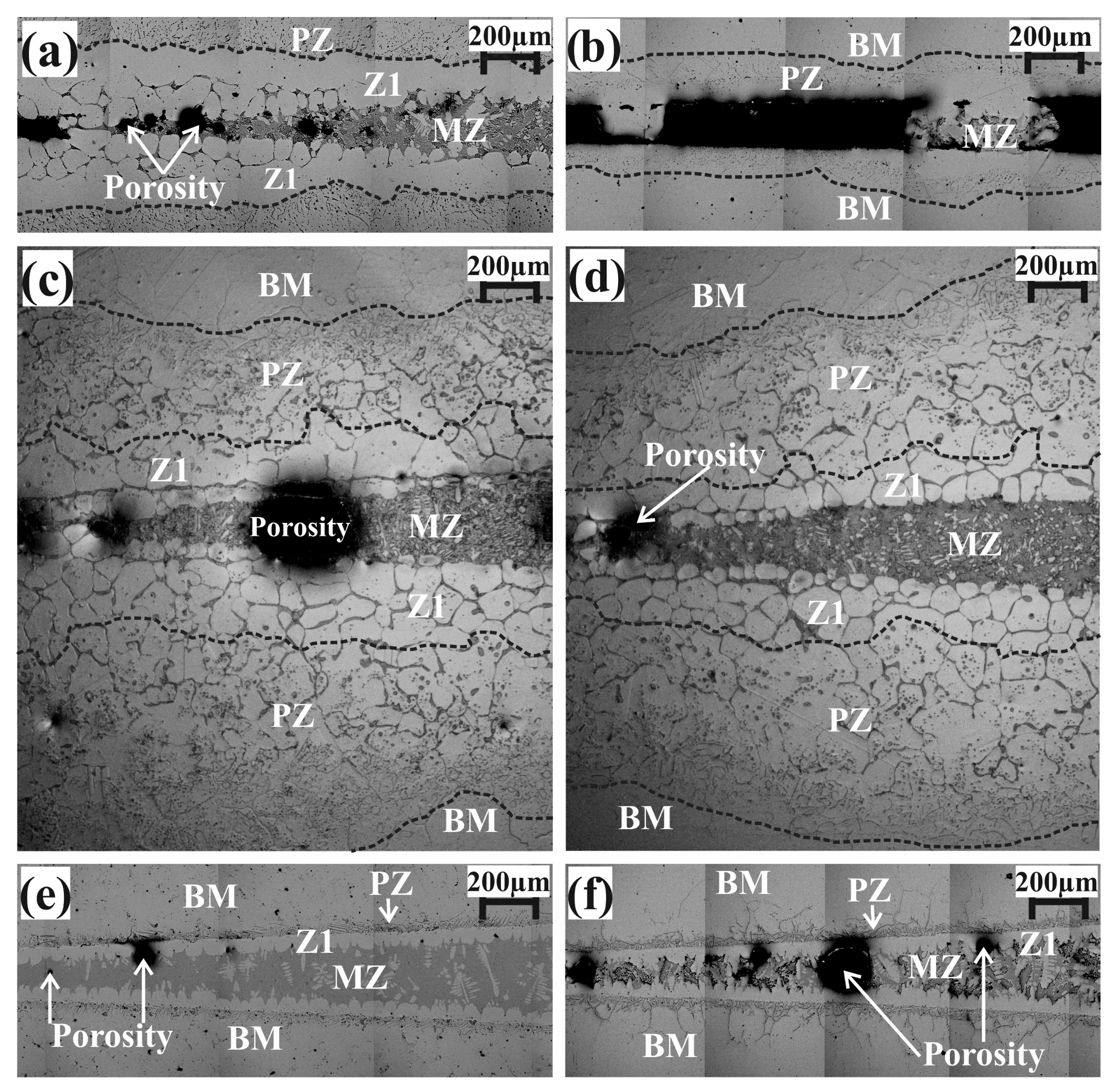
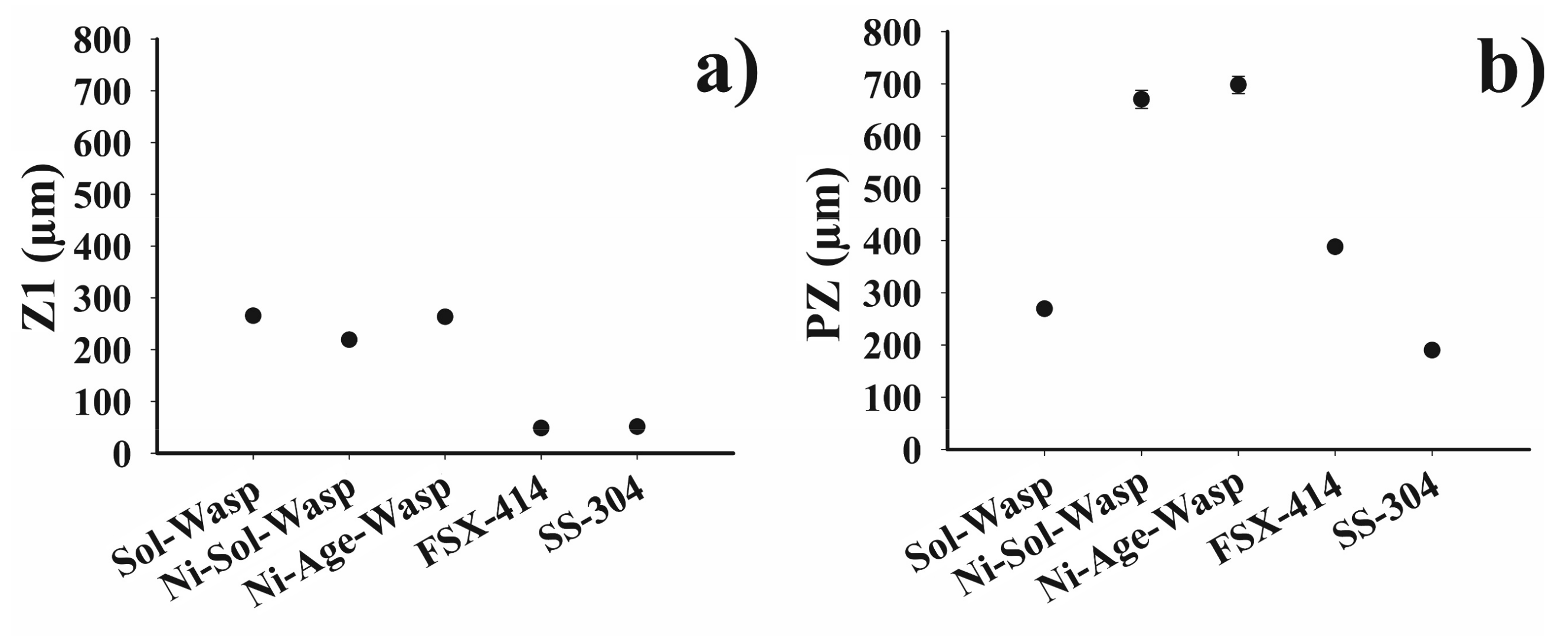
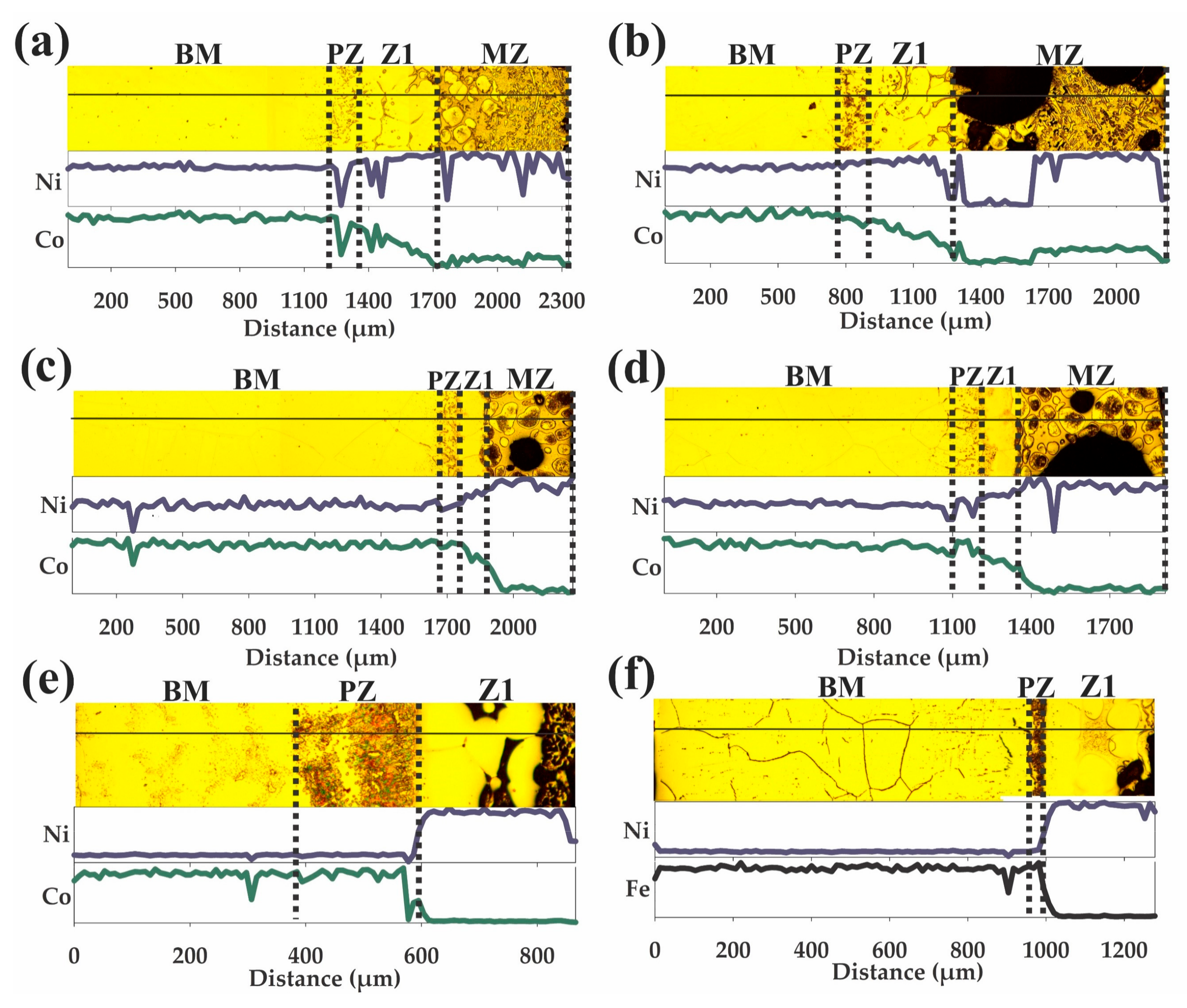
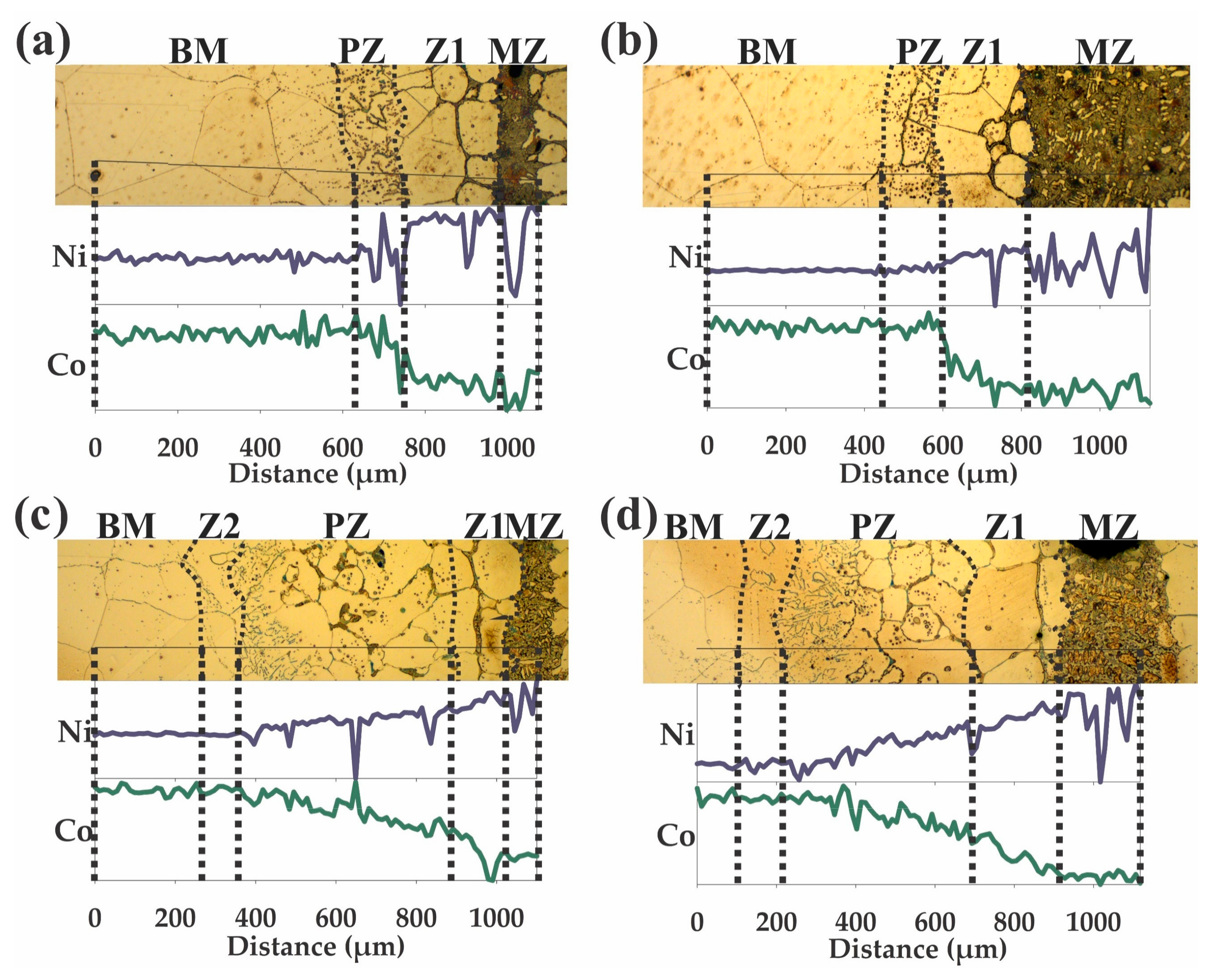
3.1. Wedge-Samples
3.2. Drop-Sample Characterization
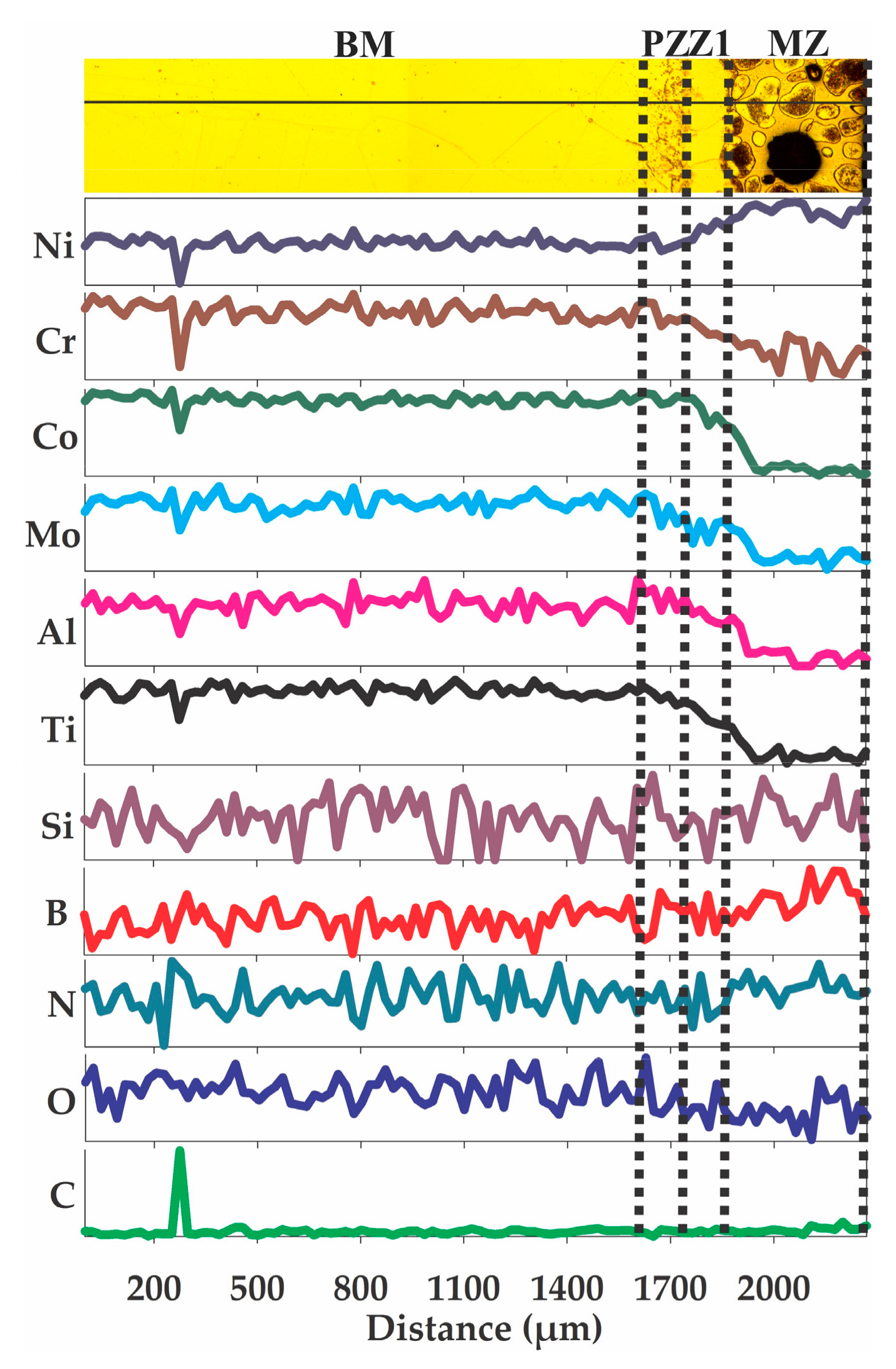
3.3. Chemical Microanalyses
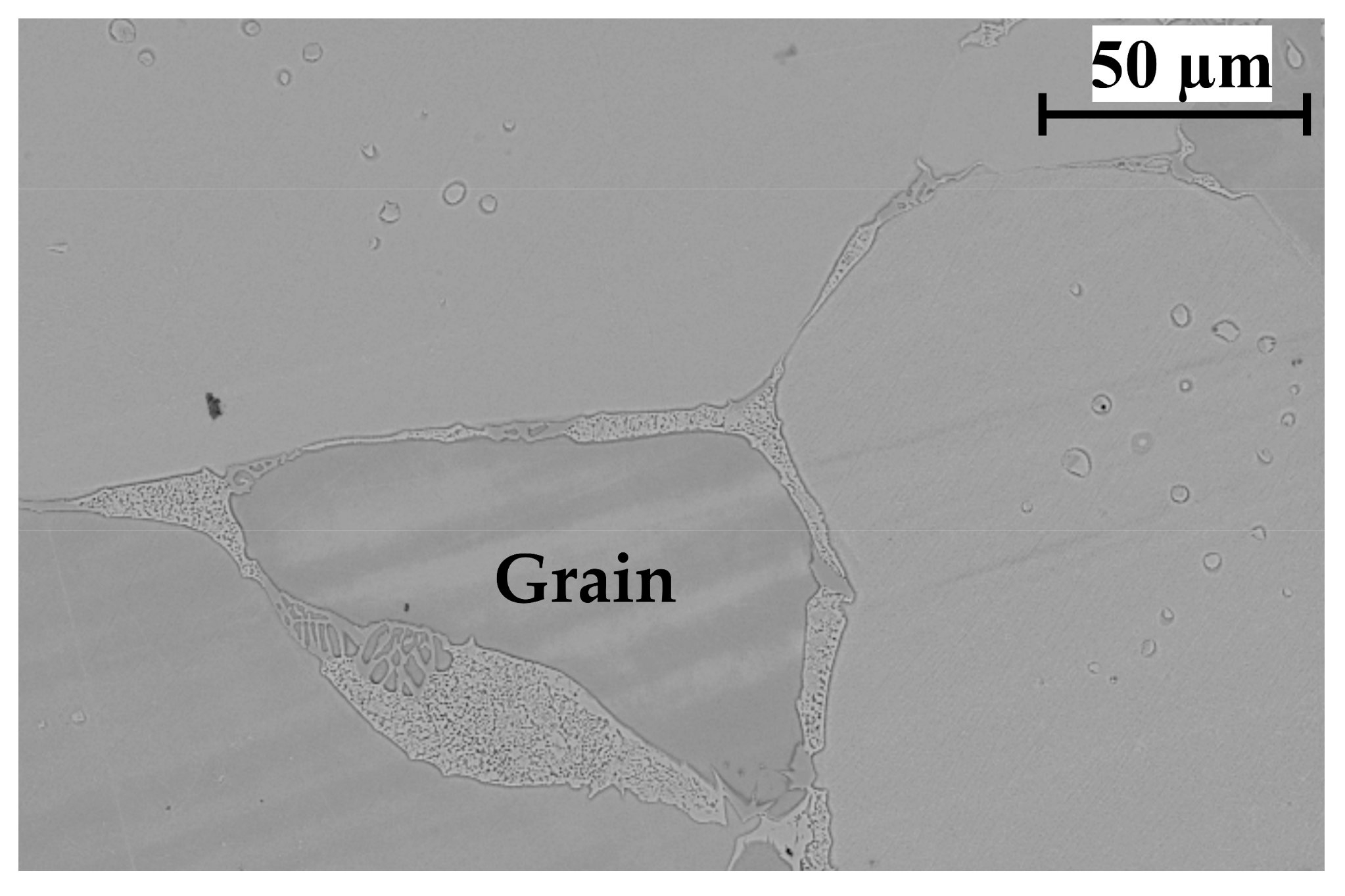
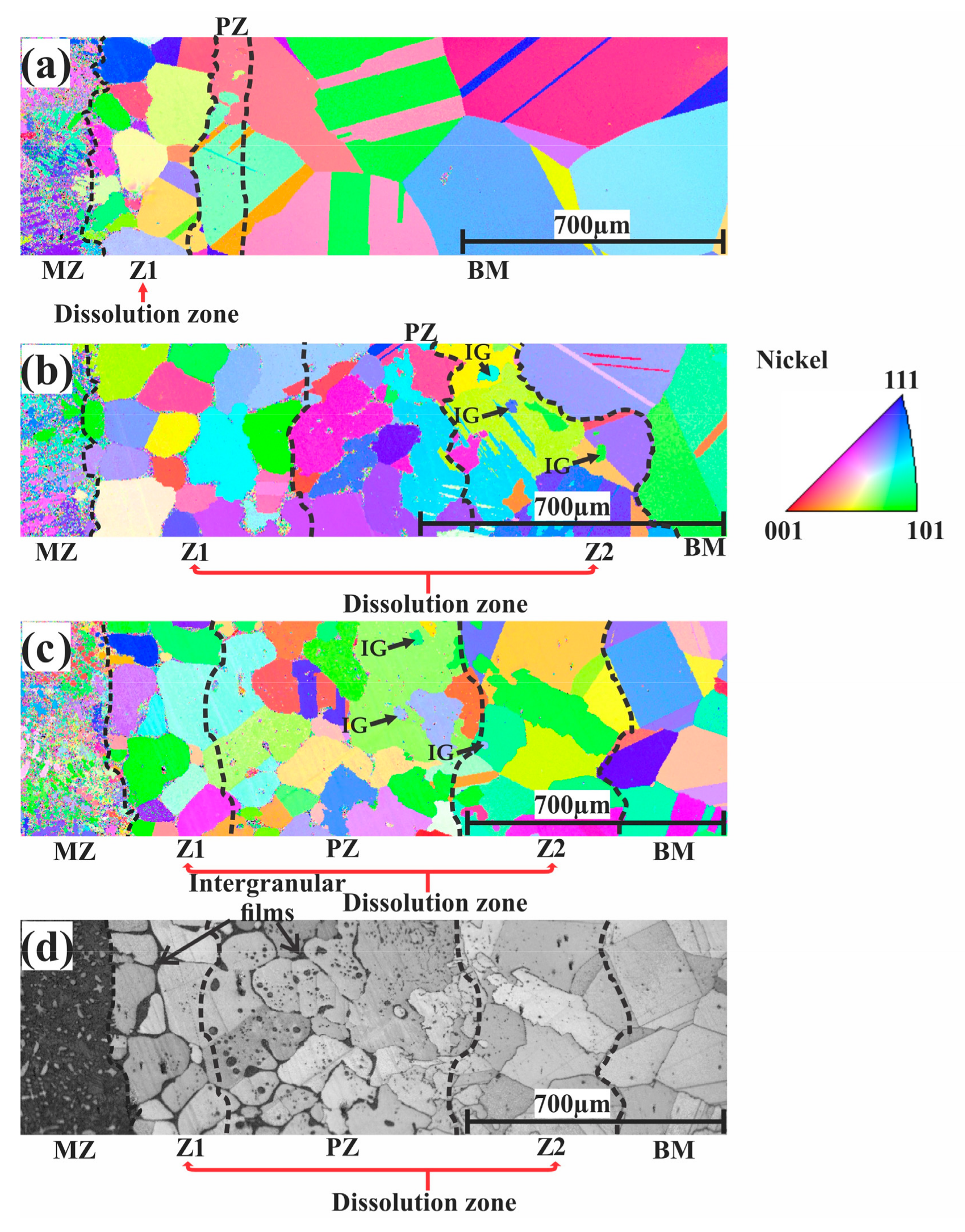
3.4. Evaluation of the Microstructural Orientation of the Brazed Waspaloy by EBSD
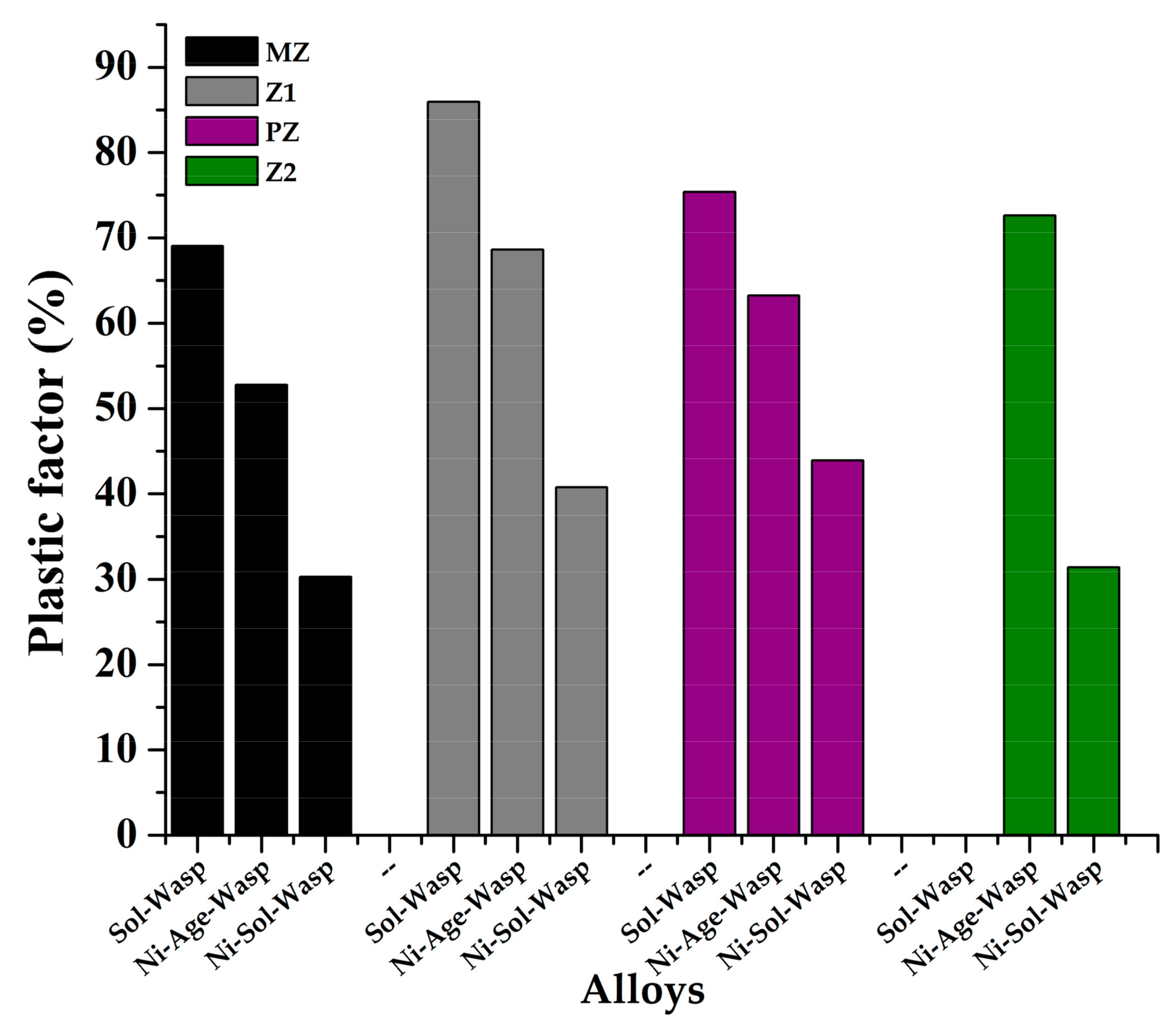
3.5. Evaluation of the Mechanical Properties of the Brazed Waspaloy by Nanoindentation
4. Discussion
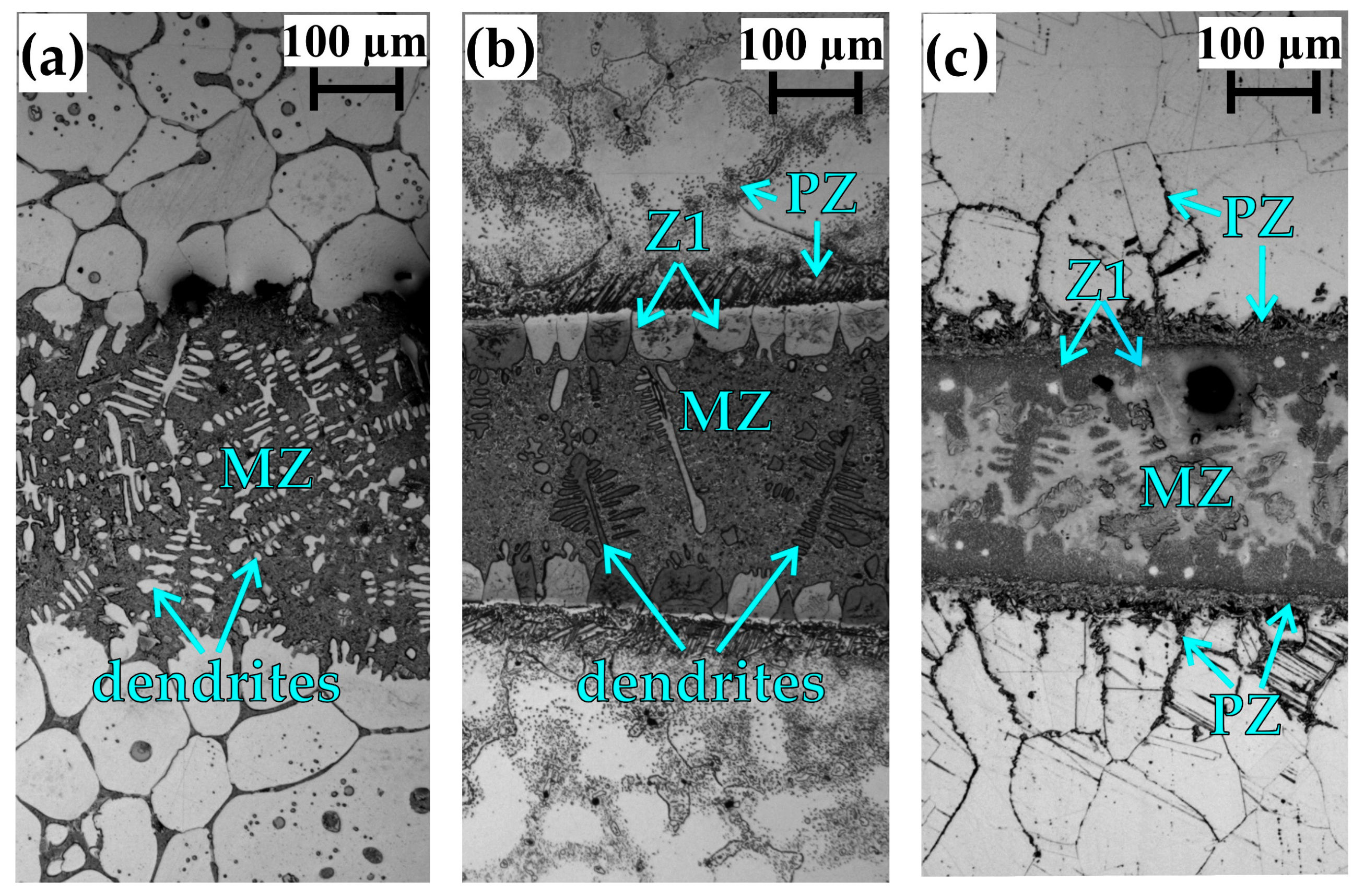
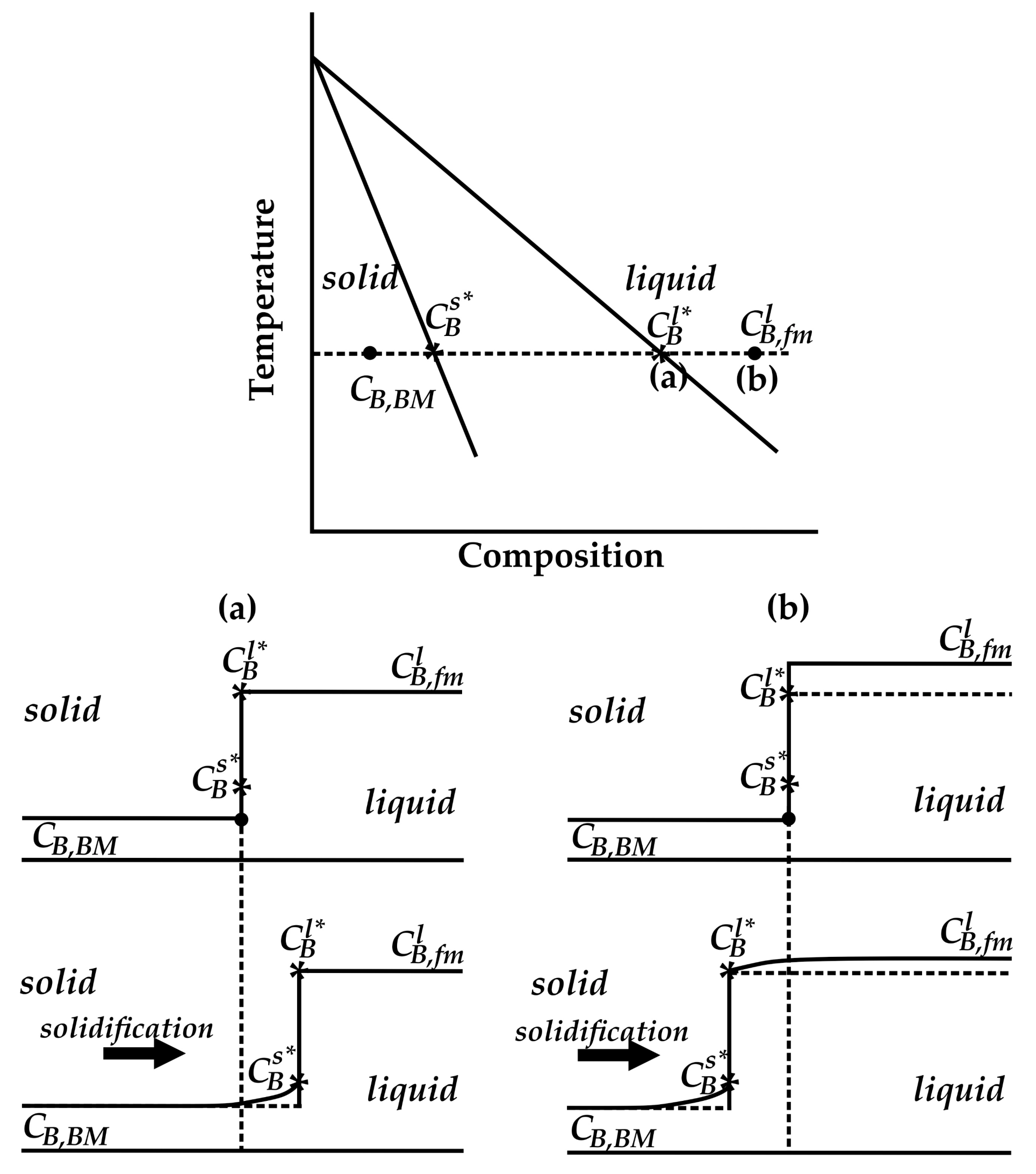
4.1. Microstructural Evolution of Z1
4.2. Microstructural Evolution of the MZ
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rabinkin, A. High-temperature brazing: Filler metals and processing. In Advances in Brazing; Elsevier: Amsterdam, The Netherlands, 2013; pp. 121–159. [Google Scholar]
