The Microstructure and Properties of an Al-Mg-0.3Sc Alloy Deposited by Wire Arc Additive Manufacturing
Abstract
:1. Introduction
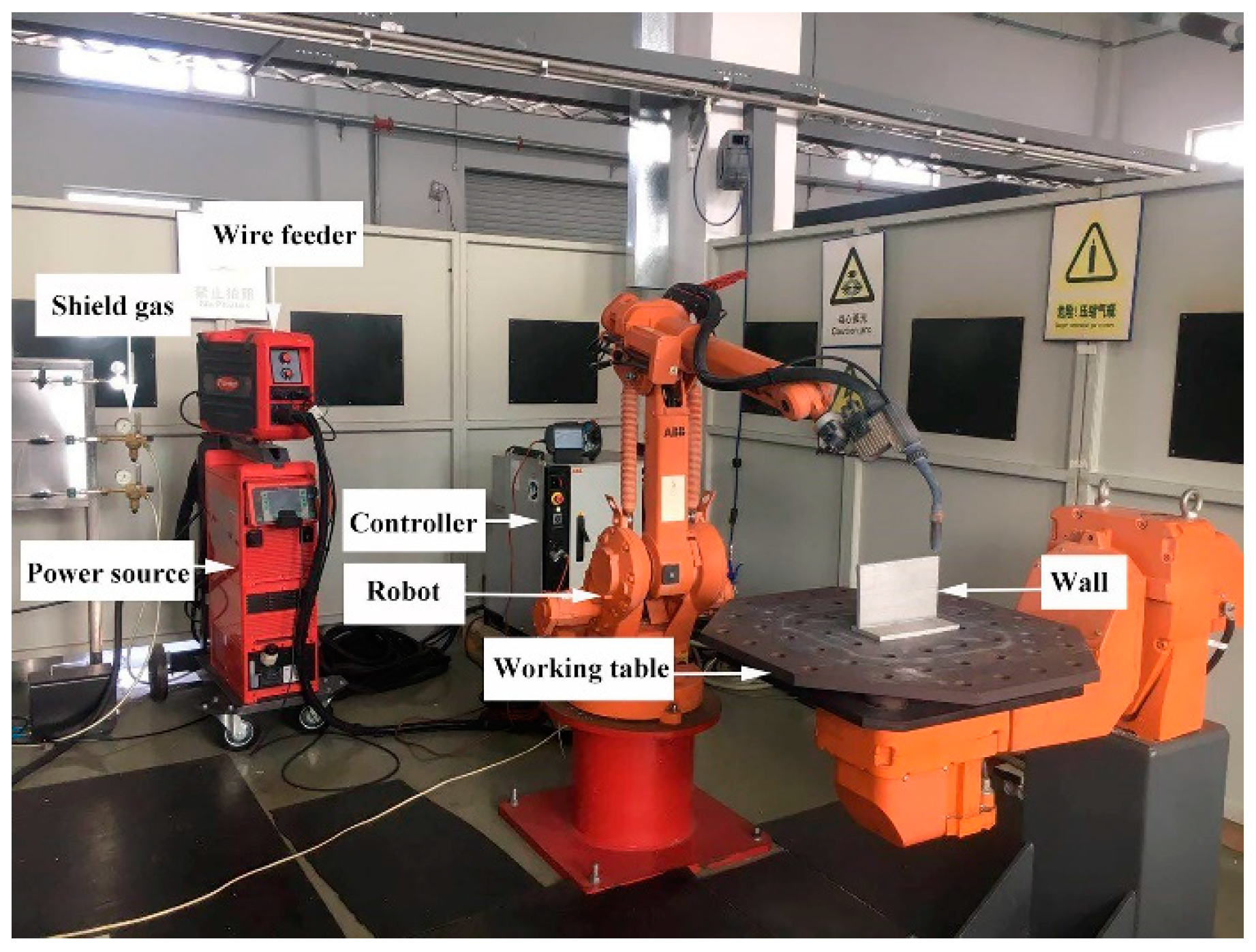
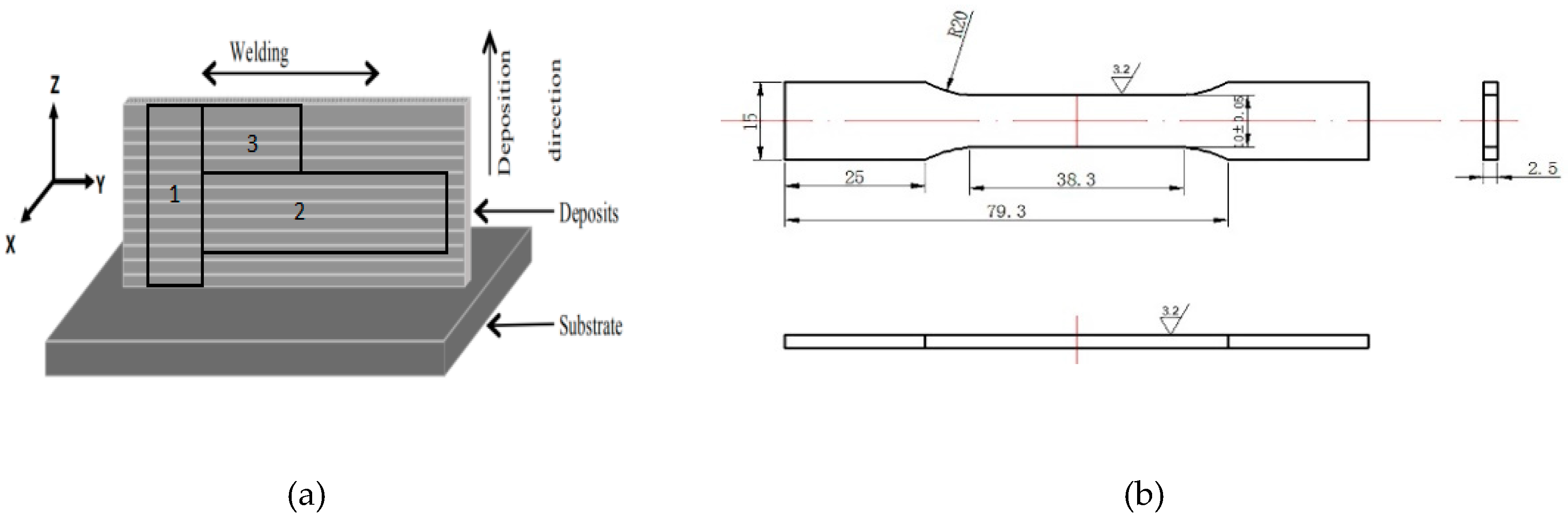
2. Materials and Methods
3. Results and Discussion
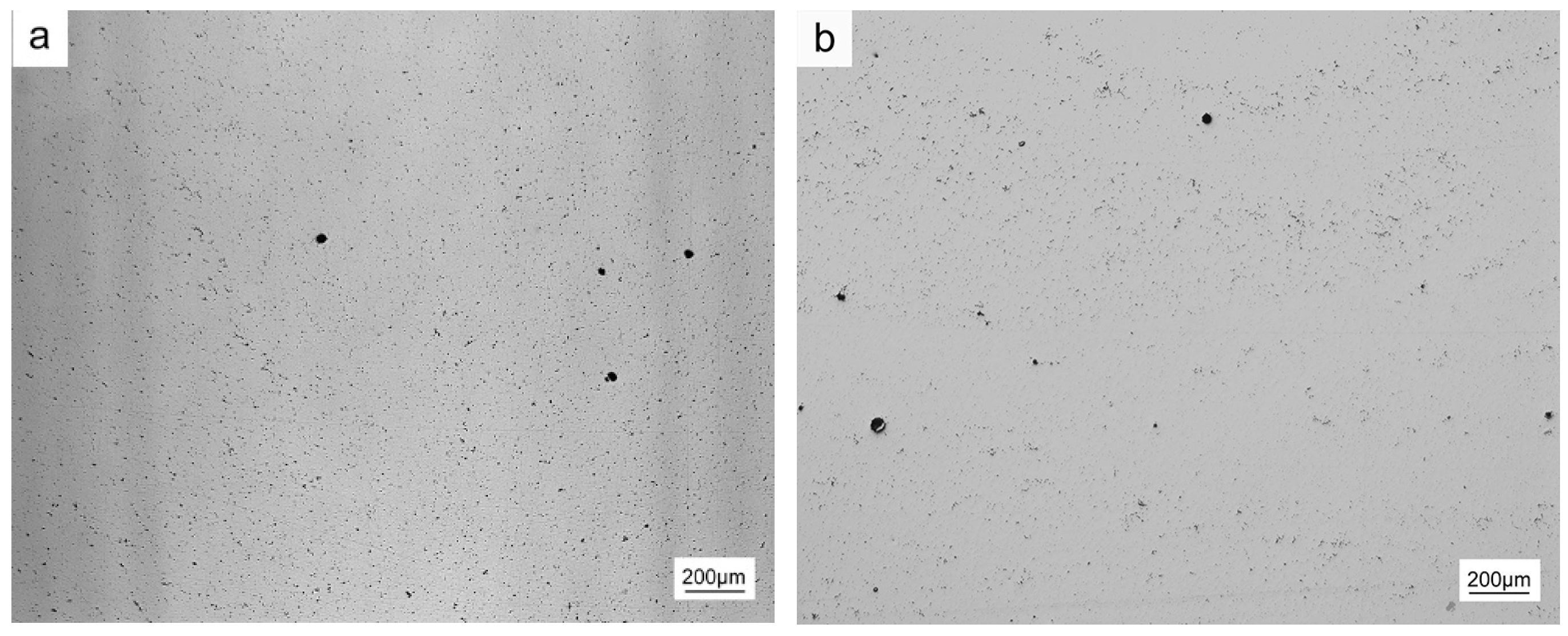
3.1. Pores
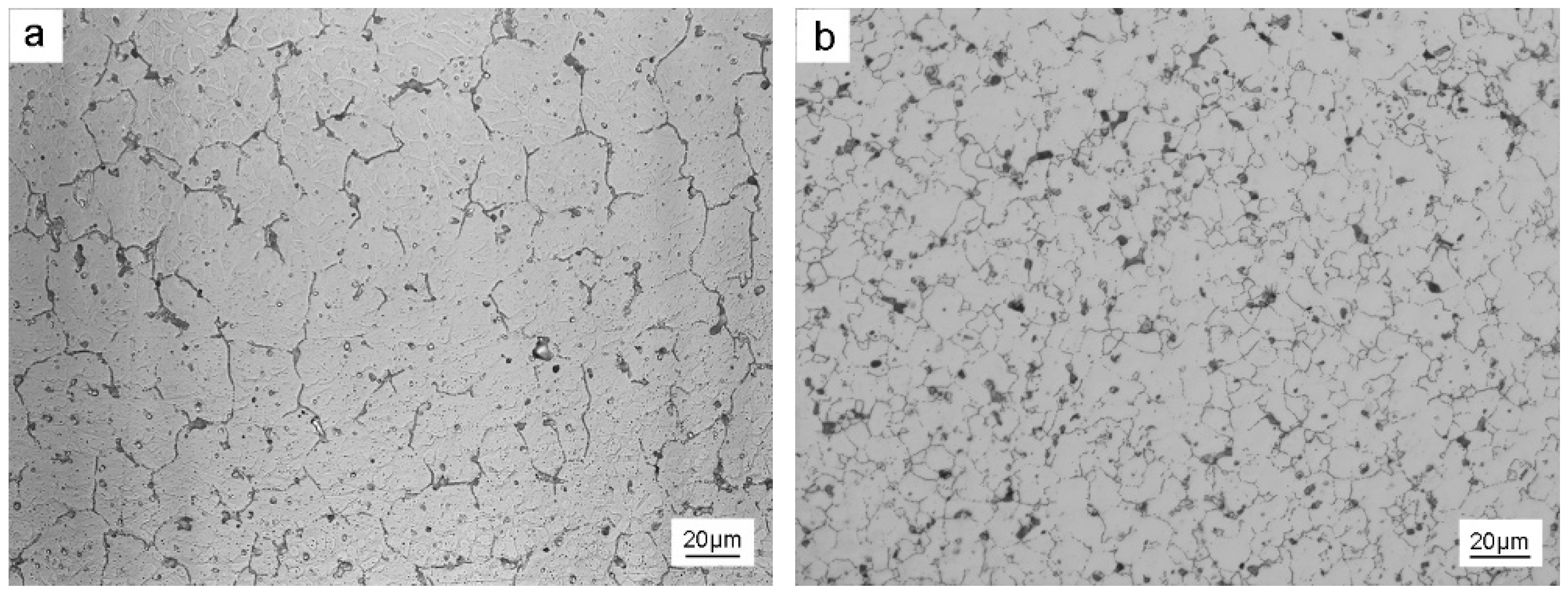
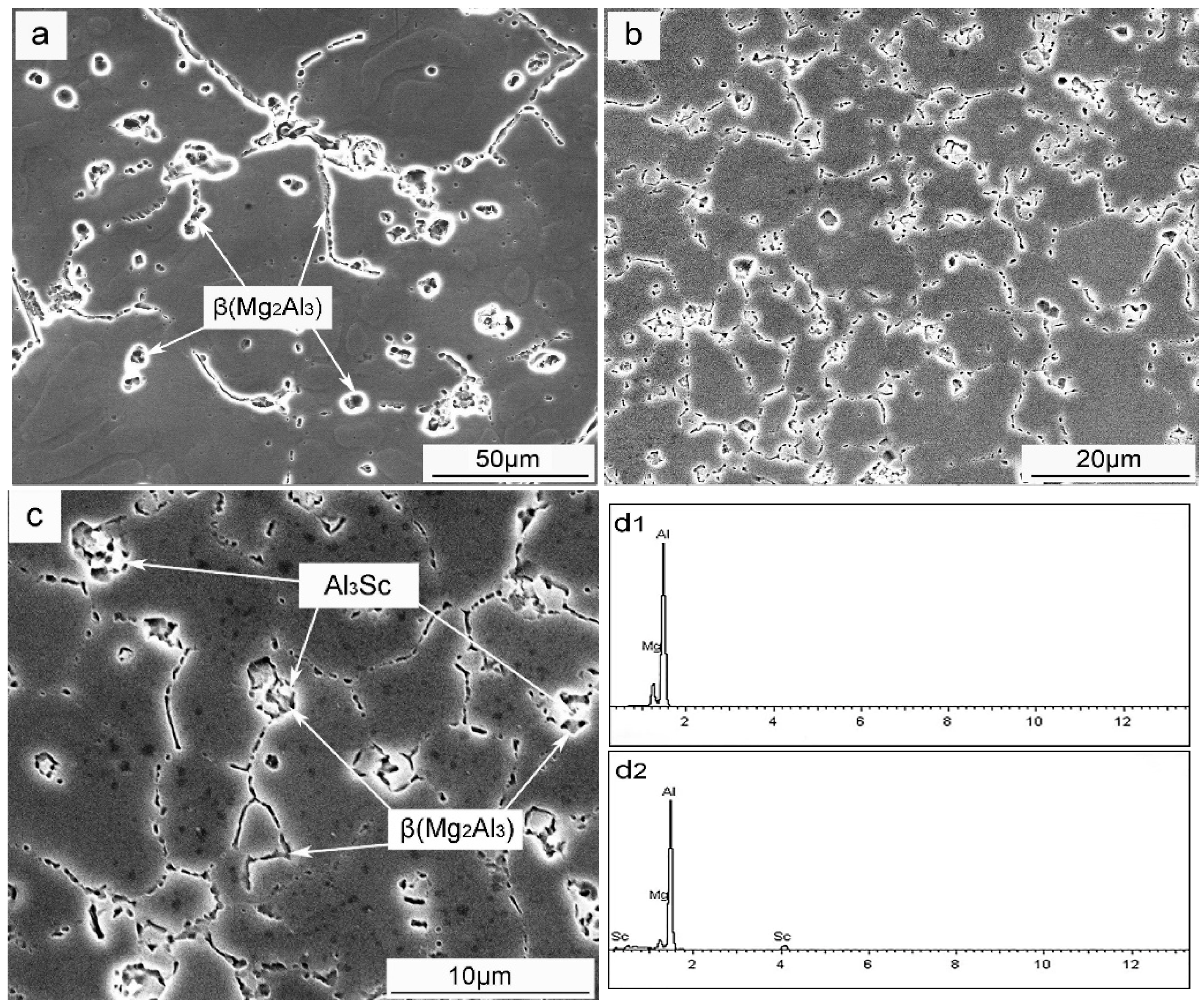
3.2. Primary Al3Sc Phase
3.3. Secondary Al3Sc Phase
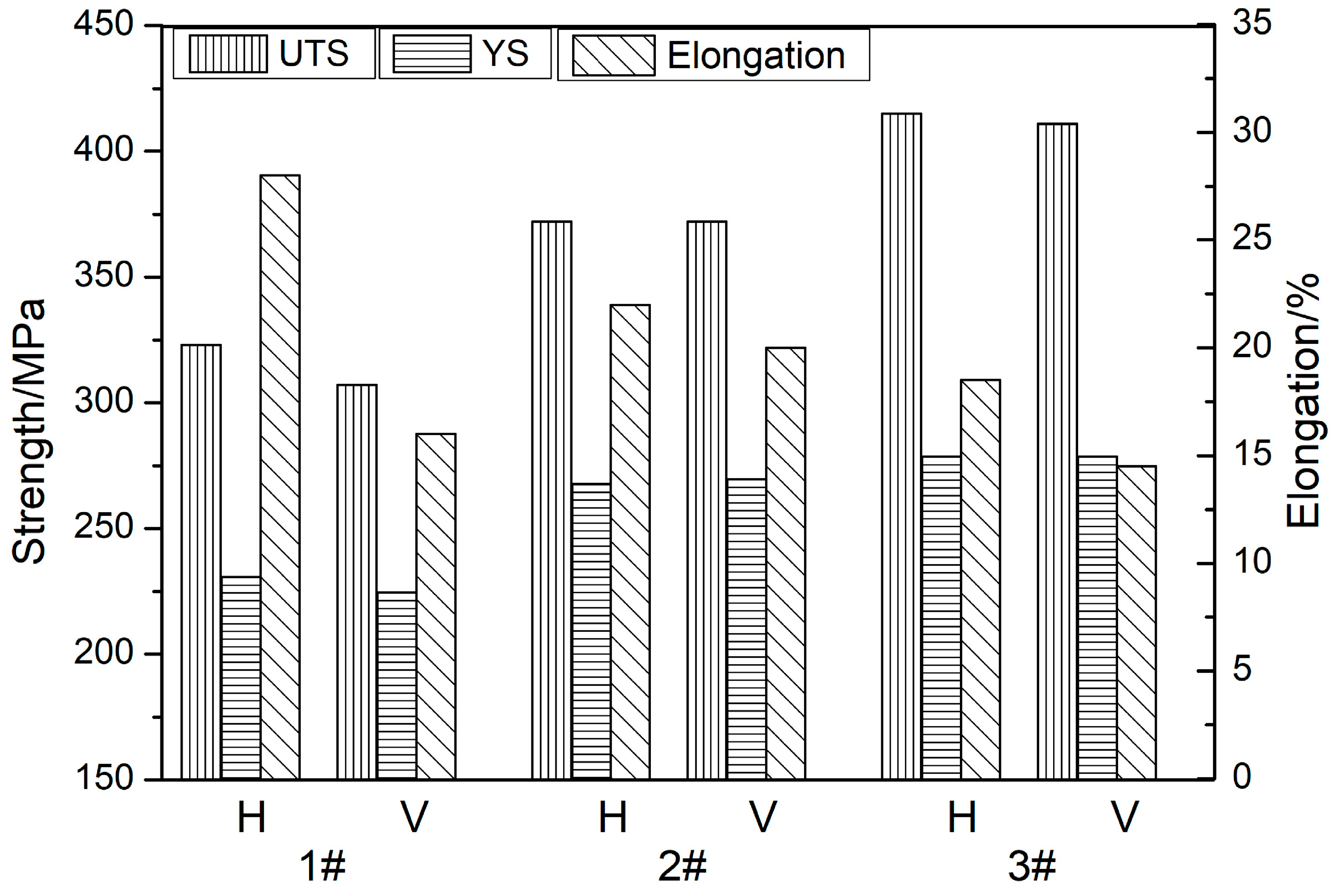
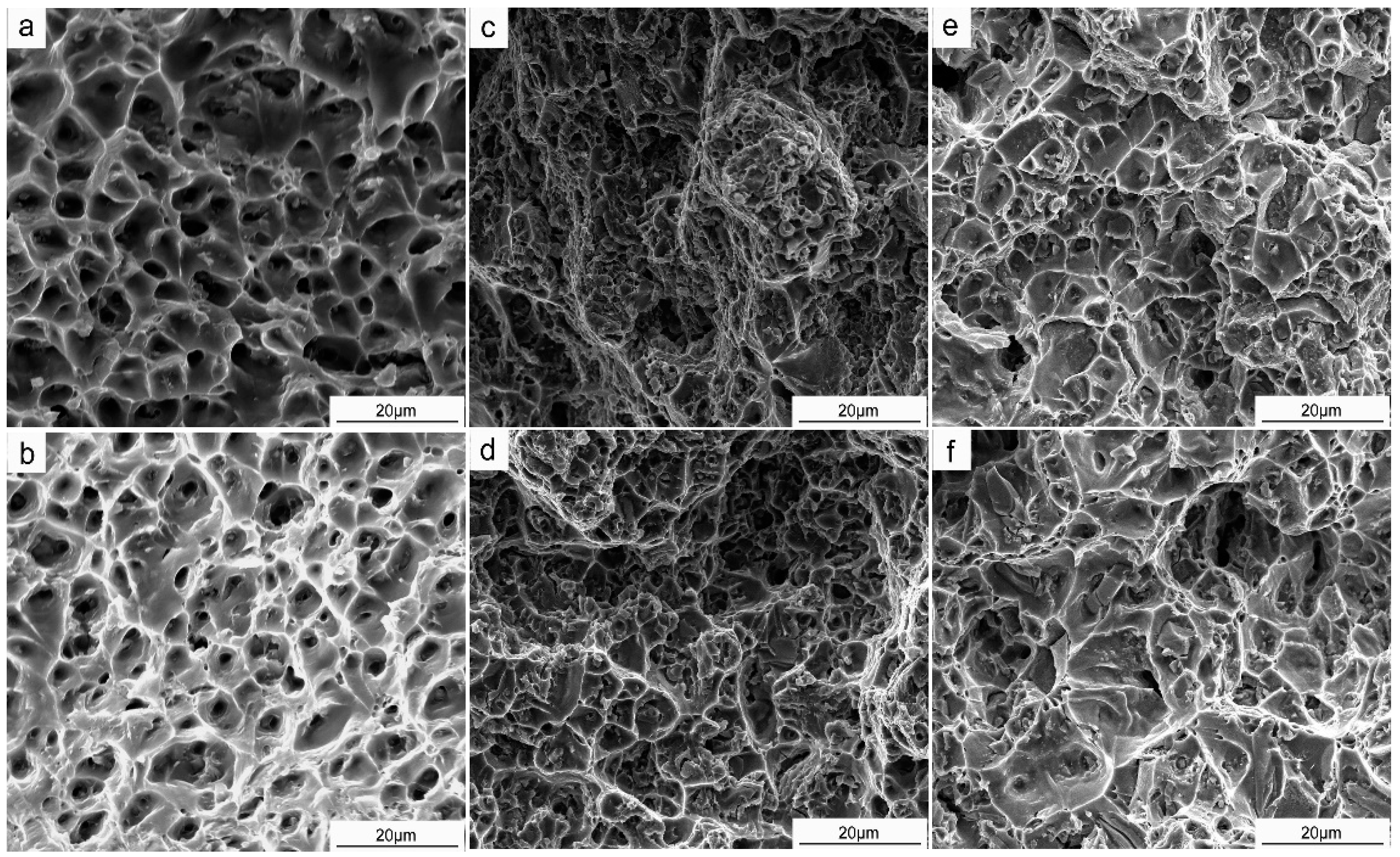
3.4. Mechanical Properties and Fracture Morphology
4. Conclusions and Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Williams, S.W.; Martina, F.; Addison, A.C.; Ding, J.; Pardal, G.; Colegrove, P. Wire plus Arc Additive Manufacturing. Mater. Sci. Technol. 2016, 32, 641–647. [Google Scholar] [CrossRef] [Green Version]