- Van Esch, H.; Stover, J.T. Review of the State of the Art of High-Temperature Brazing for Combustion Turbine Components; Electric Power Research Institute (EPRI): Palo Alto, CA, USA, 2003. [Google Scholar]
- Nicolaus, M.; Möhwald, K.; Maier, H.J. A Combined Brazing and Aluminizing Process for Repairing Turbine Blades by Thermal Spraying Using the Coating System NiCrSi/NiCoCrAlY/Al. J. Therm. Spray Technol. 2017, 26, 1659–1668. [Google Scholar] [CrossRef]
- Nicolaus, M.; Möhwald, K.; Maier, H.J. Regeneration of high pressure turbine blades. Development of a hybrid brazing and aluminizing process by means of thermal spraying. Procedia CIRP 2017, 59, 72–76. [Google Scholar] [CrossRef] [Green Version]
- Nicolaus, M.; Rottwinkel, B.; Alfred, I.; Möhwald, K.; Nölke, C.; Kaierle, S.; Maier, H.J.; Wesling, V. Future regeneration processes for high-pressure turbine blades. CEAS Aeronaut. J. 2018, 9, 85–92. [Google Scholar] [CrossRef]
- Alfred, I.; Nicolaus, M.; Hermsdorf, J.; Kaierle, S.; Möhwald, K.; Maier, H.-J.; Wesling, V. Advanced high pressure turbine blade repair technologies. Procedia CIRP 2018, 74, 214–217. [Google Scholar] [CrossRef]
- Ye, Y.; Zou, G.; Long, W.; Jia, Q.; Bai, H.; Wu, A.; Liu, L. Diffusion brazing repair of IN738 superalloy with crack-like defect: Microstructure and tensile properties at high temperatures. Sci. Technol. Weld. Join. 2019, 24, 52–62. [Google Scholar] [CrossRef]
- Bai, K.; Lan Ng, F.; Tan, T.L.; Li, T.; Pan, D. Understanding non-parabolic solidification kinetics in Ni-based alloys during TLP bonding via thermo-kinetic modelling. J. Alloys Compd. 2017, 699, 1084–1094. [Google Scholar] [CrossRef]
- Arhami, F.; Mirsalehi, S.E.; Sadeghian, A.; Johar, M.H. The joint properties of a high-chromium Ni-based superalloy made by diffusion brazing: Microstructural evolution, corrosion resistance and mechanical behavior. J. Manuf. Process. 2019, 37, 203–211. [Google Scholar] [CrossRef]
- Wikstrom, N.P.; Egbewande, A.T.; Ojo, O.A. High temperature diffusion induced liquid phase joining of a heat resistant alloy. J. Alloys Compd. 2008, 460, 379–385. [Google Scholar] [CrossRef]
- Tokoro, K.; Wikstrom, N.P.; Ojo, O.A.; Chaturvedi, M.C. Variation in diffusion-induced solidification rate of liquated Ni–Cr–B insert during TLP bonding of Waspaloy superalloy. Mater. Sci. Eng. A 2008, 477, 311–318. [Google Scholar] [CrossRef]
- Riggs, B.; Alexandrov, B.; Benatar, A.; Xu, R. Metallurgical characterisations of CMSX-4 vacuum-brazed with BNi-2 and BNi-9. Sci. Technol. Weld. Join. 2017, 22, 227–235. [Google Scholar] [CrossRef]
- Riggs, B.; Alexandrov, B.; Benatar, A.; Xu, R. Microstructural characterization of single crystal superalloy CMSX-4 brazed joints with BNi-2 and BNi-9 filler metals. In Proceedings of the International Brazing and Soldering Conference, Long Beach, CA, USA, 19–22 April 2015. [Google Scholar]
- Riggs, B.B.; Alexandrov, B.B.; Benatar, A.A.; Xu, R.R. Thermodynamic and kinetic simulations of high temperature brazing: Microstructure evolution in CMSX-4 joints. Sci. Technol. Weld. Join. 2017, 22, 428–437. [Google Scholar] [CrossRef]
- Hdz-García, H.M.; Muñoz-Arroyo, R.; Martinez, A.I.; González, M.A.; Granda-Gutiérrez, E.E.; Acevedo-Dávila, J.L.; Castro-Roman, M.; García-Vázquez, F. Aging Thermal Treatment in the Inconel 725 Brazed Incorporating Tungsten Nanoparticles. J. Nanomater. 2016. [Google Scholar] [CrossRef]
- Bakhtiari, R.; Ekrami, A. The effect of gap size on the microstructure and mechanical properties of the transient liquid phase bonded FSX-414 superalloy. Mater. Des. 2012, 40, 130–137. [Google Scholar] [CrossRef]
- Yuan, X.J.; Kang, C.Y. Dissolution and Its Activation Energy during Transient Liquid Phase Bonding of Duplex Stainless Steel: Materials Research Innovations. Volume 17. No sup1. Available online: https://www.tandfonline.com/doi/full/10.1179/1432891713Z.000000000234 (accessed on 4 July 2019).