- Szost, B.A.; Terzi, S.; Martina, F.; Boisselier, D.; Prytuliak, A.; Pirling, T.; Hofmann, M.; Jarvis, D.J. A comparative study of additive manufacturing techniques: Residual stress and microstructural analysis of CLAD and WAAM printed Ti–6Al–4V components. Mater. Des. 2016, 89, 559–567. [Google Scholar] [CrossRef] [Green Version]
- Zhan, Q.; Liang, Y.; Ding, J.; Williams, S. A wire deflection detection method based on image processing in wire + arc additive manufacturing. Int. J. Adv. Manuf. Technol. 2017, 89, 755–763. [Google Scholar] [CrossRef] [Green Version]
- Gu, J.; Ding, J.; Williams, S.W.; Gu, H.; Bai, J.; Zhai, Y.; Ma, P. The strengthening effect of inter-layer cold working and post-deposition heat treatment on the additively manufactured Al–6.3Cu alloy. Mater. Sci. Eng. A 2016, 651, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Gu, H.; Wang, W.; Li, C.; Ren, L.; Wang, Z.; Zhai, Y.; Ma, P. Microstructure and mechanical properties of aluminum alloy (ZL205A) wall produced by wire arc additive manufacturing method. Rare Met. Mater. Eng. 2019, 48, 2910–2916. [Google Scholar]
- Li, C.; Gu, H.; Wang, W.; Ming, Z.; Wang, S.; Ren, L.; Wang, Z.; Zhai, Y. Investigation on microstructure and properties of aluminum alloy (ZL114A) by wire arc additive manufacturing (WAAM). Rare Met. Mater. Eng. 2019, 48, 2917–2922. [Google Scholar]
- Anyalebechi, P.N. Hydrogen-induced gas porosity formation in Al-4.5wt % Cu-1.4 wt % Mg alloy. J. Mater. Sci. 2013, 48, 5342–5353. [Google Scholar] [CrossRef]