- Hdz-García, H.M.; Pech-Canul, M.I.; Muñoz-Arroyo, R.; Mtz-Enriquez, A.I.; Acevedo-Dávila, J.L.; Castro-Román, M.J.; Reyes-Valdés, F.A. 304 stainless steel brazing incorporating tungsten nanoparticles. J. Mater. Process. Technol. 2015, 215, 1–5. [Google Scholar] [CrossRef]
- Hdz-García, H.M.; Martinez, A.I.; Muñoz-Arroyo, R.; Acevedo-Dávila, J.L.; García-Vázquez, F.; Reyes-Valdés, F.A. Effects of Silicon Nanoparticles on the Transient Liquid Phase Bonding of 304 Stainless Steel. J. Mater. Sci. Technol. 2014, 30, 259–262. [Google Scholar] [CrossRef]
- Abbasi, K.B.; Asghari, G.; Bakhtiari, R. TLP bonding of dissimilar FSX-414/IN738 system with MBF80 interlayer: Prediction of solid/liquid interface location. Trans. Nonferrous Met. Soc. China 2014, 24, 996–1003. [Google Scholar]
- Abbasi, K.B.; Jahanbakhsh, A.; Bakhtiari, R. TLP bonding of dissimilar FSX-414/IN-738 system with MBF-80 interlayer: The effect of homogenizing treatment on microstructure and mechanical properties. Mater. Sci. Eng. A 2016, 651, 93–101. [Google Scholar] [CrossRef]
- Wu, X.W.; Chandel, R.S.; Seow, H.P.; Li, H. Wide gap brazing of stainless steel to nickel-based superalloy. J. Mater. Process. Technol. 2001, 113, 215–221. [Google Scholar] [CrossRef]
- Sadeghian, M.; Ekrami, A.; Jamshidi, R. Transient liquid phase bonding of 304 stainless steel using a Co-based interlayer. Sci. Technol. Weld. Join. 2017, 22, 666–672. [Google Scholar] [CrossRef]
- Ramirez, J.E.; Liu, S. Diffusion brazing in the nickel-boron system. Weld 1992, 71, 365–376. [Google Scholar]
- Yuan, X.; Kim, M.B.; Kang, C.Y. Characterization of transient-liquid-phase-bonded joints in a duplex stainless steel with a Ni–Cr–B insert alloy. Mater. Charact. 2009, 60, 1289–1297. [Google Scholar] [CrossRef]
- Idowu, O.A.; Richards, N.L.; Chaturvedi, M.C. Effect of bonding temperature on isothermal solidification rate during transient liquid phase bonding of Inconel 738LC superalloy. Mater. Sci. Eng. A 2005, 397, 98–112. [Google Scholar] [CrossRef]
- Ojo, O.A.; Richards, N.L.; Charturvedi, M.C. Effect of gap size and process parameters on diffusion brazing of Inconel 738. Sci. Technol. Weld. Join. 2004, 9, 209–220. [Google Scholar] [CrossRef]
- Ojo, O.A.; Richards, N.L.; Chaturvedi, M.C. Isothermal solidification during transient liquid phase bonding of Inconel 738 superalloy. Sci. Technol. Weld. Join. 2004, 9, 532–540. [Google Scholar] [CrossRef]
- Ojo, O.A.; Abdelfatah, M.M. On deviation from parabolic behaviour during transient liquid phase bonding. Mater. Sci. Technol. 2008, 24, 739–743. [Google Scholar] [CrossRef]
- Egbewande, A.T.; Chukwukaeme, C.; Ojo, O.A. Joining of superalloy Inconel 600 by diffusion induced isothermal solidification of a liquated insert metal. Mater. Charact. 2008, 59, 1051–1058. [Google Scholar] [CrossRef]
- Abdelfatah, M.M.; Ojo, O.A. On the extension of processing time with increase in temperature during transient-liquid phase bonding. Metall. Mater. Trans. A 2009, 40, 377–385. [Google Scholar] [CrossRef]
- Tung, S.K.; Lim, L.C.; Lai, M.O. Solidification phenomena in nickel base brazes containing boron and silicon. Scr. Mater. 1996, 34, 763–769. [Google Scholar] [CrossRef]
- Henhoeffer, T.; Huang, X.; Yand, S.; Au, P.; Nagy, D. Microstructure and high temperature tensile properties of wide gap brazed cobalt based superalloy X-40. Mater. Sci. Technol. 2010, 26, 431–439. [Google Scholar] [CrossRef]
- Ye, Y.; Wei, J.; Zou, G.; Long, W.; Bai, H.; Wu, A.; Liu, L. Microstructure of diffusion-brazing repaired IN738LC superalloy with uneven surface defect gap width. Sci. Technol. Weld. Join. 2017, 22, 617–626. [Google Scholar] [CrossRef]
- Wall Colmonoy Nicrobraz 150 MSDS—Google Search. Available online: https://www.google.com/search?q=Wall+Colmonoy+Nicrobraz+150+MSDS&oq=Wall+Colmonoy+Nicrobraz+150+MSDS&aqs=chrome.69i57j69i60l2.765j0j4&sourceid=chrome&ie=UTF-8 (accessed on 14 August 2019).
- Committee, A.I.H. ASM Handbook: Heat Treating; ASM International: Novelty, OH, USA, 1991. [Google Scholar]
- Watts, O.P. Rapid nickel plating. Trans. Am. Electrochem. Soc. 1916, 29, 395–403. [Google Scholar] [CrossRef]
- Wu, Y.Q.; Yan, M.F. Plasticity characterization of the modified layer produced by plasma nitrocarburizing of nanocrystallized 18Ni maraging steel. Vacuum 2011, 86, 119–123. [Google Scholar] [CrossRef]
- Pouranvari, M. Isothermal solidification during transient liquid-phase bonding of GTD-111/Ni-Si-B/GTD-111. Mater. Tehnol. 2014, 48, 113–118. [Google Scholar]
- Binesh, B.; Jazayeri, G.A. Transient Liquid Phase Bonding of IN738LC/MBF-15/IN738LC: Solidification Behavior and Mechanical Properties. J. Mater. Sci. Technol. 2016, 32, 1137–1151. [Google Scholar] [CrossRef]
- Duan, H.; Koçak, M.; Bohm, K.-H.; Ventzke, V. Transient liquid phase (TLP) bonding of TiAl using various insert foils. Sci. Technol. Weld. Join. 2004, 9, 513–518. [Google Scholar] [CrossRef]
- Cook, G.O.; Sorensen, C.D. Overview of transient liquid phase and partial transient liquid phase bonding. J. Mater. Sci. 2011, 46, 5305–5323. [Google Scholar] [CrossRef] [Green Version]
- LESOULT, G. Solidification - Cristallisation et microstructures. Available online: https://www.techniques-ingenieur.fr/base-documentaire/materiaux-th11/etude-des-metaux-et-des-alliages-etat-metallique-42345210/solidification-m58/ (accessed on 27 December 2019).
- Lin, T.; Li, H.; He, P.; Yang, X.; Huang, Y.; Li, L.; Han, L. Effect of bonding parameters on microstructures and properties during TLP bonding of Ni-based super alloy. Trans. Nonferrous Met. Soc. China 2012, 22, 2112–2117. [Google Scholar] [CrossRef]
- Pouranvari, M.; Ekrami, A.; Kokabi, A.H. Role of base-metal composition in isothermal solidification during diffusion brazing of nickel-based superalloys. Sci. Technol. Weld. Join. 2018, 23, 13–18. [Google Scholar] [CrossRef]
- Pouranvari, M.; Ekrami, A.; Kokabi, A.H. Diffusion brazing of cast INCONEL 718 superalloy utilising standard heat treatment cycle. Mater. Sci. Technol. 2014, 30, 109–115. [Google Scholar] [CrossRef]
- Gale, W.F.; Wallach, E.R. Microstructural development in transient liquid-phase bonding. Metall. Trans. A 1991, 22, 2451–2457. [Google Scholar] [CrossRef]
- Kokawa, H.; Lee, C.H.; North, T.H. Effect of grain boundaries on isothermal solidification during transient liquid phase brazing. Metall. Trans. A 1991, 22, 1627–1631. [Google Scholar] [CrossRef]
- Oyen, M.L.; Cook, R.F. A practical guide for analysis of nanoindentation data. J. Mech. Behav. Biomed. Mater. 2009, 2, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Fischer-Cripps, A.C. Nanoindentation, 3rd ed.; Mechanical Engineering Series; Springer: New York, NY, USA, 2011; ISBN 978-1-4419-9871-2. [Google Scholar]
- Shuman, D.J.; Costa, A.L.; Andrade, M.S. Calculating the elastic modulus from nanoindentation and microindentation reload curves. Mater. Charact. 2007, 58, 380–389. [Google Scholar] [CrossRef]
- Poirier, D.R.; Geiger, G.H. Transport Phenomena in Materials Processing; Wiley: Hoboken, NJ, USA, 1998; ISBN 978-0-87339-272-3. [Google Scholar]
- Gale, W.F.; Butts, D.A. Transient liquid phase bonding. Sci. Technol. Weld. Join. 2004, 9, 283–300. [Google Scholar] [CrossRef]
- Liu, Z.-K.; Ågren, J. On the transition from local equilibrium to paraequilibrium during the growth of ferrite in Fe-Mn-C austenite. Acta Metall. 1989, 37, 3157–3163. [Google Scholar]
- Hillert, M. Paraequilibrium and other restricted equilibria. MRS Online Proc. Libr. Arch. 1982, 19. [Google Scholar] [CrossRef]
- Flemings, M.C. Solidification processing. Metall. Trans. 1974, 5, 2121–2134. [Google Scholar] [CrossRef]
- MacDonald, W.D.; Eagar, T.W. Isothermal solidification kinetics of diffusion brazing. Metall. Mater. Trans. A 1998, 29, 315–325. [Google Scholar] [CrossRef]
- Kurz, W.; Fisher, D.J. Fundamentals of solidification. Trans. Tech. Publ. Switz. 1989. [Google Scholar] [CrossRef] [Green Version]
- Lippold, J.C.; Kiser, S.D.; DuPont, J.N. Welding Metallurgy and Weldability of Nickel-Base Alloys; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 978-1-118-21003-1. [Google Scholar]
- Cortial, F.; Corrieu, J.M.; Vernot-Loier, C. Influence of heat treatments on microstructure, mechanical properties, and corrosion resistance of weld alloy 625. Metall. Mater. Trans. A 1995, 26, 1273–1286. [Google Scholar] [CrossRef]
- Brŭna, M.; Sládek, A.; Kucharčík, L. Formation of porosity in Al-Si alloys. Arch. Foundry Eng. 2012, 12, 5–8. [Google Scholar] [CrossRef]
- Yue, Q.; Liu, L.; Yang, W.; Huang, T.; Zhang, J.; Fu, H.; Zhao, X. Influence of withdrawal rate on the porosity in a third-generation Ni-based single crystal superalloy. Prog. Nat. Sci. Mater. Int. 2017, 27, 236–243. [Google Scholar] [CrossRef]
- Whitesell, H.S.; Overfelt, R.A. Influence of solidification variables on the microstructure, macrosegregation, and porosity of directionally solidified Mar-M247. Mater. Sci. Eng. A 2001, 318, 264–276. [Google Scholar] [CrossRef]
- Sung, P.K.; Poirier, D.R.; Felicelli, S.D.; Poirier, E.J.; Ahmed, A. Simulations of microporosity in IN718 equiaxed investment castings. J. Cryst. Growth 2001, 226, 363–377. [Google Scholar] [CrossRef]