- Gu, J.L. Study on Microstructure and Mechanical Properties of Additively Manufactured Al-Cu-(Mg) Alloys with the CMT Process; Northeastern University: Shenyang, China, 2016. [Google Scholar]
- Horgar, A.; Fostervoll, H.; Nyhus, B.; Ren, X.; Eriksson, M.; Akselsen, O.M. Additive manufacturing using WAAM with AA5183 wire. Mater. Process. Technol. 2018, 259, 68–74. [Google Scholar] [CrossRef]
- Geng, H.; Li, J.; Xiong, J.; Lin, X.; Zhang, F. Geometric limitation and tensile properties of wire and arc additive manufacturing 5A06 aluminum alloy parts. J. Mater. Eng. Perform. 2017, 26, 621–629. [Google Scholar] [CrossRef] [Green Version]
- Rostova, T.D.; Davydov, V.G.; Yelagin, V.I.; Zakharov, V.V. Effect of Scandium on Recrystallization of Aluminum and its alloys. Mater. Sci. Forum 2000, 793–798. [Google Scholar] [CrossRef]
- Brodova, I.G.; Shirinkina, I.G.; Antonova, O.V.; Chirkova, A.V.; Dobatkin, S.V.; Zakharov, V.V. Evolution of the Structure and Phase Composition of Rapidly Solidified Al-Mg-Mn-Sc-Zr Alloys during Deformation in a Bridgman Anvil. Promis. Mater. Technol. 2010, 4, 328–334. [Google Scholar] [CrossRef]
- Marquis, E.A.; Seidman, D.N. Coarsening kinetics of nanoscale Al3Sc precipitates in an Al-Mg-Sc alloy. Acta Mater. 2005, 53, 4259–4268. [Google Scholar] [CrossRef]
- Taendl, J.; Orthacker, A.; Amenitsch, H.; Kothleitner, G.; Poletti, C. Influence of the degree of scandium supersaturation on the precipitation kinetics of rapidly solidified Al-Mg-Sc-Zr alloys. Acta Mater. 2016, 117, 43–50. [Google Scholar] [CrossRef]
- Marquis, E.A.; Seidman, D.N. Nanostructural evolution of Al3Sc precipitates in an Al-Sc-Mg alloy by three-dimensional atom probe microscopy. Surf. Interface Anal. 2004, 36, 559–563. [Google Scholar] [CrossRef]
- Du, Y.; Su, X.; Zou, J. Research Progress of Al3Sc Precipitates in Al-Sc Alloy. Heat Treat. Met. 2007, 32, 12–15. [Google Scholar] [CrossRef]
- Hyde, K.B.; Norman, A.F.; Prangnell, P.B. The effect of cooling rate on the morphology of primaryAl3Sc intermetallic particles in Al-Sc alloys. Acta Mater. 2001, 49, 1327–1337. [Google Scholar] [CrossRef]
- Ren, L.; Gu, H.; Wang, W.; Wang, S.; Li, C.; Wang, Z.; Zhai, Y.; Ma, P. Effect of interpass cooling on material properties of wire arc additive manufactured Al-6.3Mg alloy. 3D Print. Addit. Manuf. 2019, 6, 344–353. [Google Scholar] [CrossRef]
- Tao, H.; Li, S.; Liu, J.; Zhou, X.; Kang, N.; Yin, Z. Micro-alloying mechanism of Sc in aluminum alloys. Mater. Sci. Eng. Powder Metall. 2008, 13, 249–259. [Google Scholar]
- Knipling, K.E.; Dunand, D.C.; Seidman, D.N. Criteria for developing castable, creep-resistant aluminum-based alloys—A review. Int. J. Mater. Res. 2006, 97, 246–265. [Google Scholar] [CrossRef] [Green Version]
- Li, X.C. Microstructure and Metallographic Atlas of Aluminum Alloy Materials; Metallurgical Industry Press: Beijing, China, 2010; pp. 257–261. [Google Scholar]

| Alloys | Si | Fe | Mn | Mg | Ti | Sc | Al |
|---|---|---|---|---|---|---|---|
| Al-Mg | 0.0191 | 0.0889 | 0.745 | 6.32 | 0.137 | - | balance |
| Al-Mg-0.3Sc | 0.0179 | 0.0905 | 0.724 | 6.39 | 0.134 | 0.28 | balance |
| Process Parameters | |
|---|---|
| Current | 90 A |
| Arc voltage | 10 V |
| Travel speed | 8 mm/s |
| Wire feed speed | 5.5 mm/min |
| Argon(99.999%) flow rate | 25 L/min |
| Interpass temperature | 60–80 °C |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, L.; Gu, H.; Wang, W.; Wang, S.; Li, C.; Wang, Z.; Zhai, Y.; Ma, P. The Microstructure and Properties of an Al-Mg-0.3Sc Alloy Deposited by Wire Arc Additive Manufacturing. Metals 2020, 10, 320. https://doi.org/10.3390/met10030320
Ren L, Gu H, Wang W, Wang S, Li C, Wang Z, Zhai Y, Ma P. The Microstructure and Properties of an Al-Mg-0.3Sc Alloy Deposited by Wire Arc Additive Manufacturing. Metals. 2020; 10(3):320. https://doi.org/10.3390/met10030320
Chicago/Turabian StyleRen, Lingling, Huimin Gu, Wei Wang, Shuai Wang, Chengde Li, Zhenbiao Wang, Yuchun Zhai, and Peihua Ma. 2020. "The Microstructure and Properties of an Al-Mg-0.3Sc Alloy Deposited by Wire Arc Additive Manufacturing" Metals 10, no. 3: 320. https://doi.org/10.3390/met10030320