- Mancha, H.; Herrera-Trejo, M.; García, G.; Méndez, M.; Ablitzer, D. Solubility of nitrogen in liquid ASTM F-75 alloys. Mater. Des. 1996, 17, 103–106. [Google Scholar] [CrossRef]
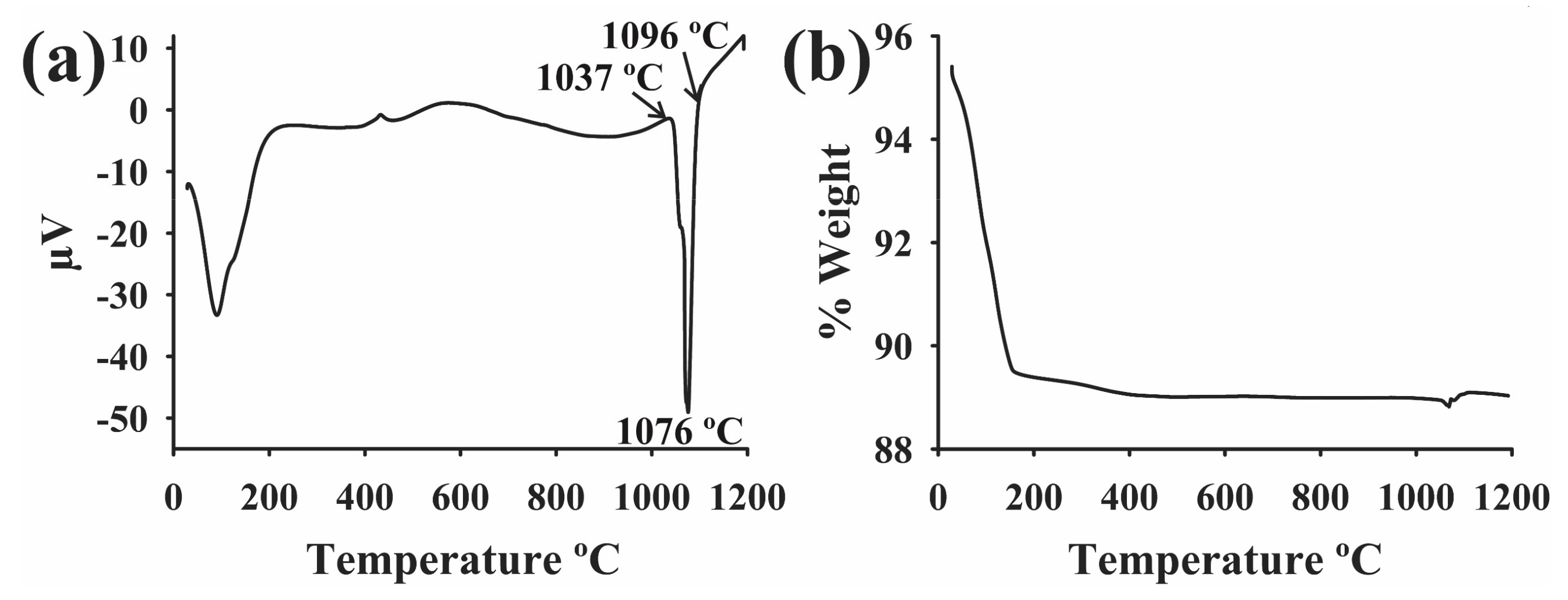
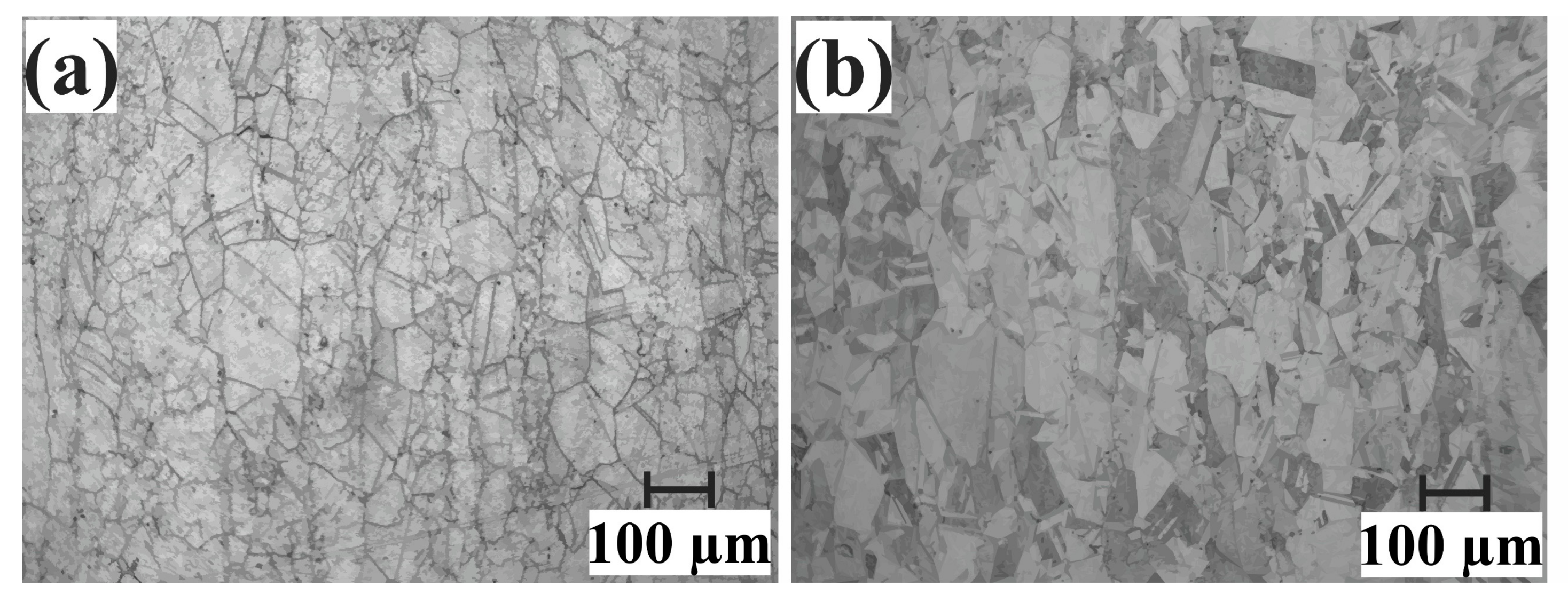
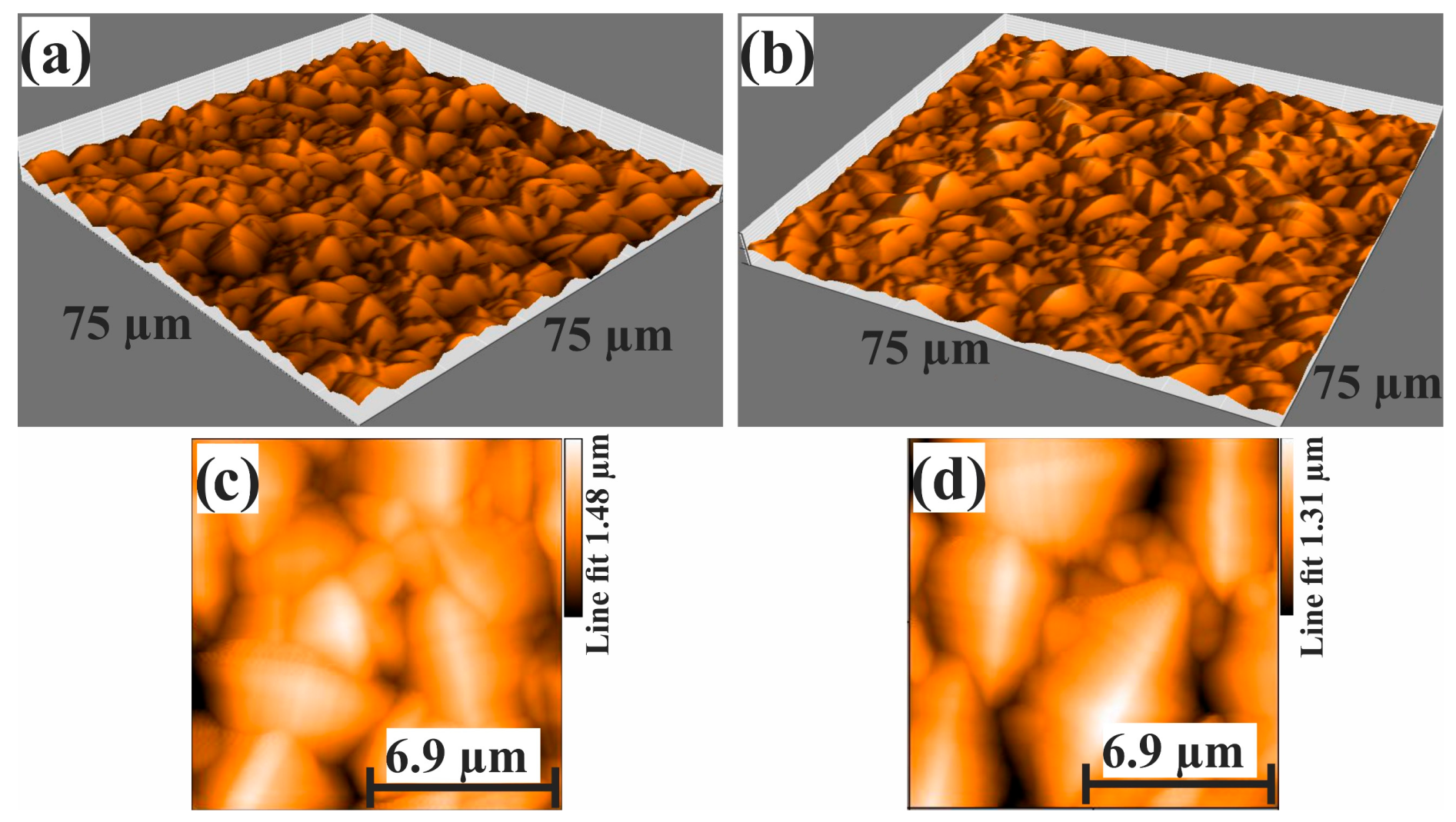
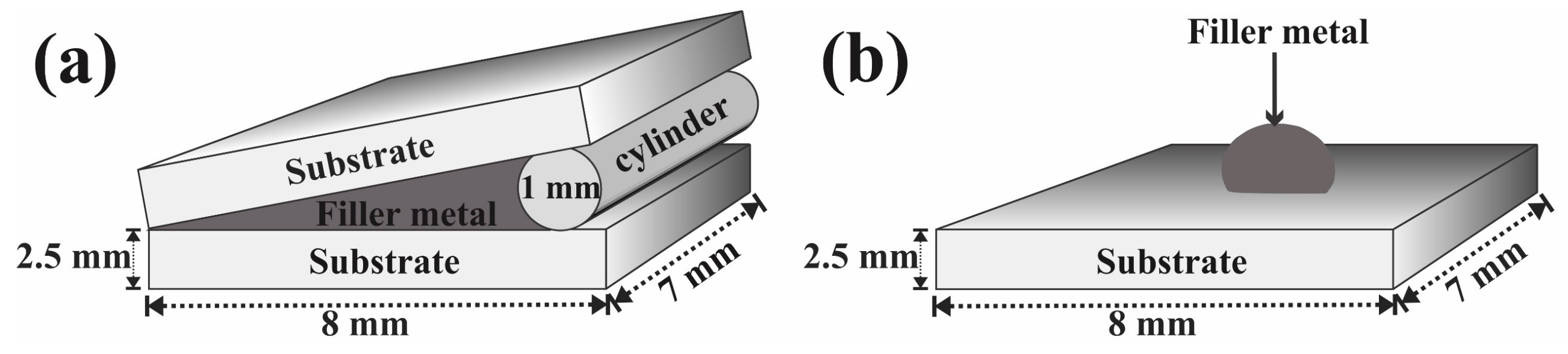



| Base Alloy | Alloys | Filler Metal, Thickness | Brazing Microstructures | Brazing Conditions | References |
|---|---|---|---|---|---|
| Nickel | IN 718 | BNi-9 foil 40 µm | Full solidified at 6 min | 1.33 × 10−3 Pa 1090 °C 60–3600 s | Bai et al. [8] |
| IN 939 | BNi-9 foil 75 µm | Full solidified at 90 min | 1.33 × 10−2 Pa 1120 °C 45, 60, 75, and 90 min | Arham et al. [9] | |
| Waspaloy | BNi-9 foil 100 µm | Full solidified at 6 h 1145 °C at 8 h 1100 °C | 1.33 × 10−3 Pa 1100–1225 °C 1–8 h | Wikstrom et al. [10] | |
| Waspaloy | BNi-9 foil 80 and 100 µm | 80 µm full solidified at 300 min 1065 °C, and at 240 min-1080 °C, 100 µm full solidified at 360 min 1145 °C | 1.33 × 10−3 Pa 20, 240, and 300 min at 1065 °C 1145, 1175, 1200 and 1225 °C during 360 min | Tokoro et al. [11] | |
| CMSX-4 | BNi-9 powder 80-100 µm | Partial solidified at 10 min | <1.33 × 10−3 Pa 1121 °C 10 min | Riggs et al. [12,13,14] | |
| IN 725 | BNi-9 paste 1 mm | Partial solidified at 2 h | Ar 1150 °C 2 h | Hernandez et al. [15] | |
| Cobalt | FSX-414 | BNi-9 foil 25, 50, 75 and 100 µm | 50 µm interlayer full solidified at 5 min | 1.33 × 10−2 Pa 1150 °C 1–30 min | Backhtiari et al. [16] |
| Stainless Steel | Duplex SS | BNi-9 foil 50 and 200 µm | Partial solidified in all conditions | 1.33 × 10−2 Pa 1075–1115 °C 0–600 s | Yuan et al. [17] |
| 304-SS | BNi-9 paste 1 mm | Partial solidified in all conditions | Ar 1200 °C 10, 20, 30 and 60 min | Hernandez et al. [18,19] | |
| Dissimilar | FSX-414/IN738 | BNi-9 foil 50 µm | Partial solidified in all conditions | 2.66 × 10−3 Pa 1050, 1075, 1100 and 1150 °C 1200, 600 and 300 s | Abbasi et al. [20,21] |
| Element | Base Metals | Filler Metal Paste | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Waspaloy | FSX-414 | 304-SS | BNi-9 | ||||||
| Std. UNS N07001 | Meas. | Std. | Meas. | Std. ASTM A240 | Meas. | Std. AWS 5.8 | Meas. | Calcinated | |
| Ni | Bal. | Bal. | 9.5–11.5 | 10.81 | 8–10.5 | 9.25 | - | Bal. | Bal. |
| Co | 12–15 | 12.55 | Bal. | Bal. | - | - | - | 0.005 | <0.02 |
| Fe | <2 | 0.186 | <2 | 1.36 | Bal. | Bal. | - | 0.36 | 0.396 |
| Cr | 18–21 | 19.52 | 27–31 | 28.32 | 18–20 | 19.50 | 14 | 12.89 | 13.38 |
| Mo | 3.5–5 | 3.98 | - | 0.211 | - | - | - | - | - |
| Al | 1.2–1.6 | 1.27 | - | 0.0058 | - | - | - | 0.0015 | 0.019 |
| Ti | 2.75–3.25 | 2.81 | - | - | - | - | - | <0.007 | <0.005 |
| Nb | - | - | - | 0.053 | - | - | - | - | - |
| Cu | <0.5 | 0.004 | - | 0.028 | - | - | - | - | - |
| Mn | <1 | 0.0054 | <1 | 0.62 | <2 | 2 | - | - | - |
| Si | <0.75 | 0.031 | <1 | 0.709 | <0.75 | 1 | - | - | - |
| W | - | - | 6-8 | 7.68 | - | - | - | - | - |
| Zr | 0.02–0.12 | 0.019 | - | - | - | - | - | <0.01 | 0.0013 |
| V | - | 0.0002 | - | <0.007 | - | - | - | - | - |
| B | 0.003–0.01 | - | <0.012 | - | - | - | 3 | 2.83 | 3.29 |
| C | 0.02–0.10 | 0.04 | 0.2–0.3 | - | <0.07 | 0.07 | <0.1 | 1.03 | 0.147 |
| P | <0.030 | <0.002 | <0.035 | - | <0.045 | 0.045 | 0.02 | 0.0075 | - |
| S | <0.03 | 0.009 | <0.015 | 0.022 | <0.03 | 0.015 | - | 0.0133 | 0.018 |
| Element/Alloy | Sol-Wasp | Age-Wasp | FSX-414 | 304-SS | BNi-9 (Filler Metal Paste) | |
|---|---|---|---|---|---|---|
| Paste | Calcined Paste | |||||
| Oxygen | 11.4 PPM | 7.9 PPM | 201 PPM | 98.7 PPM | 50580 PPM | 3050 PPM |
| Hydrogen | 2.5 PPM | 3.8 PPM | 92 PPM | 4.7 PPM | >1000 PPM | 270 PPM |
| Nitrogen | 19.2 PPM | 19.8 PPM | 1189 PPM | 722.3 PPM | 740 PPM | 130 PPM |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Escareño, N.Y.; Castro-Román, M.d.J.; Hernández-García, H.M.; Herrera-Trejo, M. Metallurgical Interaction among BNi-9 and Waspaloy, FSX-414 or 304-Type Stainless Steel under TLP Cycle. Metals 2020, 10, 306. https://doi.org/10.3390/met10030306
Flores-Escareño NY, Castro-Román MdJ, Hernández-García HM, Herrera-Trejo M. Metallurgical Interaction among BNi-9 and Waspaloy, FSX-414 or 304-Type Stainless Steel under TLP Cycle. Metals. 2020; 10(3):306. https://doi.org/10.3390/met10030306
Chicago/Turabian StyleFlores-Escareño, Norma Yolanda, Manuel de Jesús Castro-Román, Héctor Manuel Hernández-García, and Martín Herrera-Trejo. 2020. "Metallurgical Interaction among BNi-9 and Waspaloy, FSX-414 or 304-Type Stainless Steel under TLP Cycle" Metals 10, no. 3: 306. https://doi.org/10.3390/met10030306






